Abstract
The ability to survive winter temperatures is a key determinant of insect distributional ranges and population dynamics in temperate ecosystems. Although many insects overwinter in a state of diapause, the hemlock woolly adelgid [Adelges tsugae (Annand)] is an exception and instead develops during winter. We studied a low density population of A. tsugae, which undergoes two generations per year, in a forested area in which its only available host plant, eastern hemlock (Tsuga canadensis), was patchy and scarce. In January 2014, this area also experienced an exceptionally cold winter due to a southward shift in the North Polar Vortex. We used 3 years of systematic sampling prior to the 2014 cold wave, and 1 year following, to quantify the effect of the 2014 cold wave on A. tsugae population dynamics. We observed a strong negative correlation between the number of days below sub-zero temperature thresholds and A. tsugae, and estimated that the 2014 cold wave resulted in at least a 238% decrease in its population growth rate. However, we also observed that the detrimental effect of the 2014 cold wave to A. tsugae was short-lived, as populations measured in the late summer of 2014 rebounded to pre-2014 cold wave densities. This study highlights the effect that cold winter weather events can have on a winter active insect species, and the speed at which populations can recover from stochastic mortality events.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Distributional range boundaries of insect species are often delineated by suboptimal and supraoptimal temperature constraints. Many insects undergo a winter diapause, which allows poikilothermic species to survive subfreezing winter conditions and synchronizes life stages in the following spring (Tauber and Tauber 1976; Denlinger 2002). Consequently, many insect species that inhabit temperate ecosystems are able to withstand temperatures <−20 °C during the winter when in a diapausing state (Salt 1961; Bale 1991, 1993). However, not all insect species undergo a winter diapause, and although the lack of a diapause state is more common in tropical species that are not exposed to harsh winter conditions, there are exceptions in some temperate zone insect species. One exception is the hemlock woolly adelgid, Adelges tsugae (Annand), which undergoes a summer aestivation but does not diapause in winter and instead, actively feeds and develops during winter on photosynthetically active hemlock (Tsuga spp.). Adelges tsugae was introduced to eastern North America from Japan at least by 1951 (Havill et al. 2006, 2016), and since then has caused decline and mortality of eastern (T. canadensis) and Carolina (T. caroliniana) hemlock, from Georgia to Vermont (Orwig et al. 2002; Eschtruth et al. 2006; Nuckolls et al. 2009; Vose et al. 2013; Levy and Walker 2014).
Populations of A. tsugae in North America are parthenogenetic and consist of two generations per year. The progrediens generation occurs during spring-to-early summer, and their progeny are the sistens generation, which occur from summer to the follow spring. Eggs of both generations hatch into a crawler stage, which are ambulatory and settle and feed by inserting their stylet at the base of the petiole of a hemlock needle. During the summer the sistens enter aestivation until mid-Autumn. After terminating aestivation, the sistens generation feeds on hemlock until they reach the adult stage in the following spring. Active nymphs from both generations feed in the ray parenchyma cells, and develop into a sessile adult (Young et al. 1995). Adult females produce a single ovisac containing up to 150 eggs (McClure 1989; Paradis 2011). Although A. tsugae is active throughout the winter, past work has suggested that cold winter temperatures could be a barrier to its northern range expansion (e.g., in New York and New England states) (Parker et al. 1999; Skinner et al. 2003; Paradis et al. 2008; Trotter and Shields 2009). On the other hand, other studies have suggested that high summer temperatures to which aestivating nymphs are exposed can affect A. tsugae survivorship, which could also be a barrier to its range expansion (Paradis 2011; Sussky and Elkinton 2015). The central Appalachian Mountains, which is part of the Appalachian Mountain range between Tennessee and West Virginia, generally experiences a milder winter than the northeastern United States. However, winter temperatures at the higher elevations in the central Appalachians, whose peaks reach 1400–2000 meters above sea level (MSL), could still pose a challenge to A. tsugae survival and population growth.
In addition to the average winter temperatures of the central Appalachian Mountains that could be a constraint to A. tsugae population growth, stochastic winter weather events could also affect A. tsugae, albeit over a smaller temporal window. Beginning in 2010, we monitored what initially was a very low density A. tsugae population in the Monongahela National Forest in Randolph County, West Virginia (Fig. 1). This population grew over time until we sampled in early spring 2014. Immediately prior to that, beginning in January 2014, much of the northern and eastern United States experienced a weather event colloquially known as the 2014 North American cold wave. This weather event, caused by the shift southward in the North Polar Vortex, resulted in sustained cold temperatures that were below historical averages. We used 3 years (2010–2013) of systematic monitoring data to derive A. tsugae population density estimates and population growth rates prior to the 2014 North American cold wave, and 1 year (2014) following this weather event to quantify its short-term effect on A. tsugae populations in a Central Appalachian forest.
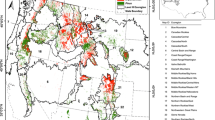
a Locator map of the region containing our study sites in West Virginia, USA (indicated by times symbol), and b location of sites used in this study, including the site of initial detection in spring 2010, initial pilot sites that were sampled in summer 2010, and additional sites that were added in summer 2011
Methods
Study region
We conducted this study in an >80-year-old second growth forest in the Monongahela National Forest in Randolph County, West Virginia (Fig. 1). During spring 2010, as part of a separate study aimed at measuring the effect of deer density on oak (Quercus spp.) regeneration, a single A. tsugae ovisac was serendipitously spotted on a fallen branch below a single dominant eastern hemlock (38°58′44″N, 79°35′45″W). This hemlock tree (59.9 cm diameter at breast height, DBH) was within a stand that was otherwise dominated by northern red oak (Q. rubra), red maple (Acer rubrum), and black cherry (Prunus serotina), which collectively comprised approximately 79% of the basal area in this forest; in contrast, hemlock comprised <1% of the basal area (Miller et al. 2004). A subsequent pilot sampling effort revealed that hemlock was indeed spatially patchy and infrequent in this forest, and A. tsugae was at very low but detectable densities. The management of this portion of the Monongahela National Forest, located at approximately 900–1100 MSL, falls under the Forest Service 2001 Roadless Rule (US Code of Federal Regulations, Title 36, Chap. 2, Part 294), and thus public access is restricted to foot traffic through gated entry points accessible from a county road. Given the low amount of hemlock and limited public access, we were intrigued by the presence of A. tsugae, even at low densities. We thus initiated a study to quantify the dynamics of A. tsugae under these initial population density conditions, and when its host plant was sparsely distributed.
Adelges tsugae sampling
We initiated a pilot sampling project in early August 2010 to quantify the densities of A. tsugae ovisacs produced by the progrediens generation. We sampled three hemlock trees from each of four sites in the vicinity of the hemlock on which we detected the first ovisac, which was included in our sampling (Fig. 1). From each tree, we sampled one branch from each of four cardinal directions (N–S–E–W). Samples were taken from approximately 3–10 m above the ground, depending on branch accessibility, using a telescoping pole pruner. We also sampled from a site approximately 2 km from these four sites, which was only approximately 200 m from a publically-accessible road. At this site, hemlock was younger and branches were accessible from the ground; thus, samples were taken from the ground using hand pruners. Here, we randomly sampled one hemlock branch from each of ten randomly chosen hemlock trees. On all cut branches, we counted, under a microscope, the number of viable A. tsugae ovisacs (i.e., ovisacs containing eggs or signs of hatched eggs) from the tips of the branches (≤15 cm in length), which generally constituted the new growth over the last year that would have been available for the preceding generation. We also measured the length of the suitable habitat of each sampled branch, which we defined as hemlock growth <2 years old; branchlets <2 years old are the branch age most preferred by A. tsugae (McClure 1991; Lagalante et al. 2006). We then calculated the number of viable ovisacs per cm of suitable habitat.
Based upon data from this pilot effort, we expanded our sampling while including sites sampled in 2010. We used aerial photographic images taken during leaf-off to initially identify patches of hemlock, as this forested area is dominated by deciduous trees (Miller et al. 2004). We selected nine additional sites that were located at various directions and distances from the location of initial A. tsugae discovery. At each site, we randomly selected and marked up to three hemlock trees for sampling. The total sample size at each sampling interval included 14 sites, a total of 42 trees, and a total of approximately 138 branches. A map of all sampling locations is presented in Fig. 1.
In our expanded sampling efforts, we also took samples twice per year: once in spring from March to April (i.e., spring sampling intervals) to quantify the densities of viable ovisacs produced by the sistens generation, and again in summer from August to September (i.e., summer sampling intervals) to quantify the densities of viable ovisacs produced by the progrediens generation. Sampling efforts began in summer 2011 and concluded in summer 2014. Branches were sampled as in 2010, and processed in the laboratory as before to estimate the number of viable ovisacs per cm of suitable habitat at both sampling intervals. Although this forested area was initially infested with A. tsugae at seemingly low abundance, we still sought to quantify the effect of A. tsugae on hemlock new growth. Thus, during the summer sampling intervals, beginning in 2011, we measured the amount of new hemlock growth on each sampled branch. We did this by measuring the length of the five longest new growth shoots per branch. Overall, our sampling efforts provided us with 3 years, and six generations, of A. tsugae dynamics prior to the 2014 North American cold wave, which began in January 2014, and 1 year, and two generations, of A. tsugae dynamics immediately following the cold wave. This gave us a unique opportunity to quantify the effect of the 2014 North American cold wave on A. tsugae populations through the use of data provided by systematic sampling at regular intervals.
Temperature data
Daily minimum and maximum surface temperatures were obtained from the National Centers for Environmental Information (2017) from a station located approximately 10 km from the approximate center of all stands that also reflected a similar elevation as our study sites (39°00′47″N, 79°28′27″W, 1033.3 MSL). We obtained temperature data from 2009 to 2014 for use in this analysis. Based upon these data, and when excluding the temperatures recorded during the 2014 North American cold wave, the mean annual temperature in this study area was 8.3 °C with a mean daily maximum and minimum of 13.2 and 3.5 °C, respectively. Across all winter seasons (November to March) prior to the 2014 cold wave, the lowest daily minimum temperature ranged from −16.6 to −22.2 °C (Fig. 2). During the cold wave, the lowest recorded daily minimum temperature from our study area was −26.7 °C and was recorded on 7 January 2014.
Minimum daily temperatures from 1 November 2009 to 31 October 2014. Black lines indicate the winter period (1 November to 31 March) from which we calculated the number of days below temperature thresholds (cf. Table 1)
Stand composition data
Although large-scale, generalized stand composition data for this forested area were provided by Miller et al. (2004), we sought to better quantify stand composition at and in the vicinity of our specific study sites. In summer 2014, from each single hemlock tree, or from the approximate center of a stand from which we sampled three hemlock trees, we measured the DBH of all stems ≥2 cm DBH within an 11.3 m radius (plot area 404 m2) and identified woody stems to species. We followed the same protocol in 1–2 additional 404 m2 plots; these plots were approximately 30 m away, and we randomly chose 1–2 azimuths (N, NE, E, SE, S, SW, W, and NW) to locate these additional plots from each sampled hemlock tree or stand. We sampled a total of fifty-six 404 m2 plots.
Data analyses
At each sampling interval, we estimated A. tsugae population densities (i.e., the number of viable ovisacs per cm of suitable habitat) for each site. Population trends over time were modeled using locally weighted polynomial regression in R (R Development Core Team 2017). To assess the relationship between A. tsugae population densities and winter temperatures, we considered daily minimum temperatures during active feeding by A. tsugae nymphs during the winter. We defined the winter as a period from 1 November to 31 March in the following year, which includes the lowest temperatures to which A. tsugae would be exposed. For each of these periods from November 2009 to March 2014 (e.g., 2009–2010, 2010–2011, ..., 2013–2014), we calculated the number of days in which the minimum daily temperature was ≤ −10, −15, and −20 °C. These thresholds were chosen based upon prior work that reported a relationship between temperature and A. tsugae mortality at temperatures between −15 and −40 °C (Parker et al. 1999; Skinner et al. 2003). These temperature thresholds also represent the range of winter temperatures that A. tsugae was exposed to at our study sites (Table 1). For each period, we then measured the Pearson correlation coefficient between the number of days below each temperature threshold and A. tsugae population densities in the subsequent spring sampling interval (R Development Core Team 2017). To iterate, the spring sampling interval measured the density of viable ovisacs produced by thsistens generation per cm of suitable habitat.
To further understand the relationship between winter temperatures and A. tsugae population dynamics, we also estimated the population growth rate at each site according to
where N is the estimated A. tsugae population density (i.e., the number of viable ovisacs per cm of suitable habitat) at the current (t) or previous sampling interval (t–1). A value of 0.01 was added to all values of N due to the occasional presence of 0 data, and data were transformed using loge to normalize the distribution of growth rates. Population growth rates were estimated for each pair of sampling intervals from summer 2010 to summer 2014. We tested for differences in the growth rate at each pair of sampling intervals using analysis of variance, and conducted post hoc analyses using Tukey’s HSD (α = 0.05) (R Development Core Team 2017).
As a complement to our primary question regarding the effect of the 2014 North American cold wave on A. tsugae populations, we also examined year-to-year variation in summer temperatures to which aestivating A. tsugae would be exposed given past work suggesting that high summer temperatures can affect their survivorship (Paradis 2011; Sussky and Elkinton 2015). We used daily maximum temperatures from 1 July to 31 August, which are the warmest months in this region (National Centers for Environmental Information 2017). Also, we selected 1 July as a starting point based upon observations from a previous study in West Virginia, located approximately 95 km northeast of this study site, that suggested nymphs were aestivating by mid-to-late June (Tobin et al. 2013). Using year as a nominal variable (2010–2014), we analyzed the daily maximum temperatures using an analysis of variance, and conducted post hoc analyses using Tukey’s HSD (α = 0.05) (R Development Core Team 2017).
To measure the relationship between A. tsugae population density and new hemlock growth, we summed the length of new growth from the five longest new growth shoots on each branch. We then estimated the Pearson correlation coefficient (r) between A. tsugae population density and new hemlock growth per branch using data collected during the summers of 2011, 2012, 2013, and 2014 (R Development Core Team 2017).
Results and discussion
Across our study period, the 2014 cold wave resulted in both the coldest minimum daily temperature and the most prolonged cold period (Table 1; Fig. 2). During the cold wave, eight of the 11 days in which the temperature was ≤−20 °C occurred in January 2014, with six of those days occurring during the last 8 days of January. We also noted that the lowest recorded temperature during the cold wave (–26.7 °C) occurred on 7 January 2014. A prior study that examined cold tolerance of A. tsugae showed that life stages were most tolerant of cold temperatures during January relative to March (Skinner et al. 2003). Skinner et al. (2003) also noted that mortality was observed following exposure to −15 °C, that <10% were able to survive exposure to −25 °C, and that <3% were able to survive exposure to −30 °C. The coldest temperatures that were observed from our sites during 2014 cold wave were generally between −20 and −25 °C (Table 1). Thus, although high mortality of A. tsugae in the central Appalachian Mountains was expected to occur during the 2014 cold wave, the cold temperatures, in retrospect, would not be expected to result in complete mortality.
Indeed, the temporal dynamics of A. tsugae population densities from our study highlights the decline in populations following the 2014 cold wave but not complete mortality (Fig. 3). For example, prior to the 2014 cold wave, the mean (±SD) number of viable ovisacs per cm of suitable habitat across sites was 0.152 (0.073) whereas in the spring following the cold wave, the mean (±SD) decreased to 0.027 (0.020), which represents a mean decrease in population density of 82.2%. We also measured significant negative correlations between A. tsugae population densities and the number of days that were below −10 °C (r = −0.38; P < 0.01), −15 °C (r = −0.35; P < 0.01), and −20 °C (r = −0.47; P < 0.01). This suggests that even exposure to temperatures below −10 °C could induce mortality. This is not surprising given the study by Skinner et al. (2003), who reported approximately 40% mortality following exposure to −15 °C in January.
In addition to the decline in population density from summer 2013 to spring 2014 (Fig. 3), the population growth rates reveal perhaps a stronger effect of the 2014 cold wave. Prior to the 2014 cold wave, we generally recorded the highest population growth rates at our study sites when estimated from the summer sampling interval to the spring sampling interval; this represents the growth rate from the density of viable ovisacs produced by the progrediens generation to the density of viable ovisacs produced by the subsequent sistens generation (Fig. 4). In contrast, population growth rates from the spring to the summer sampling intervals (i.e., from the density of viable ovisacs produced by the sistens generation to the density of viable ovisacs produced by the progrediens generation) were lower (Fig. 4). This supports prior studies conducted under field conditions in Massachusetts in which the authors observed higher mortality during the summer aestivation period, during which aestivating nymphs were more likely to be exposed to supraoptimal temperatures, than over the winter (Paradis 2011; Sussky and Elkinton 2015). A comparison of population growth rates when measured during the spring and summer sampling intervals, and before and after the 2014 cold wave, is shown in Table 2. Relative to the observed mean population growth rate in spring prior to the cold wave, the observed population growth rate from summer 2013 to spring 2014 indicates a mean decrease of 238% in the expected spring population growth rate at our study sites as a consequence of the 2014 cold wave.
Box plots of population growth rates (Eq. 1) across sites and at each sampling interval. Grey boxes indicate the population growth rate estimated in spring (Sp), which is based on the number of viable ovisacs per cm of suitable habitat produced by the progrediens generation from the prior year to the number of viable ovisacs per cm of suitable habitat produced by the sistens generation after winter. The population growth rate estimated in summer (Su) is based on the number of viable ovisacs per cm of suitable habitat produced by the sistens generation to the number of viable ovisacs per cm of suitable habitat produced by the progrediens generation in the same year. A population growth rate of 0 indicates no change in the population density from one generation to the next. Different letters over boxes indicate significant differences (α = 0.05)
We also observed one of the highest population growth rates from spring to summer 2014 (Fig. 4; Table 2). These growth rate dynamics suggest that although A. tsugae populations significantly declined following the cold wave, some individuals persisted and were able to rebound within the same year (Fig. 3). It is possible that surviving individuals benefited from both reduced intraspecific competition, and an ample supply of new hemlock growth given the association between A. tsugae population density and new hemlock growth (see below). A previous study presented convincing evidence of a density-dependent decrease in both A. tsugae fecundity and survivorship (Sussky and Elkinton 2014); however, we note that this prior study was conducted at much higher densities than what we observed in our study sites. Past work has also shown that even extremely low densities of A. tsugae are capable of sustaining themselves (Tobin et al. 2013), which is perhaps not unexpected given its ability to reproduce parthenogenetically. Another possibility for the high population growth rates from spring to summer 2014 (Fig. 4; Table 2) could be favorable summer temperatures in 2014 during aestivation. However, although the mean maximum summer temperatures (1 July to 31 August) in 2014 were significantly less than 2010, 2011, and 2012, they were not significantly different than summer temperatures in 2013 (Table 3), which was a year in which growth rates were not significantly different than 2011 and 2012 (Fig. 4). Moreover, mean daily maximum summer temperatures in our study area ranged from 22.6 to 24.4 °C across all years (Table 3); thus, summer conditions during aestivation in heavily-forested areas of the central Appalachian Mountains may not, at present, be a limiting factor in A. tsugae population growth.
The highest density observed at any site during any sampling period prior to the 2014 cold wave was 0.37 viable ovisacs per cm of suitable habitat. However, despite these low population densities, we still observed a negative correlation between A. tsugae densities and the amount of new hemlock growth (r = −0.11, P = 0.03) (Fig. 5). We observed a range of new growth lengths, from a mean of 0 cm per shoot to 20.5 cm per shoot. Most of our observed A. tsugae densities were below 0.4 ovisacs per cm of suitable habitat (Fig. 5), at which the mean new growth length per shoot was 3.1 cm. The negative association between A. tsugae densities and new hemlock growth suggests that even low A. tsugae densities can adversely affect hemlock growth. A previous study also observed a strong relationship between A. tsugae densities and new hemlock growth, albeit at higher A. tsugae densities than what we observed at our study sites (Sussky and Elkinton 2015).
A prior study estimated that hemlock comprised < 1% of the total basal area in this forested area (Miller et al. 2004). We sampled a total of 2.27 ha from which we measured 1568 stems, of which 120 stems were eastern hemlock. Hemlock in our plots represented 8.2% of the total basal area we recorded, but is an overestimate of the overall amount of hemlock in this forested area due to our non-random sampling that specifically targeted hemlock. Across all our sampled plots, the most numerically dominant tree species were American beech (Fagus grandifolia, 443 stems), sugar maple (Acer saccharum, 221 stems), and yellow poplar (Liriodendron tulipifera, 137 stems), while in terms of basal area, the dominant tree species were yellow poplar (25.8% of total basal), black cherry (13.1% of total basal), and red oak (11.0% of total basal). Thus, even when we specifically located study sites centered on hemlock, it was still a minority component at our sites.
Given that this forested area is not easily accessible by the public, other than by foot, and the scarcity of hemlock, it is intriguing that A. tsugae populations are present at detectable levels. The anthropogenic movement of life stages would seem to be an unlikely pathway given the lack of accessibility and the absence of human settlements in our study area. The area is frequented year-round by recreationists, hunters, and fishermen, but it seems unlikely that this would be a mechanism though which, for example, hemlock infested with A. tsugae would be introduced. A previous study by McClure (1990) in Connecticut showed that a number of factors, including wind and humans, can contribute to A. tsugae dispersal. McClure (1990) also observed that wildlife, such as birds, can serve as a transport vector for A. tsugae eggs and newly emerged crawlers. Although the precise mechanism of A. tsugae introduction in this forested area will likely never be known, its presence does underscore the ability of this invasive species to infest new and remote areas, even when human access is limited, movement of infested hemlock by humans would seem to be unlikely, and hemlock host plants are rare.
Many insect species native to this region of the United States undergo diapause to survive winter temperatures. Diapause confers protection against cold temperatures, even those that are <−20 °C. Although A. tsugae is active in winter, Skinner et al. (2003) observed that some individuals survived exposure to −30 °C. We believe this study to be one of the first to quantify the effect of the 2014 North American cold wave on an insect population under natural conditions and through the use of data collected on population densities prior to and immediately following this winter weather event. Our data highlight the effect that stochastic winter events can have on a winter-feeding insect species that is otherwise reasonably cold tolerant. Our data also demonstrate the ability of A. tsugae to recover from such a dramatic decline in population densities due to stochastic mortality, with important implications in both conservation and invasion ecology.
References
Bale JS (1991) Insects at low temperature: a predictable relationship? Funct Ecol 5:291–298
Bale JS (1993) Classes of insect cold hardiness. Funct Ecol 7:751–753
Denlinger DL (2002) Regulation of diapause. Annu Rev Entomol 47:93–122
Eschtruth AK, Cleavitt NL, Battles JJ, Evans RA, Fahey TJ (2006) Vegetation dynamics in declining eastern hemlock stands: 9 years of forest response to hemlock woolly adelgid infestation. Can J For Res 36:1435–1450
Havill N, Montgomery M, Yu G, Shiyake S, Caccone A (2006) Mitochondrial DNA from hemlock woolly adelgid (Hemiptera: Adelgidae) suggests cryptic speciation and pinpoints the source of the introduction to eastern North America. Ann Entomol Soc Am 99:195–203
Havill NP, Shiyake S, Lamb Galloway A, Foottit RG, Yu G, Paradis A, Elkinton J, Montgomery ME, Sano M, Caccone A (2016) Ancient and modern colonization of North America by hemlock woolly adelgid, Adelges tsugae (Hemiptera: Adelgidae), an invasive insect from East Asia. Mol Ecol 25:2065–2080
Lagalante A, Lewis N, Montgomery M, Shields K (2006) Temporal and spatial variation of terpenoids in Eastern Hemlock (Tsuga canadensis) in relation to feeding by Adelges tsugae. J Chem Ecol 32:2389–2403
Levy F, Walker ES (2014) Pattern and rate of decline of a population of Carolina hemlock (Tsuga caroliniana Engelm.) in North Carolina. Southeast Nat 13:46–60
McClure MS (1989) Evidence of a polymorphic life cycle in the hemlock woolly adelgid, Adelges tsugae (Homoptera: Adelgidae). Ann Entomol Soc Am 82:50–54
McClure MS (1990) Role of wind, birds, deer, and humans in the dispersal of hemlock woolly adelgid (Homoptera: Adelgidae). Environ Entomol 19:36–43
McClure MS (1991) Density dependent feedback and population cycles in Adelges tsugae (Homoptera: Adelgidae) on Tsuga canadensis. Environ Entomol 20:258–264
Miller GW, Kochenderfer JN, Gottschalk KW (2004) Effect of pre-harvest shade control and fencing on Northern red oak seedling development in the Central Appalachians. In: Spetich MA (ed) Upland oak ecology symposium: history, current conditions, and sustainability. USDA Forest Service General Technical Report SRS-73, Asheville, pp 182–189
National Centers for Environmental Information (2017) http://www.ncdc.noaa.gov. Accessed 5 May 2017
Nuckolls AE, Wurzburger N, Ford CR, Hendrick RL, Vose JM, Kloeppel BD (2009) Hemlock declines rapidly with hemlock woolly adelgid infestation: impacts on the carbon cycle of southern Appalachian forests. Ecosystems 12:179–190
Orwig DA, Foster DR, Mausel DL (2002) Landscape patterns of hemlock decline in New England due to the introduced hemlock woolly adelgid. J Biogeogr 29:1475–1487
Paradis A (2011) Population dynamics of hemlock woolly adelgid (Adelges tsugae) in New England. Ph.D. dissertation. University of Massachusetts, Amherst, p 114
Paradis A, Elkinton J, Hayhoe K, Buonaccorsi J (2008) Role of winter temperature and climate change on the survival and future range expansion of the hemlock woolly adelgid (Adelges tsugae) in eastern North America. Mitig Adapt Strateg Glob Chang 13:541–554
Parker BL, Skinner M, Gouli S, Ashikaga T, Teillon HB (1999) Low lethal temperature for hemlock woolly adelgid (Homoptera: Adelgidae). Environ Entomol 28:1085–1091
R Development Core Team (2017) The R project for statistical computing, Vienna. http://www.R-project.org/. Accessed 5 May 2017
Salt RW (1961) Principles of insect cold-hardiness. Annu Rev Entomol 6:55–74
Skinner M, Parker BL, Gouli S, Ashikaga T (2003) Regional responses of hemlock woolly adelgid (Homoptera: Adelgidae) to low temperatures. Environ Entomol 32:523–528
Sussky EM, Elkinton JS (2014) Density-dependent survival and fecundity of hemlock woolly adelgid (Hemiptera: Adelgidae). Environ Entomol 43:1157–1167
Sussky EM, Elkinton JS (2015) Survival and near extinction of hemlock woolly adelgid (Hemiptera: Adelgidae) during summer aestivation in a hemlock plantation. Environ Entomol 44:153–159
Tauber MJ, Tauber CA (1976) Insect seasonality: diapause maintenance, termination, and postdiapause development. Annu Rev Entomol 21:81–107
Tobin PC, Turcotte RM, Snider DA (2013) When one is not necessarily a lonely number: initial colonization dynamics of Adelges tsugae on eastern hemlock, Tsuga canadensis. Biol Invasions 15:1925–1932
Trotter RT, Shields KS (2009) Variation in winter survival of the invasive hemlock woolly adelgid (Hemiptera: Adelgidae) across the eastern United States. Environ Entomol 38:577–587
Vose JM, Wear DN, Mayfield AE, Nelson CD (2013) Hemlock woolly adelgid in the southern Appalachians: control strategies, ecological impacts, and potential management responses. For Ecol Manag 291:209–219
Young RF, Shields KS, Berlyn GP (1995) Hemlock woolly adelgid (Homoptera: Adelgidae): stylet bundle insertion and feeding sites. Ann Entomol Soc Am 88:827–835
Acknowledgements
We thank Josh Bolyard, Josh Embrey, Tesia Gnagey, Gino Luzader, and Eric Walberg (USDA Forest Service, Northern Research Station); and Kandance Burton, Will Harris, Amy Hill, Craig Larcenaire, Danielle Martin, Lindsay Wolfe, and Tara Spinos (USDA Forest Service, State and Private Forestry, Northeastern Area) for valuable field and laboratory assistance. We are grateful to Angela Mech (University of Washington) for helpful comments on an earlier draft.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tobin, P.C., Turcotte, R.M., Blackburn, L.M. et al. The big chill: quantifying the effect of the 2014 North American cold wave on hemlock woolly adelgid populations in the central Appalachian Mountains. Popul Ecol 59, 251–258 (2017). https://doi.org/10.1007/s10144-017-0589-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10144-017-0589-y