Abstract
The emerald ash borer (EAB), Agrilus planipennis (Coleoptera: Buprestidae), a phloem-boring beetle native to Asia, was first discovered in 2002 in the USA and Canada, causing widespread mortality of ash trees (Fraxinus spp.). A classical biological control program against EAB was implemented with the first regulatory approvals for environmental releases of three hymenopteran parasitoids from China in 2007: Tetrastichus planipennisi (Eulophidae), Spathius agrili (Braconidae), and Oobius agrili (Encyrtidae), and a fourth parasitoid, Spathius galinae (Braconidae) from the Russian Far East in 2015. We analyzed literature from the Scopus Database to examine the ecological premises of protection of North American ash trees with biocontrol, with a particular focus on the population dynamics of EAB and its biocontrol agents and implications for protecting and conserving native ash in the aftermath of EAB invasion. To date, the introduced parasitoids have been released in over 360 counties in 31 EAB-infested states, Washington D.C., and three Canadian provinces. Three of the parasitoids, T. planipennisi, S. galinae, and O. agrili, have successfully established self-sustaining populations in many release areas in the northeastern and Midwestern USA. In several regions where releases were made early, these agents have now spread to nearby forests and resulted in significant suppression of the target pest to low densities. Survival of regenerating ash due to suppression of EAB by parasitoids has also been observed in some sites with early parasitoid releases. The suppression of EAB is likely to expand geographically and thus contribute to North American ash recovery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The emerald ash borer (EAB), Agrilus planipennis Fairmaire (Coleoptera: Buprestidae), native to Asia, is a destructive pest of ash trees, Fraxinus spp. (Oleaceae). This beetle was first discovered in southern Michigan, USA and nearby Ontario, Canada in 2002 and has since become the most destructive pest of North American ash-dominated hardwood forests (Herms and McCullough 2014). Attempts to eradicate EAB in North America by USA and Canadian regulatory agencies were abandoned a few years after its first detection because EAB became too widespread (GAO 2006). Ongoing research, development, and implementation of EAB-management strategies were subsequently directed towards management via biological control, regulatory restriction of movement of EAB-infested wood or plant materials, insecticide treatment or physical destruction of infested trees, and EAB-resistant ash genotypes (e.g., Liu et al. 2003, 2007; Bauer et al. 2015; Koch et al. 2015; McCullough et al. 2015; Mercader et al. 2015).
In 2020, the USA federal regulatory effort to contain the spread of EAB was discontinued because of high implementation costs, a lack of effective EAB-surveillance tools, and the inability to prevent EAB from both short-distance natural dispersal or long-distance spread by human transport of infested ash materials (Federal Register 2020). Although highly effective systemic insecticides are available to protect high value, landscape ash (Herms et al. 2009; Sadof et al. 2021), costs and environmental concerns prevent widespread use of chemical controls against EAB in natural forests. Therefore, classical biological control via the discovery, introduction, release, and establishment of self-propagating and dispersing host-specific natural enemies is currently the most promising strategy for sustainable management of EAB to conserve native Fraxinus spp. in the forests of North America. Previous reviews by Bauer et al. (2015) and Duan et al. (2018) reported the progress in earlier phases of the classical biocontrol program against EAB in North America, including foreign exploration for Asian parasitoids, host specificity testing and regulatory approval of discovered agents, as well as release and establishment recovery in the USA and Canada. Here, we examine the ecological premises of protecting North American ash trees against EAB by the introduced natural enemies, with a particular focus on the population dynamics of the pest and introduced agents and implications for protecting and conserving native Fraxinus species in the aftermath of EAB invasion. In addition, we review recent progress and challenges in the implementation and evaluation of the biological control program against EAB as the target pest continues to spread throughout North America.
For this review, we first searched online data bases of both Scopus and ISI Web of Science using the key words “emerald ash borer or Agrilus planipennis” and found that both databases produced similar number of documents (904–922). Then, we conducted further searches of the Scopus online database using key words “emerald ash borer” or “Agrilus planipennis” in combination with “parasitoid”, “natural enemies”, “biological control” or “biocontrol”. A total of 228 unique publications were found that focused on biological control or biocontrol of the emerald ash borer with parasitoids or other natural enemies from the Scopus records. All these publications were read and analyzed for data and content related to the objectives of this review, namely ecological data on ash protection and conservation, and/or data on population dynamics of EAB and natural enemies. Data from relevant original research articles and significant reviews that addressed these objectives were used and cited in this review. In addition, we also contacted colleagues in China for any historical Chinese literatures that might be relevant to this review.
Ecological premise for protecting North American ash trees using classical biocontrol
Ash tree mortality risk from the EAB invasion
Ash, Fraxinus spp. (Oleaceae) trees were relatively free of serious diseases and insect pests in North America before the invasion of EAB (Pugh et al. 2011). Since it was first identified as the sole factor causing ash tree mortality in southeastern Michigan and nearby Ontario in 2002, however, the spread and establishment of EAB has killed hundreds of millions of North American ash trees in 36 states and Washington, D.C. in USA and five Canadian provinces (Canadian Food Inspection Agency 2022; Emerald Ash Borer Information 2022). The potential economic costs associated with the EAB invasion were estimated to be $1 billion per year from 2009 to 2019 (Kovacs et al. 2010), and the ecological impacts on North American forests are severe and widespread, including threats to vertebrate and invertebrate ash specialist herbivores (e.g., Wagner and Todd 2016); composition of arthropod communities associated with ash (Jennings et al. 2017); altered forest composition and structure (Morin et al. 2017); impacts on soil microbial communities, understory vegetation, and invasive plants (Klooster et al. 2018); altered riparian forest structure and function (Engelken and McCullough 2020; Engelken et. al. 2020); impacts on tidal swamps and coastal river habitat (Jacobsen 2020); and facilitation of secondary invasions (Baron and Rubin 2021).
All ash species native to North America encountered by EAB to date appear to be susceptible (Anulewicz et al. 2008; Herms and McCullough 2014), including the most common species: green (Fraxinus pensylvanica Marsh.), white (F. americana L.), and black (F. nigra Marsh.) as well as the less common blue (F. quadrangulata Michx.) and pumpkin ash (F. profunda [Bush] Bush). However, the degree of ash susceptibility to EAB varies among ash species and may be related to differences in bark texture, host volatiles, and nutritional or defensive compounds (e.g., Chen et al. 2011a, 2011b; Cipollini et al. 2011; Whitehill et al. 2011, 2012; Tanis and McCullough 2012, 2015; Koch et al. 2015; Rigsby et al. 2015; Villari et al. 2016; Qazi et al. 2018). Other ecological factors such as tree age, physiological condition, habitat type, and natural enemies may also play a role in ash susceptibility to EAB (Tluczek et al. 2011; Knight et al. 2014; Duan et al. 2021). For example, blue ash appears to be much less susceptible to EAB infestation than other North American ash trees, possibly due to a combination of differences in volatile emissions (Pureswaran and Poland 2009) and defense compounds (Qazi et al. 2018), as well as its smooth bark, which makes the tree less suitable for EAB oviposition (Tanis and McCullough 2012, 2015; Spei and Kashian 2017).
Although at some sites nearly 100% of ash trees > 2.5 cm in diameter at breast height (DBH) in infested stands have been attacked and killed by EAB (Klooster et al. 2014), invading EAB populations in North America appear to first kill mature (canopy) ash trees as compared to smaller understory ash trees, saplings, and seedlings (Cappeart et al. 2005; Tanis and McCullough 2015). Moreover, smaller ash saplings with DBH < 2.5 cm are rarely attacked by EAB (Marshall et al. 2013). It is conceivable that younger ash trees have both physical (e.g., smooth-bark surface) and chemical (secondary defense compounds) characteristics that are less attractive to EAB oviposition than canopy ash trees (e.g., Marshall et al. 2013). It is also possible that the smaller area of phloem in these young ash trees limits EAB colonization.
Klooster et al. (2014) found that the three most common North American ash species, green, white, and black, are equally vulnerable to severe levels of mortality when EAB populations are high. To date, however, few studies have determined the threshold EAB density that kills an ash tree of a given species at a given age in a specific habitat. A study in urban forests in Canada suggests that infested ash trees could recover from a density of 10 EAB larvae per m2 of phloem (MacQuarrie and Scharbach 2015). A more recent study in Michigan reported on the abundance of surviving ash saplings and young trees (DBH ~ 2.5–5.8 cm) in natural forests, where EAB densities averaged 2–7 larvae per m2 of phloem (Duan et al. 2017). It is plausible that the threshold EAB density that kills an ash tree varies with levels of tree resistance or tolerance, which are themselves influenced by a whole host of factors such as tree species, age, climates, and forest habitat conditions (e.g., MacQuarrie and Sabarbach 2015; Tennis and McCullough 2015; Dang et al. 2021).
Rationale for classical biocontrol
In contrast to the enormous economic and ecological impacts of EAB in North America, early Chinese literature only reported occasional damage to stressed or weakened Asian ash trees or susceptible North American ash trees in China (CASIZ 1986). Although at the time little was known about the biology of EAB and factors regulating its populations in Asia, the ability of North American ash trees to survive without significant mortality in China strongly suggested the possibility of effective top down EAB population control by specialized natural enemies. Subsequent discovery of a complex of hymenopteran parasitoids attacking EAB eggs and larvae in northern China and the Russian Far East further supports this premise of top-down suppression of EAB populations by the co-evolved natural enemies in its native range (see next Section).
Shortly after detection of EAB in North America, field surveys for native North American natural enemies attacking EAB were conducted in Michigan and other newly infested regions. Parasitism by native North American parasitoids was minimal (< 5%) in these invaded regions (e.g., see reviews in Bauer et al. 2015 and Davidson and Rieske 2016; Jennings et al. 2016; Duan et al. 2018). Relatively high levels of larval parasitism by generalist North American native parasitoids were observed at some heavily infested sites in Michigan and Ontario, with up to 71% parasitism by Atanycolus cappaerti Marsh and Strazanac (Hymenoptera: Braconidae) (Cappaert and McCullough 2009) and ~ 40% parasitism by Phasgonophora sulcata Westwood (Hymenoptera: Chalcididae) (Roscoe et al. 2016). Augmentative releases of the native natural enemy P. sulcata are also currently under investigation in Canada (Gaudon and Smith 2020). To date, however, no studies have demonstrated the effectiveness of these native North American parasitoids in regulating EAB population dynamics at low densities. Furthermore, no native North American parasitoids have been found attacking EAB eggs (Bauer et al. 2015; Duan et al. 2018), justifying exploration for co-evolved Asian natural enemies for implementation of classical biocontrol.
Implementation of an emerald ash borer biocontrol program
Bauer et al. (2015) and Duan et al. (2018) reviewed the progress in earlier phases of the classical biocontrol program against EAB in North America. Briefly, foreign explorations in the pest’s native range were conducted from 2003 to 2012 and led to discovery of three major hymenopteran parasitoids, an egg parasitoid Oobius agrili Zhang and Huang (Encyrtidae) (Zhang et al. 2005) and two larval parasitoids, Tetrastichus planipennisi Yang (Eulophidae) and Spathius agrili Yang (Braconidae) (Yang et al. 2005, 2006; Liu et al. 2007) in northeast China. While surveys for EAB natural enemies in Japan and Mongolia were unproductive because of the lack of detectable EAB populations, field work in the Russian Far East later resulted in discovery of an egg parasitoid Oobius primorskyensis Yao and Duan (Encyrtidae) and two larval parasitoids (Braconidae), Spathius galinae Belokobylskij & Strazanac and Atanycolus nigriventris Vojnovskaja-Krieger (Belokobylskij et al. 2012; Duan et al. 2012a; Yao et al. 2016). Additional exploration work in South Korea in areas with low density EAB populations infesting species of Asiatic ash in Daejeon and in Yangsuri recovered three natural enemy species including S. galinae, Tetrastichus telon Graham (Eulophidae), and a beetle Teneroides maculicollis Lewis (Cleridae) (Gould et al. 2015).
After reviewing host range data generated from both quarantine testing in the USA and China (Table 1), USDA APHIS issued environmental release permits for O. agrili, S. agrili, and T. planipennisi in 2007 and later for S. galinae in 2015. To produce large number of these parasitoids for environmental releases throughout EAB-infested regions in the USA, a mass-rearing facility was subsequently constructed by 2010 in Brighton, Michigan and field release guidelines for the introduced biocontrol agents were published (Gould et al. 2015; Duan et al. 2018; USDA–APHIS/ARS/FS 2021). The Brighton EAB biocontrol rearing facility currently produces ~ 400,000 female T. planipennisi, ~ 170,000 O. agrili, and ~ 100,000 female S. galinae annually. However, production of S. agrili was purposely reduced because releases were discontinued in northern regions after 2012 due to its lack of establishment at higher latitudes (USDA–APHIS/ARS/FS 2021). To improve the likelihood of establishment for both T. planipennisi and S. galinae, high numbers are being released at more northerly sites and at higher elevations where their synchrony with EAB larval host stages is confirmed (Gould et al. 2020; USDA–APHIS/ARS/FS 2021). By fall of 2022, one or more of these four biocontrol agents were released in > 360 counties in 31 EAB-infested states, Washington D.C., and three Canadian provinces (Mapbiocontrol 2022; Supplementary figure S1, S2, and S3; Butler et al. 2022).
After parasitoid releases were made, establishment and spread of the released agents were evaluated with various sampling methods in the field, including ash tree-harvesting and rearing of parasitoids from large bolts (Butler et al. 2022), debarking of EAB-infested ash trees (Duan et al. 2013; Jennings et al. 2013), field deployment of sentinel EAB larvae and/or sentinel eggs as traps (Jennings et al. 2018; Rutledge et al. 2021; Quinn et al. 2022a, b), and use of yellow pan traps (Parisio et al. 2017; Petrice et al. 2021). Field recovery efforts revealed that all three of the biocontrol agents from China (O. agrili, T. planipennisi, and S. agrili) were recovered from EAB one year after release, indicating reproductive and overwintering success. However, only O. agrili and T. planipennisi were consistently recovered for two or more years after their last release in an area, and these two species are now considered firmly established and spreading naturally beyond their initial release sites. Recent studies on S. galinae in Michigan, Connecticut, Massachusetts, and New York, USA, where it was released from 2015 to 2017, have also shown that S. galinae is well established and spreading widely (Duan et al. 2019b, 2020; Rutledge et al. 2021; Quinn et al. 2022a). The establishment of S. agrili (i.e., recovery two years after the final release) is currently unconfirmed in northern states and most of the mid-Atlantic region. However, its reproduction in EAB larvae was confirmed one or two years after releases at a few sites south of the 40th parallel, where this species is still being released (Hooie et al. 2015; Jennings et al. 2016; Aker et al. 2022). Spathius agrili has not yet established well at any locations.
Several parasitoid recovery studies in the northern USA have documented rapid long-distance spread of T. planipennis and S. galinae following releases. For example, Jones et al. (2019) captured T. planipennisi in yellow pan traps deployed along the entirety of a 20 km transect in New York for three years following parasitoid release in a localized EAB outbreak. As EAB spread south along the ash corridor, T. planipennisi populations followed. Tetrastichus planipennisi in Michigan was found to have spread up to 3 km from release sites one year after its field releases (Duan et al. 2013). Using sentinel green ash logs infested with EAB larvae, Quinn et al. (2022a) detected both T. planipennisi and S. galinae 14 km away from the release sites 3–4 years after their last field releases in New York and Connecticut. Most recently, Aker et al. (2022) detected multiple established populations of S. galinae in Maryland at sites up to 90 km from the nearest release point approximately three year after release, indicating rapid, long-distance spread. In contrast to T. planipennisi and S. galinae, the egg parasitoid O. agrili appears to spread much more slowly in forests possibly because of its smaller size (~ 1 mm) and lower reproduction potential. For example, Abell et al. (2014) did not detect O. agrili at the non-release (control) sites 1.5–3 km away from the closest release sites until three years after the parasitoid’s release. Most recently, Quinn et al. (2022b) studied the dispersal ability of O. agrili by attaching freshly laid EAB eggs (sentinel hosts) on both green ash and white fringe trees located at various distances from the release point. Adult O. agrili were recovered at least 45 m from the release point within 4–5 days, and the dispersal distance was affected only by the time after initial release and not by hosts’ food plant species.
Evaluation of impacts of biocontrol on emerald ash borer and ash regeneration
Impact of introduced parasitoids on EAB population dynamics
Following environmental releases of T. planipennisi, O. agrili, and S. agrili, six long-term study sites, each comprised of a release and non-release control plot, were established in either 2007 or 2010 in southern Michigan, USA to monitor EAB population dynamics and mortality factors. At each release plot, ~ 1000–3000 female adults each of O. agrili, S. agrili, and T. planipennisi were released, and, in subsequent years, infested ash trees were sampled to estimate EAB egg and larval parasitism, and other causes of larval mortality (for details see Duan et al. 2013; Abell et al. 2014). Starting in 2015 after APHIS issued environmental release permits, S. galinae was also released for two consecutive years at each of these Michigan sites (Mapbiocontrol 2022).
EAB egg parasitism by O. agrili increased over the first five years after release at these Michigan sites, averaging ~ 1 to 4% from 2008 to 2011 and ~ 28% by 2014 in release plots. The natural spread of O. agrili from the release plots to the control plots occurred but was generally slow (Abell et al. 2014). The overall spread of O. agrili and its impact in suppressing EAB population growth have yet to be determined because sampling EAB eggs from ash bark layers and crevices is labor intensive and difficult to standardize (Abell et al. 2014; Petrice et al. 2021). Moreover, parasitism of EAB eggs by O. agrili is patchy. Therefore, more intensive sampling is needed to recover it and quantify its impact on EAB population dynamics (Petrice et al. 2021).
EAB larval parasitism by T. planipennisi in the Michigan plots was also low at first, averaging 1 to 6% from 2008 to 2011, but then increased to ~ 30% by 2014 in both the release and control plots (Duan et al. 2012b, 2013, 2015b). Life table analyses of seven years of data from the six Michigan study sites revealed that T. planipennisi contributed significantly to reducing net EAB population growth rates in smaller diameter trees in the aftermath of the initial EAB outbreak (Duan et al. 2015b). During the initial outbreak phase, native generalist natural enemies including parasitoids (Atanycolus spp.) and woodpeckers (such as Dryobates pubescens L., Leuconotopicus villosus L. and/or Melanerpes carolinus L.) contributed to declines in invasive EAB populations (Duan et al. 2014; Jennings et al. 2016). However, it was the introduced specialist T. planipennisi that became the dominant source of EAB larval mortality in small ash trees in the aftermath of the EAB invasion in Michigan (Duan et al. 2015b; 2017). A similar study in white ash forests of New York showed that the combination of woodpecker predation and parasitism by T. planipennisi significantly reduced the net reproductive rate of EAB in regenerating ash trees. At six sites in western New York the net reproductive rate was reduced to zero (Gould et al. 2022).
EAB larval parasitism by T. planipennisi was found to be concentrated in smaller diameter ash trees in field surveys in China, the Russian Far East, and the USA (Liu et al. 2007; Abell et al. 2012; Duan et al. 2012a; Jennings et al. 2016). The ability of T. planipennisi to parasitize EAB in larger ash trees is constrained by its short ovipositor (average 2 to 2.5 mm long), which cannot reach EAB larvae feeding under the thick bark (> 3.2 mm) found on the lower boles of ash trees that are > 12 cm DBH (Abell et al. 2012). Thus, T. planipennisi has a greater impact on EAB larval mortality in small diameter trees.
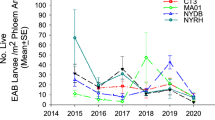
While releases of T. planipennisi and O. agrili are continuing, efforts are now increasingly focused on establishing S. agrili and S. galinae in North America to control EAB more successfully in larger ash trees, where their longer ovipositors allow them to reach EAB larvae under thicker bark. For example, the ovipositor of S. galinae averages 4 to 6 mm long, more than twice that of T. planipennisi. Consequently, S. galinae can attack EAB larvae feeding in ash trees up to 30 cm DBH (Duan et al. 2012a; Murphy et al. 2017). Based on their native distributions, S. agrili is presumed to be more adapted to southern climates and S. galinae to more northern climates (Jones et al. 2020). However, the lack of persistent recoveries of S. agrili from many of the previous release sites in Michigan (Duan et al. 2013, 2015b), New York and Maryland (Jennings et al. 2016), Tennessee (Hooie et al. 2015), and Kentucky (Davidson and Rieske 2016) suggests that this parasitoid may not be well adapted to North American hardwood forests for EAB biocontrol. In contrast, releases of S. galinae that began in the summer of 2015, primarily in Michigan and several Northeastern and Mid-Atlantic states, have resulted in successful establishment. Recent field studies from both Michigan and several northeastern USA states showed that S. galinae has established self-sustaining populations in release areas where T. planipennis had already been released or established (Duan et al. 2021, 2022). Based on recent life-table analyses, S. galinae alone caused a 31–57% reduction in the net population growth rates of EAB during the outbreak phase (Duan et al. 2022). Spathius galinae has now become the dominant parasitoid species, and, along with local generalist natural enemies and T. planipennisi, it reduced average EAB larval densities from 30 live EAB larvae per m2 of tree phloem in 2015 to less than seven in 2020 (Duan et al. 2022). This level of reduction has contributed to ash recovery and regeneration in the aftermath of EAB invasion waves (Duan et al. 2022). Life table analysis of EAB population dynamics at these biocontrol study sites indicates that the net population growth rate of EAB was at or below replacement levels (Figs. 1 and 2) (Duan et al. 2017, 2022).
Net population growth rates (R0) of emerald ash borer (Agrilus planipennis) infesting ash saplings (diameter at breast height or DBH = 2.5–5.8 cm), averaged from six different study sites in southern Michigan where the introduced larval parasitoids are well established since their releases from 2007 to 2010. Solid line represents R0 estimated using life table analysis by including all sources of the observed larval mortalities. Dashed line represents R0 estimated by the same lifetable analysis after excluding T. planipennisi from the life table, assuming mortality rates from other factors would not change due to increases in EAB densities (Duan et al. 2017)
Net population growth rates (R0) of emerald ash borer (Agrilus planipennis) infesting pole-size ash trees (diameter at breast height or DBH = 8–27 cm), averaged across different study sites in northeastern states (Connecticut, New York, and Massachusetts), where the introduced larval parasitoid Spathius galinae is well established since its release in 2016 and 2017. Arrows indicate the timing of S. galinae releases: the small arrow represents low release numbers and the large arrow high release numbers. Solid line represents R0 estimated using life table analysis of the observed EAB larval survival and mortalities caused by S. galinae and other mortality factors (e.g., woodpeckers, other native parasitoids). Dashed line represents R0 estimated by the same lifetable analysis after removing parasitism by S. galinae from the life table, assuming mortality rates from other factors would not change due to increases in EAB densities (Duan et al. 2022)
Recent studies suggest that the success or effectiveness of the current EAB biocontrol program in North America may be influenced by the interaction of EAB and parasitoid lifecycles. Jones et al. (2020) found that S. galinae and T. planipennisi are well synchronized with a lifecycle where some EAB take two years to develop as is the case in the northern USA. However, in more southern states, where most EAB overwinter as mature larvae in pupation chambers out of reach of parasitoids emerging in early spring, parasitoid populations are less likely to persist. In fact, Gould et al. (2020) modeled the likelihood of establishment of T. planipennisi and found that as summer temperatures increased, the percentage of EAB overwintering under the bark and thus available to spring emerging parasitoids decreased, and the likelihood of establishment by T. planipennisi also declined. Spathius galinae, unlike S. agrili which emerges in mid-summer (Yang et al. 2010), also emerges early in the spring (Jones et al. 2020) and is likely to establish more poorly in southern states. Oobius agrili has two generations per year. The first generation emerges from diapause and produces the second generation progenies that are able to reproduce without requiring diapause. Non-diapause adults of the second generation reproduces and their offspring enters winter diapause. Petrice et al. (2019) found that O. agrili has a critical daylength threshold for entering diapause, and that predicted synchrony with EAB egg laying is affected by latitude and thus daylength throughout the summer. Oobius primorskyensis, which has different diapause requirements than O. agrili, might be able to survive and thrive where O. agrili does not establish (Larson and Duan 2016; Duan et al. 2019a).
Ash recovery and regeneration in the aftermath of EAB invasion with biocontrol
Because the high abundance of susceptible North American ash species facilitates rapid EAB population growth rates, it would be extremely difficult to rapidly protect susceptible overstory ash trees against EAB in newly invaded-areas solely through the introduction and establishment of limited numbers of specialized natural enemies from Asia. In post-EAB invaded hardwood forests of North America, however, where EAB populations are lower and ash densities have been dramatically reduced, establishment of the introduced EAB parasitoids may effectively conserve surviving ash by moderating the frequency and amplitude of future EAB outbreaks, as occurs in EAB’s native range (see previous section). This in turn should allow these surviving trees to increase in age and their reproduction should lead to higher ash densities over time.
In southeastern Michigan, where establishment and spread of T. planipennisi and O. agrili have been confirmed since 2012, densities of ash and other native saplings were higher in forests closer to parasitoid release sites (Margulies et al. 2017). In another study of ash health in long-term EAB biocontrol study sites in 2012 and 2015, lower tree mortality and greater diameter growth were observed in large diameter ash trees growing in release plots vs. those in control plots (Kashian et al. 2018). Moreover, researchers found that many relatively healthy ash saplings (4–16 per 100 m2) and pole-size young trees (2–9 per 100 m2) have persisted, despite formerly high EAB densities that resulted in loss of most overstory ash trees by 2010 (Duan et al. 2017; Gould et al. 2022; JJD, TRP unpublished data). However, recovery of North American ash in the post-EAB invasion forests will take time even after EAB densities are successfully reduced by the introduced agents. This is because tree regrowth and regeneration are very slow processes, normally taking more than two decades for these ash trees to reach the overstory.
Concluding remarks
Since its first detection in the USA in 2002, EAB has continued to spread and cause economic damage to ash nursery stock and the lumber industry, degradation of ash forests, and reduction in ecosystem functions in ash forests in North America. The classical biocontrol program against EAB, which started over a decade ago with the introduction and establishment of co-evolved natural enemies from the pest’s native range, has shown the ability to suppress EAB to lower densities, which is allowing North American ash species in northern hardwood forests to recover and regenerate in the aftermath of the EAB invasion. This program has now documented successful establishment of the egg parasitoid O. agrili and the two larval parasitoid T. planipennisi and S. galinae in EAB-infested forests at most release sites in the northern USA, in areas where surveys to document parasitoid establishment have been conducted. While the role of O. agrili in suppressing EAB population growth requires continued evaluation, the larval parasitoids T. planipennisi (from China) and S. galinae (from the Russian Far East) have become the dominant biotic factors suppressing EAB population growth rates and significantly reducing EAB densities in the aftermath forests in Michigan and several northeastern states, where these parasitoids were released between 2007 and 2017 (Duan et al. 2015b, 2017; 2021; Margulies et al. 2017; Kashian et al. 2018). EAB densities at these biocontrol study sites are now sufficiently low (< 10 larvae per m2 phloem area) to allow the surviving trees and saplings to recover and grow to canopy trees, reaching the overstory (Duan et al. 2015b, 2017, 2021). We expect that the suppression of EAB densities is likely to expand geographically as established populations of O. agrili, T. planipennisi, and S. galinae increase and spread to new areas and parasitoids are released as part of the ongoing biocontrol release effort. However, tree regrowth and regeneration are very slow processes, normally taking decades. Even after EAB densities are successfully reduced and the frequency of EAB outbreaks moderated by the introduced agents, long-term monitoring studies will be needed to fully assess the contribution of EAB biocontrol to the recovery and regeneration of North American ash in the post-EAB invasion forests. We recommend expanding the current EAB biocontrol research to (1) quantify the long-term impact of EAB biocontrol on ash community and forest recovery in the aftermath of EAB as ash trees grow to canopy size, (2) determine parasitoid establishment in EAB populations in warmer regions (southern United States), (3) explore different regions of Asia for EAB natural enemies adapted to climate zones similar to those in the southern and western USA where EAB is now invading, and (4) evaluate the potential of area-wide EAB control with integration of host plant resistance and/or selective insecticide use (e.g., Davidson and Rieske 2016; Koch et al. 2021).
References
Abell KJ, Duan JJ, Bauer LS, Lelito JP, Van Driesche RG (2012) The effect of bark thickness on the effectiveness of Tetrastichus planipennisi (Hymen: Eulophidae) and Atanycolus spp. (Hymen: Braconidae) two parasitoids of emerald ash borer (Coleop: Buprestidae). Biol Control 63:320–325
Abell KJ, Bauer LS, Duan JJ, Van Driesche RG (2014) Long-term monitoring of the introduced emerald ash borer (Coleoptera: Buprestidae) egg parasitoid, Oobius agrili (Hymenoptera: Encyrtidae), in Michigan, USA and evaluation of a newly developed monitoring technique. Biol Control 79:36–42
Aker SA, de Andrade RB, Duan JJ, Gruner DS (2022) Rapid spread of an introduced parasitoid for biological control of emerald ash borer (Coleoptera: Buprestidae) in Maryland. J Econ Entomol 115:381–386
Anulewicz AC, McCullough DG, Cappaert DL, Poland TM (2008) Host range of the emerald ash borer (Agrilus planipennis Fairmaire) (Coleoptera: Buprestidae) in North America: results of multiple-choice field experiments. Environ Entomol 37:230–241
Baron JN, Rubin BD (2021) Secondary invasion? Emerald ash borer (Agrilus planipennis) induced ash (Fraxinus spp.) mortality interacts with ecological integrity to facilitate European buckthorn (Rhamnus cathartica). Can J for Res 51:455–464
Bauer LS, Duan JJ, Gould JR, Van Driesche RG (2015) Progress in the classical biological control of Agrilus planipennis Fairmaire (Coleoptera: Buprestidae) in North America. Can Entomol 147:300–317
Belokobylskij SA, Yurchenko GI, Strazanac JS, Zaldívar-Riverón A, Mastro V (2012) A new emerald ash borer (Coleoptera: Buprestidae) parasitoid species of Spathius Nees (Hymenoptera: Braconidae: Doryctinae) from the Russian Far East and South Korea. Ann Entomol Soc Am 105:165–178
Butler S, Dedes J, Jones G, Hughes C, Ladd T, Martel V, Ryall K, Sweeney J, MacQuarrie CJK (2022) Introduction and establishment of biological control agents for control of emerald ash borer (Agrilus planipennis) in Canada. Can Entomol 154:e47
Canadian Food Inspection Agency (2022) Emerald Ash Borer—Agrilus planipennis. Canadian food inspection agency, http://www.inspection.gc.ca/plants/plant-pests-invasive-species/insects/emerald-ash-borer/eng/1337273882117/1337273975030 (Accessed 15 Dec 2022)
Cappaert DL, McCullough DG (2009) Occurrence and seasonal abundance of Atanycolus cappaerti (Hymenoptera: Braconidae) a native parasitoid of emerald ash borer, Agrilus planipennisi (Coleoptera: Buprestidae). Great Lakes Entomol 51:152–165
Cappaert D, McCullough DG, Poland TM, Siegert NW (2005) Emerald ash borer in North America: a research and regulatory challenge. Am Entomol 51:152–165
Chinese Academy of Science, Institute of Zoology (1986) Agrilus marcopoli Obenberger. In: Editorial committee (eds.), Agriculture insects of China part I. China Agriculture Press, Beijing, 445
Chen Y, Ciaramitaro TM, Poland TM (2011a) Moisture content and nutrition as selection forces for emerald ash borer larval feeding behaviour. Ecol Entomol 36:344–354
Chen Y, Whitehill JGA, Bonello P, Poland TM (2011b) Differential response in foliar chemistry of three ash species to emerald ash borer adult feeding. J Chem Ecol 37:29–39
Cipollini D, Wang Q, Whitehill JGA, Powell JR, Bonello P, Herms DA (2011) Distinguishing defense characteristics in the phloem of ash species resistant and susceptible to emerald ash borer. J Chem Ecology 37:450–459
Dang Y, Wei K, Wang XY, Duan JJ, Jennings DE, Poland TP (2021) Introduced plants induce outbreaks of a native pest and facilitate invasion in the plants’ native range: evidence from the emerald ash borer. J Ecology 110:593–604
Davidson D, Rieske LK (2016) Establishment of classical biological control targeting emerald ash borer is facilitated by use of insecticides, with little effect on native arthropod communities. Biol Control 101:78–86
Duan JJ, Yurchenko G, Fuester RW (2012a) Occurrence of emerald ash borer (Coleoptera: Buprestidae) and biotic factors affecting its immature stages in the Russian Far East. Environ Entomol 41:245–254
Duan JJ, Bauer LS, Abell KJ, Van Driesche RG (2012b) Population responses of hymenopteran parasitoids to the emerald ash borer (Coleoptera: Buprestidae) in recently invaded areas in north central United States. BioControl 57:199–209
Duan JJ, Bauer LS, Abell KJ, Lelito JP, Van Driesche RG (2013) Establishment and abundance of Tetrastichus planipennisi (Hymenoptera: Eulophidae) in Michigan: potential for success in classical biocontrol of the invasive emerald ash borer (Coleoptera: Buprestidae). J Econ Entomol 106:1145–1154
Duan JJ, Bauer LS, Abell KJ, Van Driesche RG (2014) Natural enemies implicated in the regulations of an invasive pest: a life table analysis of the population dynamics of the invasive emerald ash borer. Ag Entomol 16:406–416
Duan JJ, Gould JR, Fuester RW (2015a) Evaluation of the host specificity of Spathius galinae (Hymenoptera: Eulophidae), a larval parasitoid of the emerald ash borer (Coleoptera:Buprestidae) in Northeast Asia. Biol Control 89:91–97
Duan JJ, Bauer LS, Abell KJ, Ulyshen MD, Van Driesche RG (2015b) Population dynamics of an invasive forest insect and associated natural enemies in the aftermath of invasion: implications for biological control. J Appl Ecol 52:1246–1254
Duan JJ, Bauer LS, Van Driesche RG (2017) Emerald ash borer biocontrol in ash saplings: the potential for early stage recovery of North American ash trees. Ecol Manag 394:64–72
Duan JJ, Bauer LS, Van Driesche RG, Gould JR (2018) Progress and challenges of protecting North American ash trees from the emerald ash borer using biological control. Forests 9(3):142
Duan JJ, Schmude JM, Larson KM, Fuester RW, Gould JR, Ulyshen MD (2019a) Field parasitism and host specificity of Oobius primorskyensis (Hymenoptera: Encyrtidae), an egg parasitoid of the emerald ash borer (Coleoptera:Buprestidae) in the Russian Far East. Biol Control 10:44–50
Duan JJ, Van Driesche RG, Crandall RS, Schmude JM, Rutledge CE, Slager BH, Gould JR, Elkinton JS (2019b) Establishment and early impact of Spathius galinae (Hymenoptera: Encyrtidae) on emerald ash borer (Coleoptera: Buprestida) in the Northeastern United States. J Econ Entomol 112:2121–2130
Duan JJ, Bauer LS, Van Driesche RG, Schmude JM, Petrice T, Chandler JL, Elkinton J (2020) Effects of extreme low winter temperatures on the overwintering survival of the introduced larval parasitoids Spathius galinae and Tetrastichus planipennisi: implications for biological control of emerald ash borer in North America. J Econ Entomol 113:1145–1151
Duan JJ, Van Driesche RG, Schmude JM, Petrice T, Chandler JL, Elkinton JS (2021) Niche partitioning and coexistence of parasitoids of the same feeding guild introduced for biological control of an invasive forest pest. Biol Control 160:104698
Duan JJ, Van Driesche RG, Schmude J, Crandall R, Rutlege C, Quinn N, Slager BH, Gould JR, Elkinton JS (2022) Significant suppression of invasive emerald ash borer by introduced parasitoids: potential for North American ash recovery. J Pest Sci 95:1081–1090
Emerald Ash Borer Information (2022) Emerald ash borer information network. Available online: http://www.emeraldashborer.info/ (Accessed on 15 Aug 2022).
Engelken P, McCullough DG (2020) Riparian forest conditions along three northern Michigan rivers following emerald ash borer invasion. Can J Res 50:800–810
Engelken P, Benbow ME, McCullough DG (2020) Legacy effects of emerald ash borer on riparian forest vegetation and structure. Forest Ecol Manag 457:117684
Federal Register (2007) Availability of an environmental assessment for the proposed release of three parasitoids for the biological control of the emerald ash borer Agrilus planipennis in the Continental United States. Fed Regist 2007(72):28947–28948
Federal Register (2015) Availability of an environmental assessment for field release of the parasitoid Spathius galinae for the biological control of the emerald ash borer (Agrilus planipennis) in the contiguous United States. Fed. Regist. 2015, 80, 7827-7828, [docket number APHIS–2014–0094]. Available online: https://www.regulations.gov/docket?D=APHIS-2014-0094 (Accessed on 23 Jan 2022)
Federal Register (2020) Removal of emerald ash borer quarantine regulations. Fed. Regist. 85-FR-81085, [Docket No. APHIS-2017-0056]. Available online: https://www.federalregister.gov/documents/2020/12/15/2020-26734/removal-of-emerald-ash-borer-domestic-quarantine-regulations (Accessed on 23 Jan 2022)
GAO - Government Accountability Office (2006) Invasive forest pests: lessons learned from three recent infestations may aid in managing future efforts. Report of the United States Government Accounting Office. 2006, GAO–06–353. Available online: https://www.gao.gov/assets/250/249776.pdf (Accessed on 23 Jan 2022).
Gaudon JM, Smith SM (2020) Augmentation of native North American natural enemies for the biological control of the introduced emerald ash borer in central Canada. BioControl 65:71–79
Gould JR, Warden ML, Slager BH, Murphy TC (2020) Host overwintering phenology and climate change influence the establishment of Tetrastichus planipennisi Yang (Hymenoptera: Eulophidae), a larval parasitoid introduced for biocontrol of the emerald ash borer. J Econ Entomol 6:2641–2649
Gould JR, Hickin M, Fierke MK (2022) Mortality of emerald ash borer larvae in small regenerating ash in New York Forests. J Econ Entomol 115:1442–1454
Gould JR, Bauer LS, Duan JJ, Williams D, Liu HP (2015) History of emerald ash borer biological control. In: Van Driesche, R.G., Reardon, R. (eds) The biology and control of emerald ash borer. FHTET 2014-09. USDA Forest Service, Morgantown, West Virginia, USA, Pp. 83–95. https://www.fs.usda.gov/treesearch/pubs/49321 (Accessed on 2 March 2022)
Herms DA, McCullough DG (2014) Emerald ash borer invasion of North America: history, biology, ecology, impact, and management. Ann Rev Entomol 59:13–30
Herms DA, McCullough DG, Sadof S, Miller CS, Granshaw W (2009) Insecticide options for protecting ash trees from emerald ash borer. North Central IPM Center Bulletin, Ohio, p 12 http://www.emeraldashborer.info/documents/multistate_eab_insecticide_fact_sheet.pdf. (Accessed on 27 January 2023)
Hooie NA, Wiggins GJ, Lambdin PL, Grant JF, Powell SD, Lelito JP (2015) Native parasitoids and recovery of Spathius agrili from areas of release against emerald ash borer in eastern Tennessee, USA. BioContr Sci Tech 25:345–351
Jacobsen A (2020) Emerald ash borer in the ash (Fraxinus spp.)-dominated tidal swamps of the lower Patuxent River. Md Northeast Nat 27:817–840
Jennings DE, Gould JR, Vandenberg JD, Duan JJ, Shrewsbury PM (2013) Quantifying the impact of woodpecker predation on population dynamics of the emerald ash borer (Agrilus planipennis). PLoS ONE 8(12):e83491
Jennings DE, Duan JJ, Bean D, Gould JR, Rice KA, Shrewsbury PM (2016) Monitoring the establishment and abundance of introduced parasitoids of emerald ash borer larvae in Maryland, USA. Biol Control 101:138–144
Jennings DE, Duan JJ, Bean D, Rice KA, Williams GL, Bell SK, Shurtleff AS, Shrewsbury PM (2017) Effects of the emerald ash borer invasion on the community composition of arthropods associated with ash tree boles in Maryland, USA. Agri Forest Entomol 19:122–129
Jennings DE, Duan JJ, Shrewsbury PM (2018) Comparing methods for monitoring establishment of the emerald ash borer (Agrilus planipennis, Coleoptera: Buprestidae) egg parasitoid Oobius agrili (Hymenoptera: Encyrtidae) in Maryland, USA. Forests 9(10):659
Jones MI, Gould JR, Warden ML, Fierke MK (2019) Dispersal of emerald ash borer (Coleoptera: Buprestidae) parasitoids along an ash corridor in western New York. Biol Control 128:94–101
Jones MI, Gould JR, Mahon JH, Fierke MK (2020) Phenology of emerald ash borer (Coleoptera: Buprestidae) and its introduced larval parasitoids in the northeastern United States. J Econ Entomol 113:622–632
Kashian DM, Bauer LS, Spei B, Duan JJ, Gould JR (2018) Potential impacts of emerald ash borer biocontrol on ash health and recovery in southern Michigan. Forests 9(6):296
Klooster WS, Herms DA, Knight KS, Herms CP, McCullough DG, Smith A, Gandhi KJK, Cardina J (2014) Ash (Fraxinus spp.) mortality, regeneration, and seed bank dynamics in mixed hardwood forests following invasion by emerald ash borer (Agrilus planipennis). Biol Invas 16:859–873
Klooster WS, Gandhi KJ, Long LC, Perry KI, Rice KB, Herms DA (2018) Ecological impacts of emerald ash borer in forests at the epicenter of the invasion in North America. Forests 9(5):250
Knight KS, Brown JP, Long RP (2014) Factors affecting the survival of ash (Fraxinus spp.) trees infested by emerald ash borer (Agrilus planipennis). Biol Invas 15:371–383
Koch JL, Carey DW, Mason ME, Poland TM, Knight KS (2015) Intraspecific variation in Fraxinus pennsylvanica responses to emerald ash borer (Agrilus planipennis). New For 46(5):995–1011
Koch J, Pearson DE, Huebner CD, Young MK, Sniezko RA (2021) Restoration of landscapes and habitats affected by established invasive species. In: Poland TM, Patel-Weynand T, Finch M, Miniat CF, Hayes DC, Lopez VM (eds) Invasive species in forests and rangelands of the United States. Springer, Cham, Switzerland, pp 185–202
Kovacs FK, Haight RG, McCullough DG, Mercader RJ, Siegert NW, Liebhold AM (2010) Cost of potential emerald ash borer damage in USA communities, 2009–2019. Ecol Econ 69:569–578
Larson KM, Duan JJ (2016) Differences in the reproductive biology and diapause of two congeneric species of egg parasitoids (Hymenoptera: Encyrtidae) from northeast Asia: implications for biological control of the invasive emerald ash borer (Coleoptera:Buprestidae ). Biol Control 101:39–15
Liu H, Bauer LS, Gao R, Zhao T, Petrice TR, Haack RA (2003) Exploratory survey for the emerald ash borer, Agrilus planipennis (Coleoptera: Buprestidae), and its natural enemies in China. Great Lakes Entomol 36:191–204
Liu H, Bauer LS, Miller DL, Zhao T, Gao R, Song L, Luan Q, Jin R, Gao C (2007) Seasonal abundance of Agrilus planipennis (Coleoptera: Buprestidae) and its natural enemies Oobius agrili (Hymenoptera: Encyrtidae) and Tetrastichus planipennisi in China. Biol Control 42:61–71
MacQuarrie CJK, Scharbach R (2015) Influence of mortality factors and host resistance on the population dynamics of emerald ash borer (Coleoptera: Buprestidae) in urban forests. Environ Entomol 44:160–173
Mapbiocontrol (2022) Agent release tracking and data management for federal, state, and researchers releasing biocontrol agents for management of the emerald ash borer. Available online: http://www.mapbiocontrol.org/ (Accessed on 15 Aug 2022)
Margulies E, Bauer L, Ibanez I (2017) Buying time: preliminary assessment of biocontrol in the recovery of native forest vegetation in the aftermath of the invasive emerald ash borer. Forests 8(10):369
Marshall JM, Smith EL, Mech R, Storer AJ (2013) Estimates of Agrilus planipennis infestation rates and potential survival of ash. Amer Midland Nat 169:179–193
McCullough DG, Mercader RJ, Siegert NW (2015) Developing and integrating tactics to slow ash (Oleaceae) mortality caused by emerald ash borer (Coleoptera: Buprestidae). Can Entomol 147:349–358
Mercader RJ, McCullough DG, Storer AJ, Bedford JM, Heyd R, Poland TM, Katovich S (2015) Evaluation of the potential use of a systemic insecticide and girdled trees in area wide management of the emerald ash borer. For Ecol Manag 350:70–80
Morin RS, Liebhold AM, Pugh SA, Crocker SJ (2017) Regional assessment of emerald ash borer, Agrilus planipennis, impacts in forests of the eastern United States. Biol Invas 19:703–711
Murphy TC, Van Driesche RG, Gould JR, Elkinton JS (2017) Can Spathius galinae attack emerald ash borer larvae feeding in large ash trees? Biol Control 114:8–13
Parisio MS, Gould JR, Vandenberg JD, Bauer LS, Fierke MK (2017) Evaluation of recovery and monitoring methods for parasitoids released against emerald ash borer. Biol Control 106:45–53
Petrice TR, Miller DL, Bauer LS, Poland TM, Ravlin FW (2019) Photoperiodic modulation of diapause induction and termination in Oobius agrili Zhang and Huang (Hymenoptera: Encyrtdae), an egg parasitoid of the invasive emerald ash borer. Biol Control 138:104047
Petrice TR, Bauer LS, Miller DL, Stanovick JS, Poland TM, Ravlin FW (2021) Monitoring field establishment of the emerald ash borer biocontrol agent Oobius agrili Zhang and Huang (Hymenoptera: Encyrtdae) sampling methods, sample size, and phenology. Biol Control 156:104535
Pugh SA, Liebhold AM, Morin RS (2011) Changes in ash tree demography associated with emerald ash borer invasion, indicated by regional forest inventory data from the Great Lakes States. Can J Res 41:2165–2175
Pureswaran DS, Poland TM (2009) Host selection and feeding preference of Agrilus planipennis (Coleoptera: Buprestidae) on ash (Fraxinus spp.). Environ Entomol 38:757–765
Qazi SS, Lombardo DA, Abou-Zaid MM (2018) A metabolomic and HPLC-MS/MS analysis of the foliar phenolics, flavonoids and coumarins of the Fraxinus species resistant and susceptible to emerald ash borer. Molecules 23(11):2734
Quinn NF, Gould JR, Rutledge CE, Fassler A, Elkinton JS, Duan JJ (2022a) Spread and phenology of Spathius galinae and Tetrastichus planipennisi, recently introduced for biocontrol of emerald ash borer (Coleoptera: Buprestidae) in the northeastern United States. Biol Control 165:104794. https://doi.org/10.1016/j.biocontrol.2021.104794
Quinn NF, Duan JJ, Elkinton JS (2022b) Monitoring the impact of introduced emerald ash borer biocontrol agents: factors affecting Oobius agrili dispersal and parasitization of sentinel host eggs. BioControl 67:387–394
Rigsby CM, Showalter DN, Herms DA, Koch JL, Bonello P, Cipollini D (2015) Physiological responses of emerald ash borer larvae to feeding on different ash species reveal putative resistance mechanisms and insect counter-adaptations. J Insect Physiol 78:47–54
Roscoe LE, Lyons DB, Smith SM (2016) Observations on the life-history traits of the North American parasitoid Phasgonophora sulcata Westwood (Hymenoptera: Chalcididae) attacking Agrilus planipennis (Coleoptera: Buprestidae) in Ontario, Canada. Can Ent 148:294–306
Rutledge CE, Van Driesche RG, Duan JJ (2021) Comparative efficacy of three techniques for monitoring the establishment and spread of larval parasitoids recently introduced for biological control of emerald ash borer, Agrilus planipennis (Coleoptera: Buprestidae). Biol Control 161:104704
Sadof CS, Mockus L, Gonzel MD (2021) Factors influencing efficacy of an area-wide pest management program in three urban forests. Urb For Urb Green 58:126965
Spei BA, Kashian DM (2017) Potential for persistence of blue ash in the presence of emerald ash borer in southeastern Michigan. Forest Ecol Manag 392:137–143
Tanis SR, McCullough DG (2015) Host resistance of five Fraxinus species to Agrilus planipennis (Coleoptera: Buprestidae ) and effects of paclobutrazol and fertilization. Environ Entomol 41:287–299
Tanis SR, McCullough DG (2012) Differential persistence of blue ash (Fraxinus quadrangulata) and white ash (Fraxinus americana) following emerald ash borer (Agrilus planipennis) invasion. Can J Forest Res 42:1542–1550
Tluczek AR, McCullough DG, Poland TM (2011) Influence of host stress on emerald ash borer (Agrilus planipennis Fairmaire) (Coleoptera: Buprestidae) adult density, development, and distribution in Fraxinus pennsylvanica trees. Environ Entomol 40:357–366
USDA–APHIS/ARS/FS (2021) USDA–Animal Plant Health Inspection Service–Agricultural Research Service–Forest Service. Emerald ash borer biological control release and recovery guidelines. https://www.aphis.usda.gov/plant_health/plant_pest_info/emerald_ash_b/downloads/eab-field-release-guidelines.pdf (Accessed on 23 Feb 2022)
Villari C, Herms DA, Whitehill JG, Cipollini D, Bonell P (2016) Progress and gaps in understanding mechanisms of ash tree resistance to emerald ash borer, a model for wood-boring insects that kill angiosperms. New Phytol 209:63–79
Wagner DL, Todd KJ (2016) New ecological assessment for the emerald ash borer: a cautionary tale about unvetted host-plant literature. Am Entomol 62:26–35
Whitehill JGA, Popova-Butler A, Green-Church KB, Koch JL, Herms DA, Bonello P (2011) Interspecific proteomic comparisons reveal ash phloem genes potentially involved in constitutive resistance to the emerald ash borer. PLoS ONE 6(6):e24863
Whitehill JGA, Opiyo SO, Koch JL, Herms DA, Cipollini DF, Bonello P (2012) Interspecific comparison of constitutive ash phloem phenolic chemistry reveals compounds unique to Manchurian ash, a species resistant to emerald ash borer. J Chem Ecology 38:499–511
Yang ZQ, Achterberg CV, Choi WY, Marsh PM (2005) First recorded parasitoid from China of Agrilus planipennis: a new species of Spathius (Hymenoptera: Braconidae: Doryctinae). Ann Entomol Soc Am 98:636–642
Yang ZQ, Yao YX, Wang XY (2006) A new species of emerald ash borer parasitoid from China belonging to the genus Tetrastichus (Hymneoptera: Eulophidae). Proc Entomol Soc Wash 108:550–558
Yang ZQ, Wang XY, Gould JR, Wu H (2008) Host specificity of Spathius agrili Yang (Hymenoptera: Braconidae), an important parasitoid of the emerald ash borer. Biol Control 47:216–221
Yang ZQ, Wang XY, Gould JR, Reardon RC, Zhang YN, Liu GL, Liu ES (2010) Biology and behavior of Spathius agrili, a parasitoid of the emerald ash borer, Agrilus planipennisi, in China. J Insect Science 10:30
Yao YX, Duan JJ, Hopper KR, Mottern JL, Gates MW (2016) A new species of Oobius Trjapitzin (Hymenoptera: Encyrtidae) from the Russian far east that parasitizes eggs of emerald ash borer (Coleoptera: Buprestidae). Ann Entomol Soc Am 106:629–638
Zhang YZ, Huang DW, Zhao TH, Liu HP, Bauer LS (2005) Two new species of egg parasitoids (Hymenoptera: Encyrtidae) of wood-boring beetle pests from China. Phytoparasitica 53:253–260
Acknowledgements
The authors claim no conflict of interests. We thank Travis Perkins (Michigan State University) for preparing the maps, the many entomologists, technicians, and student employees from USDA Agricultural Research Service (ARS), Forest Service (FS), and Michigan State University, University of Maryland, University of Massachusetts, state regulators and land managers for helping carry out EAB biocontrol research and implementing EAB biocontrol programs. The authors also appreciate reviews of earlier draft of this manuscript by ARS colleague Dr. Xingeng Wang and Research Leader Dr. Kim Hoelmer.
Funding
This study was supported by Agricultural Research Service, CRIS # 8010-22000-030D.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors claim no conflict of interest.
Research involving human and animals rights
The research involves no human participants and/or animals.
Informed consent
All authors have participated in drafting the manuscript and agreed to be a co-author.
Additional information
Handling Editor. Dirk Babendreier
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Duan, J.J., Gould, J.R., Quinn, N.F. et al. Protection of North American ash against emerald ash borer with biological control: ecological premises and progress toward success. BioControl 68, 87–100 (2023). https://doi.org/10.1007/s10526-023-10182-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-023-10182-w