Abstract
Emerald ash borer (EAB) Agrilus planipennis Fairmaire (Coleoptera: Buprestidae) is an invasive, wood-boring pest of North American ash trees (Fraxinus spp.). Oobius agrili Zhang and Huang (Hymenoptera: Encyrtidae), a solitary egg parasitoid, was one of the several parasitoids introduced from the pest’s native Northeast Asian range to the USA for EAB classical biocontrol. Since its introduction, this agent has been released over 31 states against EAB, yet determination of the spread and impact of this parasitoid has proved difficult partly because of its small size and cryptic host eggs. The present study examines the dispersal distance and parasitism of O. agrili shortly after release, as well as the impact of host’s food plants (trees), where the host eggs were deployed. Sentinel EAB eggs were deployed on pairs of green ash (Fraxinus pensylvanica) and white fringe (Chionanthus virginicus) trees in circles around the release point up to 45 m away. After 48 or 120 h, the eggs were retrieved and examined for parasitism. There was no significant difference in observed parasitism by distance or tree species. However, significantly more EAB eggs were parasitized in the longer deployment compared to the shorter deployment. These findings suggest that sentinel EAB eggs may be deployed on ash or white fringe trees to effectively monitor the establishment and spread of O. agrili. Future studies using sentinel host eggs in natural ash stands may yield further insights into the spread rate of O. agrili post-release and its effectiveness in suppressing the targeted pest populations over time.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Factors affecting insect establishment and dispersal after deliberate and unintentional introduction to new areas have been perennial topics of investigation. Failure to establish and spread can be caused fully or in part by many biotic and abiotic variables, including but not limited to: phenological mismatch (Ramsfield et al. 2016), insufficient propagule size and number (Lockwood et al. 2005), unavailability of hosts (Dang et al. 2021), extreme weather events (Tobin et al. 2017; Macquarrie et al. 2019), stochasticity and Allee effects (Williams et al. 2021), lack of habitat connectivity (Lustig et al. 2017), and dispersal ability (Fagan et al. 2002; Fahrner and Aukema 2018). In the context of classical biological control, determining the dispersal capacity of introduced insects is an essential aspect of selecting agents and predicting their subsequent establishment and spread. A balance between high and low dispersal ability must be sought in order to avoid the potential pitfalls of slow spread, inbreeding, and Allee effects (Heimpel and Asplen 2011). Due to the myriad factors potentially affecting dispersal and establishment, each insect of interest must be studied on a case-by-case basis.
Agrilus planipennis Fairmaire (Coleoptera: Buprestidae), or emerald ash borer (EAB), is among the most damaging invasive species in North America (Herms and McCullough 2014). The larval stage of EAB feeds on the phloem of ash trees (Fraxinus spp.), resulting in tree decline and eventual death (MacFarlane and Meyer 2005). Since its accidental introduction in Michigan, USA in 2002, it has invaded 35 US states and five Canadian provinces, causing severe economic losses and degradation of forest ecosystem functions and services (Kovacs et al. 2010; McCullough 2019). Besides utilizing Fraxinus spp. trees as food plants in both native and newly invaded ranges, this beetle has recently been discovered successfully attacking the white fringe tree, Chionanthus virginicus, native to the southern USA (Peterson and Cipollini, 2017; Olson and Rieske 2019).
Classical biological control is among the most promising long-term, low-cost methods through which to regulate invasive EAB populations (Bauer et al. 2015; Duan et al. 2018). To this end several parasitoid species from EAB’s native range were collected, tested, and selected for introduction to North America. One of these parasitoids was Oobius agrili Zhang and Huang (Hymenoptera: Encyrtidae). In its native range, O. agrili is responsible for 12–62% EAB egg mortality in infested ash trees (Liu et al. 2007). As an egg parasitoid, O. agrili prevents damage to ash trees by attacking EAB before they hatch and begin feeding on the trees (Liu et al. 2007). Since its release in North America, O. agrili attack rate on EAB infesting ash trees have been low and variable, but they have been observed to parasitize up to 21.8% of eggs (Abell et al. 2014; Duan et al. 2015). Currently, it is not known if O. agrili attacks host eggs laid on white fringe trees. Thus, there is a need for further study of factors affecting O. agrili performance.
The uncertainties around O. agrili parasitism rates are due in part to the difficulty in sampling this minute insect. Visual surveys for EAB eggs are possible, but observer effects are a concern (Abell et al. 2014; Jennings et al. 2018). Many studies of O. agrili utilize bark sifting, in which the bark of ash trees is removed and EAB eggs sifted out and examined for evidence of parasitization (Abell et al. 2014; Jennings et al. 2018; Petrice et al. 2021). This method is considered to be among the most effective (Petrice et al. 2021). Yellow pan traps are another commonly used method, and while the success rate is mixed, yellow pan traps do successfully capture O. agrili (Parisio et al. 2017; Petrice et al. 2021). A fourth sampling method, sentinel eggs, is effective, but requires laboratory production of EAB eggs in sufficient numbers and continued monitoring of the eggs after deployment (Duan et al. 2011, 2012; Jennings et al. 2014; Petrice et al. 2021). However, sentinel eggs have the benefit of providing quantitative measures of parasitoid activity and efficacy (% parasitism), as opposed to the other methods, which either: (1) focus on the detection of adults in the case of yellow pan traps, or (2) estimate biocontrol activity based on the number of eggs sampled via bark scraping or peeling where initial number of eggs present in a given year is unknown, making it difficult to accurately calculate annual percent parasitism (Duan et al. 2012). As such, developing a better understanding of the efficacy of sentinel eggs as a sample method, as well as potential ways to improve this method, are needed.
An important aspect of developing an effective classical biological control program is to determine the rate of establishment and spread of the released agent. This helps ensure sufficient propagule pressure for establishment, essential aspects of biological control (Wittmann et al. 2014). However, detecting the presence of an introduced species can prove challenging depending upon the species in question and the methods implemented (Caton et al. 2022). Compared to other EAB biocontrol agents, there are few studies of O. agrili establishment, efficacy, and dispersal in the field. Abell et al. (2014) collected relatively few O. agrili-parasitized eggs via bark sifting, but observed greater parasitization at release sites compared to control sites where releases had not taken place (18.9–21.8% vs. 3.3–4.3%, respectively), with most release and control sites located approximately 1 km apart and first detections occurring three years post-release in control sites. In a study of O. agrili establishment in Maryland, USA O. agrili was only recovered from three out of nine release sites sampled after several years of repeated releases in those areas, suggesting poor establishment or detection (Jennings et al. 2018). There have been even fewer studies examining O. agrili rate of spread at finer scale. In one such study, Parisio et al. (2014) recovered O. agrili in yellow pan traps located up to 20 m away from the release point in a wooded area during a five week period of sampling. In New York, USA no O. agrili were captured in yellow pan traps deployed at 250 m intervals along a river leading away from release sites (Jones et al. 2019). To maximize O. agrili establishment and spread, we must determine factors affecting the dispersal and parasitism rate of O. agrili when released into the field. To this end, the present study determined the short-term dispersal of newly released adult O. agrili using sentinel host eggs deployed on different host trees at various distances from the release point.
Materials and methods
Study site and trees
This study took place in a large, mowed grass field located at the USDA-ARS Beneficial Insects Introduction Unit in Newark, DE, USA (39.66812° N, 75.74087° W) from June through July 2021 on sunny, calm days (precipitation = 0–0.36 cm, maximum wind = 0–28.97 km h−1, temperature = 11.11–35.6 °C). The grass field (~ 2 ha) is also neighboring several small urban forests (0.5–1 ha) consisting of primarily maple (Acer spp), birch (Betula spp), and ash (Fraxinus spp.). A total of 21 green ash (Fraxinus pensylvanica) and 21 white fringe tree (Chionanthus virginicus) saplings were used in this study (diameter at breast height 3–5 cm). Of these trees, four green ash and three white fringe trees were planted at the edge of unmanaged wooded areas, while the rest were potted. All trees were deployed in pairs (one green ash and one white fringe tree). Figure 1 depicts our plot design. At the center of the study area, a pair of trees was designated as the release trees, where parasitoid releases would occur (Fig. 1a). Tree pairs were placed at various distances (9–45 m) away from the pair of central release trees in modified concentric circles as follows: 9.6 ± 0.4 m (n = 4), 20.0 ± 1.2 m (n = 6), and 34.6 ± 2.5 m (n = 10). Trees within the same pair were approximately 0.5–1.0 m apart (Fig. 1b).
a Overview of the study design. At the center of the study area, a pair of trees was designated as the release trees, where parasitoid releases would occur. Sentinel eggs were not deployed at the release trees. Tree pairs on which sentinel egg pouches were deployed were placed at various distances (9–45 m) away from the pair of central release trees (red pin) in modified concentric circles. Trees in group A (green pins) were 9.6 ± 0.4 m from the release point (n = 4), trees in group B (yellow pins) were 20.0 ± 1.2 m (n = 6) from the release point, and trees in group C (blue pins) were 34.6 ± 2.5 m (n = 10) from the release point. b A view of the release trees and several of the trees on which sentinel eggs were deployed. Trees within the same pair were approximately 0.5–1.0 m apart
Insects
All insects were reared at the USDA-ARS Beneficial Insects Introduction Unit in Newark, DE, USA. EAB eggs were produced as described in Duan et al. (2013). Host eggs used in this experiment were 0–4 days old, well within the window of susceptibility and acceptability for O. agrili (Duan et al. 2014). Oobius agrili used in this experiment were from a continuously reared culture of field-collected O. agrili that were originally collected in Northeast China between 2008 and 2010 (Duan et al. 2014).
Sentinel egg deployment and parasitoid release
EAB eggs oviposited on coffee filter paper were used as sentinel host eggs in this experiment. The filter paper was cut into strips containing 10–20 eggs, or several smaller strips adding up to that number of eggs, with the same number of eggs per pouch used in a given trial The eggs were then placed in 8 × 10 cm mesh (1 mm aperture size) pouches. One sentinel egg pouch was attached to each tree via garden wire at approximately breast height. Sentinel eggs were not deployed on the central release trees. Approximately 200–320 adults of O. agrili were released at the central release trees by opening the vials in which they were contained, placing the vials at the base of the release trees, and allowing the parasitoids to leave the vials of their own volition. Sentinel host eggs remained in the field for either 48 h (n = 3) or 120 h (n = 2) post O. agrili releases. At the end deployment, sentinel host eggs were retrieved from the field and then maintained in the laboratory under normal rearing conditions (25 °C, 65% RH, L:D 16:8) for approximately four weeks to evaluate parasitism. The number of remaining undamaged, parasitized, and predated eggs was recorded according to methods described in Liu et al. (2007) and Duan et al. (2012). Egg parasitism was scored based on successful development of parasitoid larvae, which were associated with darkening host eggs. Eggs were considered predated if visible evidence of the egg being consumed was present (i.e., egg was visibly pierced or chewed or fragments of chorion present). Trials were separated by at least one week.
Statistical analysis
All data were analyzed using SAS JMP Pro 15.1.0 (SAS Corporation, Cary, NC, USA). The proportion of eggs parasitized per pouch were analyzed via likelihood ratio χ2 test based on generalized linear (binomial logit link) model, with distance from release tree, tree species, and deployment time as covariables.
Results
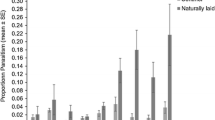
Overall, 22.6 ± 2.0% of sentinel eggs deployed were parasitized by O. agrili. Parasitism was observed throughout the deployment distances sampled, including the farthest distance, 44.8 m from the release point in both the 48 and 120 h trials. No significant effect of distance from release point on proportion of sentinel host eggs parasitized by O. agrili was observed (χ2 = 1.89, df = 1, P = 0.17) (Fig. 2). There was no significant difference in the rate of parasitism between eggs deployed on green ash and white fringe trees (χ2 = 0.05, df = 1, P = 0.83) (Fig. 3a). However, parasitization was significantly greater in EAB eggs that were deployed for 120 h compared to those that were only deployed for 48 h (χ2 = 10.91, df = 1, P = 0.001). Parasitism after 120 h deployment (mean = 29.4 ± 3.0%) was nearly double that of the parasitism observed after 48 h (mean = 16.0 ± 2.4%) (Fig. 3b). We also observed losses of deployed eggs due to undetermined predators throughout the study. However, the rate of predation was relatively low, with ~ 15.8 ± 1.6% of eggs predated.
Discussion
Our study found that while tree species and distance from the release point did not affect parasitism, O. agrili can travel as far as 45 m in as little as 48 h after release to parasitize eggs. This suggests that the potential rate of spread after release is much greater than previously thought (e.g. Abell et al. 2014) and not necessarily constrained by tree species or linear distance. These findings potentially allow for greater flexibility in the design of sampling schemes using sentinel host eggs. Future studies should continue to expand the radius of the study area and allow at least 120 h field exposure of deployed sentinel host eggs after the parasitoids are released to determine the maximum distance (or capacity) of the parasitoid dispersal and establishment.
A longer sampling window would allow the parasitoids more time to search for and attack host eggs, but would limit the precise determination of rate of spread for several reasons. More time in the field may allow for greater opportunity for predation (Jennings et al. 2014), reducing the accuracy of the estimate for in-field parasitism. Predation rates of wild EAB eggs can be as high as 37–52% in the field (Duan et al. 2011). Jennings et al. (2014) found that predation can be reduced by almost 40% compared to unprotected controls through using 1 mm aperture mesh pouches with no significant reduction in parasitism. Similar pouches were used to protect sentinel eggs from field predation in our study, but still resulted in ~ 15.8% sentinel eggs preyed upon by identified predators. Further investigation into factors that may affect predation, such as pouch deployment method, tree species, and local variation is underway (Quinn and Duan unpublished).
In addition to loss of sentinel eggs due to predation during field exposure, host suitability also decreases over time, with eggs older than two weeks being unsuitable for parasitization (Duan et al. 2014), further limiting the benefits of longer deployment. Reducing the sampling duration (i.e., the amount of time sentinel eggs exposed to the field conditions) may help determine the rate of O. agrili spread after release. In laboratory studies, an individual O. agrili will attack an average of 19–24 eggs in the first week after emergence at 25 °C, but the attack rate sharply reduces to less than five eggs after the third week (Hoban et al. 2016). The number of parasitoids released (200–320) relative to the host eggs deployed in each replicate (420–840) was approximately 1:3. This suggests that the reduced parasitism observed in the shorter duration trial is not due to the parasitoid ovipositional limit, but rather host finding or dispersal limits. Another consideration when applying our findings to field populations is that a natural, forested environment is more heterogeneous than our study design, which may complicate host finding on a local scale (Bukovinszky et al. 2007), but improve biological control at a landscape level (Cohen and Crowder 2017; Bosem Baillod et al. 2017). Cues, semiochemical or otherwise, used by O. agrili to locate A. planipennis eggs are currently unknown, although the volatile profiles of A. planipennis tree hosts have been documented (Pureswaran and Poland 2009; Peterson et al. 2020) and may be attractive to natural enemies of the emerald ash borer. Further study of O. agrili behavior in both laboratory and natural settings could provide a better understanding of factors affecting O. agrili host finding.
Ever since the documentation of successful use of C. virginicus as an alternate host (Cipollini 2015; Peterson et al. 2020), there have been concerns about the potential of C. virginicus to serve as an enemy free space for A. planipennis (Olson and Rieske 2019). Our study found no difference in parasitization between eggs deployed on F. pennsylvanica and C. virginicus. While investigations into tritrophic interactions of A. planipennis, its parasitoids, and its host trees (both new and old) are still warranted, our study may provide some measure of reassurance that, at least in the case of O. agrili, biological control activity may not be compromised should A. planipennis oviposit on C. virginicus. However, this remains to be verified in the field for the larval parasitoids, S. galinae and T. planipennisi. Recent laboratory studies suggest that both S. agrili, S. galinae (Ragozzino et al. 2021), and T. planipennisi (Hoban et al. 2018) performance is only moderately affected by host plant, further suggesting that host plant effects on biological control may be limited.
It is important to note that abiotic factors such as wind and rain can strongly impact parasitoid dispersal and activity (Weisser et al. 1997; Kristensen et al. 2013). However, given that throughout each trial maximum observed windspeed remained at 18 km h−1 or less, precipitation was minimal (< 1 cm), and temperatures were within historical norms for the time of year, it is unlikely that abiotic conditions adversely affected parasitoid foraging or dispersal in this study. Future studies examining the impact of environmental conditions on parasitoid performance will be key, especially given the potential impacts of climate change on biological control agents and our ecosystems (Furlong and Zalucki 2017).
Overall, our study has demonstrated the efficacy of sentinel host eggs in determining the spread and realized parasitism rates of O. agrili immediately after environmental release. Continued studies of parasitism, in conjunction with other sources of EAB mortality such as predation, throughout their introduced range over time will be needed to determine long-term biological control contribution. Future studies should seek to determine the efficacy and activity of O. agrili at finer distance resolution through intensive studies on foraging behavior and chemical ecology. This will improve our understanding of factors affecting this important biological control agent’s impact on A. planipennis.
References
Abell KJ, Bauer LS, Duan JJ, van Driesche R (2014) Long-term monitoring of the introduced emerald ash borer (Coleoptera: Buprestidae) egg parasitoid, Oobius agrili (Hymenoptera: Encyrtidae), in Michigan, USA and evaluation of a newly developed monitoring technique. Biol Control 79:36–42
Bauer LS, Duan JJ, Gould JR, van Driesche R (2015) Progress in the classical biological control of Agrilus planipennis Fairmaire (Coleoptera: Buprestidae) in North America. Can Entomol 147:300–317
Bosem Baillod A, Tscharntke T, Clough Y, Batáry P (2017) Landscape-scale interactions of spatial and temporal cropland heterogeneity drive biological control of cereal aphids. J Appl Ecol 54:1804–1813
Bukovinszky T, Gols R, Hemerik L, van Lenteren JC, Vet LE (2007) Time allocation of a parasitoid foraging in heterogeneous vegetation: implications for host-parasitoid interactions. J Anim Ecol 76:845–853
Caton BP, Fang H, Manoukis NC, Pallipparambil GR (2022) Quantifying insect dispersal distances from trapping detections data to predict delimiting survey radii. J Appl Entomol 146:203–216
Cipollini D (2015) White fringetree as a novel larval host for emerald ash borer. J Econ Entomol 108:370–375
Cohen AL, Crowder DW (2017) The impacts of spatial and temporal complexity across landscapes on biological control: a review. Curr Opin Insect Sci 20:13–18
Dang Y-Q, Zhang Y-L, Wang X-Y, Xin B, Quinn NF, Duan JJ (2021) Retrospective analysis of factors affecting the distribution of an invasive wood-boring insect using native range data: the importance of host plants. J Pest Sci 94:981–990
Duan JJ, Bauer LS, Ulyshen MD, van Driesche R (2011) Development of methods for the field evaluation of Oobius agrili (Hymenoptera: Encyrtidae) in North America, a newly introduced egg parasitoid of the emerald ash borer (Coleoptera: Buprestidae). Biol Control 56:170–174
Duan JJ, Bauer LS, Hansen JA, Abell KJ, van Driesche R (2012) An improved method for monitoring parasitism and establishment of Oobius agrili (Hymenoptera: Encyrtidae), an egg parasitoid introduced for biological control of the emerald ash borer (Coleoptera: Buprestidae) in North America. Biol Control 60:255–261
Duan JJ, Watt T, Taylor P, Larson K, Lelito JP (2013) Effects of ambient temperature on egg and larval development of the invasive emerald ash borer (Coleoptera: Buprestidae): implications for laboratory rearing. J Econ Entomol 106:2101–2108
Duan JJ, Jennings DE, Williams DC, Larson KM (2014) Patterns of parasitoid host utilization and development across a range of temperatures: implications for biological control of an invasive forest pest. BioControl 59:659–669
Duan JJ, Bauer LS, Abell KJ, Ulyshen MD, van Driesche RG (2015) Population dynamics of an invasive forest insect and associated natural enemies in the aftermath of invasion: implications for biological control. J Appl Ecol 52:1246–1254
Duan JJ, Bauer LS, van Driesche RG, Gould JR (2018) Progress and challenges of protecting North American ash trees from the emerald ash borer using biological control. Forests 9:142
Fagan WF, Lewis MA, Neubert MG, van Den Driessche P (2002) Invasion theory and biological control. Ecol Lett 5:148–157
Fahrner S, Aukema BH (2018) Correlates of spread rates for introduced insects. Glob Ecol Biogeogr 27:734–743
Furlong MJ, Zalucki MP (2017) Climate change and biological control: the consequences of increasing temperatures on host–parasitoid interactions. Curr Opin Insect Sci 20:39–44
Heimpel GE, Asplen MK (2011) A “Goldilocks” hypothesis for dispersal of biological control agents. BioControl 56:441–450
Herms DA, McCullough DG (2014) Emerald ash borer invasion of North America: history, biology, ecology, impacts, and management. Annu Rev Entomol 59:13–30
Hoban J, Duan JJ, Hough-Goldstein J (2016) Effects of temperature and photoperiod on the reproductive biology and diapause of Oobius agrili (Hymenoptera: Encyrtidae), an egg parasitoid of emerald ash borer (Coleoptera: Buprestidae). Environ Entomol 45:726–731
Hoban JN, Duan JJ, Shrewsbury PM (2018) Host utilization and fitness of the larval parasitoid Tetrastichus planipennisi are influenced by emerald ash borer’s food plants: implications for biological control. Biol Control 127:85–93
Jennings DE, Duan JJ, Larson KM, Lelito JP, Shrewsbury PM (2014) Evaluating a new method for monitoring the field establishment and parasitism of Oobius agrili (Hymenoptera: Encyrtidae), an egg parasitoid of emerald ash borer (Coleoptera: Buprestidae). Florida Entomol 97:1263–1265
Jennings DE, Duan JJ, Shrewsbury PM (2018) Comparing methods for monitoring establishment of the emerald ash borer (Agrilus planipennis, Coleoptera: Buprestidae) egg parasitoid Oobius agrili (Hymenoptera: Encyrtidae) in Maryland, USA. Forests 9:659
Jones MI, Gould JR, Warden ML, Fierke MK (2019) Dispersal of emerald ash borer (Coleoptera: Buprestidae) parasitoids along an ash corridor in western New York. Biol Control 128:94–101
Kovacs KF, Haight RG, McCullough DG, Mercader RJ, Siegert NW, Liebhold AM (2010) Cost of potential emerald ash borer damage in US communities, 2009–2019. Ecol Econ 69:569–578
Kristensen NP, Schellhorn NA, Hulthen AD, Howie LJ, De Barro PJ (2013) Wind-borne dispersal of a parasitoid: the process, the model, and its validation. Environ Entomol 42:1137–1148
Liu H, Bauer LS, Miller DL, Zhao T, Gao R, Song L, Luan Q, Jin R, Gao C (2007) Seasonal abundance of Agrilus planipennis (Coleoptera: Buprestidae) and its natural enemies Oobius agrili (Hymenoptera: Encyrtidae) and Tetrastichus planipennisi (Hymenoptera: Eulophidae) in China. Biol Control 42:61–71
Lockwood JL, Cassey P, Blackburn T (2005) The role of propagule pressure in explaining species invasions. Trends Ecol Evol 20:223–228
Lustig A, Worner SP, Pitt JPW, Doscher C, Stouffer DB, Senay SD (2017) A modeling framework for the establishment and spread of invasive species in heterogeneous environments. Ecol Evol 7:8338–8348
MacFarlane DW, Meyer SP (2005) Characteristics and distribution of potential ash tree hosts for emerald ash borer. For Ecol Manag 213:15–24
Macquarrie CJK, Cooke BJ, Saint-Amant R (2019) The predicted effect of the polar vortex of 2019 on winter survival of emerald ash borer and mountain pine beetle. Can J For Res 49:1165–1172
McCullough DG (2019) Challenges, tactics and integrated management of emerald ash borer in North America. Int J Res 93:197–211
Olson DG, Rieske LK (2019) Host range expansion may provide enemy free space for the highly invasive emerald ash borer. Biol Invasions 21:625–635
Parisio MS, Gould JR, Vandenberg JD, Bauer LS, Fierke MK (2014) Investigating individual dispersal capabilities of the eab parasitoids Oobius agrili and Tetrastichus planipennisi. In: Proceedings of the Emerald Ash Borer research and technology development meeting. Wooster, pp 71–72
Parisio MS, Gould JR, Vandenberg JD, Bauer LS, Fierke MK (2017) Evaluation of recovery and monitoring methods for parasitoids released against emerald ash borer. Biol Control 106:45–53
Peterson DL, Böröczky K, Tumlinson J, Cipollini D (2020) Ecological fitting: chemical profiles of plant hosts provide insights on selection cues and preferences for a major buprestid pest. Phytochemistry 176:112397
Petrice TR, Bauer LS, Miller DL, Stanovick JS, Poland TM, Ravlin FW (2021) Monitoring field establishment of the emerald ash borer biocontrol agent Oobius agrili Zhang and Huang (Hymenoptera: Encyrtidae): sampling methods, sample size, and phenology. Biol Control 156:104535
Pureswaran DS, Poland TM (2009) Host selection and feeding preference of Agrilus planipennis (Coleoptera: Buprestidae) on ash (Fraxinus spp.). Environ Entomol 38:757–765
Ragozzino M, Duan JJ, Salom S (2021) Responses of two introduced larval parasitoids to the invasive emerald ash borer (Coleoptera: Buprestidae) infesting a novel host plant, white fringe tree: Implication for biological control. Biol Control 160:104672
Ramsfield TD, Bentz BJ, Faccoli M, Jactel H, Brockerhoff EG (2016) Forest health in a changing world: effects of globalization and climate change on forest insect and pathogen impacts. Forestry 89:245–252
Tobin PC, Turcotte RM, Blackburn LM, Juracko JA, Simpson BT (2017) The big chill: quantifying the effect of the 2014 North American cold wave on hemlock woolly adelgid populations in the central Appalachian Mountains. Popul Ecol 59:251–258
Weisser WW, Volkl W, Hassell MP (1997) The importance of adverse weather conditions for behaviour and population ecology of an aphid parasitoid. J Anim Ecol 66:386–400
Williams HE, Brockerhoff EG, Liebhold AM, Ward DF (2021) Probing the role of propagule pressure, stochasticity, and Allee effects on invasion success using experimental introductions of a biological control agent. Ecol Entomol 46:383–393
Wittmann MJ, Metzler D, Gabriel W, Jeschke JM (2014) Decomposing propagule pressure: the effects of propagule size and propagule frequency on invasion success. Oikos 123:441–450
Acknowledgements
ARS agreement # 59-8010-0-003 funded by the Farm Bill Program.
Author information
Authors and Affiliations
Contributions
Project conception and coordination: Jian Duan (USDA-ARS) and Nicole Quinn (USDA-ARS and University of Massachusetts). Data collection: Nicole Quinn, Becca Robertson, Jon Schmude, Adam Scherr, Destiny Brumbaugh, Jess Faucher, Joseph Kovach, Sherri Jackson, Kelsey Miles. Original manuscript preparation: Nicole Quinn. Manuscript review: Jian Duan and Joe Elkinton (University of Massachusetts).
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial competing interests to disclose.
Ethical approval
The authors have no relevant financial or non-financial competing interests to disclose. This work was done in compliance with all BioControl ethical standards.
Additional information
Handling Editor: Stefano Colazza.
Rights and permissions
About this article
Cite this article
Quinn, N.F., Duan, J.J. & Elkinton, J. Monitoring the impact of introduced emerald ash borer parasitoids: factors affecting Oobius agrili dispersal and parasitization of sentinel host eggs. BioControl 67, 387–394 (2022). https://doi.org/10.1007/s10526-022-10149-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-022-10149-3