Abstract
This study evaluated the effects of different aeration types on water quality, shrimp growth and biofloc composition in a Litopenaeus vannamei culture. The study was conducted with three treatments: (1) PR—propeller aspirator pump aerator; (2) VP—vertical pump aerator; and (3) BL—diffused air blower. The study was performed in a greenhouse with nine 35,000-L rectangular tanks. Water quality parameters (temperature, salinity, dissolved oxygen, pH, ammonia, nitrite, nitrate, settleable and suspended solids) were measured along the 33 experimental days. Moreover, samples were collected to quantify the microorganisms present in the tanks. At the end of the study, samples of the biofloc of each tank were collected to proximal analysis. Throughout the experiment, the temperature, pH, salinity and alkalinity were maintained within the recommended levels for L. vannamei. The propeller treatment showed a concentration of total ammonia above the recommended levels and lower densities of ciliates and flagellates, most likely because of inadequate biofloc formation in this treatment. The final weight was higher in the blower and propeller treatments. However, survival was lower in the propeller treatment compared to the other treatments. The results of this study suggest that diffused air systems (air blower) improve the formation of biofloc and growth performance of L. vannamei.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The biofloc technology (BFT) system uses minimal or no water exchange, and the water quality can be improved by the formation of microbial aggregates (bioflocs). Therefore, BFT is a more environmentally friendly alternative to traditional shrimp aquaculture (De Schryver et al. 2008).
With reduced water use, BFT systems release few effluents, and as a result of increased stocking densities, shrimp production is enhanced (Krummenauer et al. 2011, 2014). In these systems, biofloc formation can be stimulated by the addition of a carbon source to maintain a C:N ratio ranging from 10 to 20:1 (Ebeling et al. 2006; Crab et al. 2007). Biofloc particles contain a variety of microorganisms, such as bacteria, microalgae, protozoa and zooplankton (Ray et al. 2010). Therefore, biofloc can complement the diet of reared animals, which uses the natural productivity in the culture system as a feed source (Wasielesky et al. 2006; Emerenciano et al. 2012). Wasielesky et al. (2006) and Otoshi et al. (2011) analyzed the effects of natural productivity in Litopenaeus vannamei juveniles and confirmed the positive effects on growth, food consumption, weight gain, feed conversion rates and shrimp survival. Thus, the influence of the management parameters like mixing intensity, dissolved oxygen, pH, temperature and organic carbon sources has a great importance to achieve a better aggregation of particles and high quality of the bioflocs (De Schryver et al. 2008).
The formation of microbial aggregates (bioflocs) is dependent on physical, chemical and biological interactions. In the early stages of a culture, there is an increase in the abundance of bacteria that use the dissolved organic matter for growth (Biddanda 1985). Thus, because of the adherent mucus production by these bacteria, there is an increase in the size of bioflocs caused by the aggregation of particles. The abundance of other microorganisms, such as flagellates, ciliates and amoeboid forms, may increase because most of these microorganisms graze on the bacteria (Biddanda and Pomeroy 1988). In addition, the cultured organisms, such as crustaceans and fish, can affect the dynamics of aggregation by grazing or movement (Dilling and Alldredge 2000). The addition of feed generates products that will be metabolized, which increases the number of bacteria if enough oxygen is available in the water. Therefore, the activity of microbial communities is defined by the rates of mixing and oxygenation generated by the aeration processes (Avnimelech 2009).
In addition to the effects on growth and performance of cultured animals, low oxygen concentrations in BFT systems may influence the biofloc microbial community (Ray et al. 2010). Furthermore, the bacterial processes of nitrogen assimilation and nitrification consume oxygen and reduce the alkalinity of the system such that it is sometimes necessary to supplement the cultures with oxygen and carbonate (Ebeling et al. 2006; Hargreaves 2006; Furtado et al. 2011).
One requirement for the formation of bioflocs is a high oxygenation of the culture tanks, which is primarily provided by aeration devices. The aeration system choice is crucial to obtain high productivity in the system. According to Avnimelech (2009), aerators are inserted into cultures for several reasons: (a) to supply oxygen for animals beyond their limitations, maintaining high stocking densities and productivity; (b) to distribute the oxygen into the culture tanks horizontally and vertically; (c) to mix the water column; and (d) to oxygenate the sediment coverage.
In the operation of ponds, different aeration equipments can be utilized depending on various factors in the management of the farms (Wheaton 1977). The main aeration devices used are paddlewheel aerators, propeller aerators, vertical pump aerators and diffused air systems in which different equipments are utilized, including aerotubes, nozzles and air stones. The diffused aeration systems optimize oxygen transfer by producing bubbles that stimulate the initial aggregation of the bioflocs. However, conventional aerators used in ponds are less expensive than diffused air systems. Furthermore, in a recent review, Crab et al. (2012) identified that one of the challenges that still deserves attention for future research in BFT system would be the selection and positioning of aerators.
In this context, the aim of this study was to evaluate the effects of the different aeration types available in the market on biofloc formation, the microbial community and water quality as well as to determine the influence of these factors on the growth parameters of Litopenaeus vannamei juveniles reared in a biofloc technology culture system.
Materials and methods
Culture conditions
The 33-day growth study was performed at the Marine Station of Aquaculture, Federal University of Rio Grande (FURG), Southern Brazil (32°12′16S, 52°10′38W) in a greenhouse with nine 35 m2 (5 × 7 m) rectangular tanks covered with a geomembrane (HDPE, 1.5 mm), and the water depth used was 1 m. Litopenaeus vannamei juveniles (Aquatec®, Canguaretama; Rio Grande do Norte State, Brazil) were stocked with a mean initial weight of 4.3 ± 0.93 g. The stocking density was 140 shrimp m−2 (approximately 4900 shrimp per tank).
Experimental design
The study was performed with three treatments (three replicates each): (1) PR, ½ HP propeller aspirator pump aerator (Trevisan Equipamentos Agroindustriais Ltda.; Palotina, Paraná, Brazil); (2) VP, ½ HP vertical pump aerator (Trevisan Equipamentos Agroindustriais Ltda.; Palotina, Paraná, Brazil); and (3) BL, ½ HP diffusion air blower (Ibram Indústria Brasileira de Máquinas Ltda.; Vila Maria, São Paulo, Brazil). The tanks of the BL treatment were equipped with 35 air stones (10 cm in length each, one stone per m2 placed at the bottom of the tank) connected with 20-mm PVC pipes and powered using a ½ HP air blower for each tank. The tanks were filled with filtered seawater, treated with a chlorine solution (10 ppm) and dechlorinated with ascorbic acid powder (1 g per 1000 L). Artificial substrates (Needlona™) were used to increase the surface area of the tanks (approximately 150 % of the lateral tank area) available for bacterial colonization. The fertilization method followed Ebeling et al. (2006) and Avnimelech (2009). To maintain the alkalinity values above 100 mg L−1, corrections were made according to Furtado et al. (2011) with hydrated lime (Ca(OH)2).
Water quality parameters
The water temperature, salinity, dissolved oxygen (DO) and pH were recorded using a multiparameter (YSI 556, YSI Inc.; Yellow Springs, OH, USA). Water samples were collected daily to quantify the concentration of total ammonia nitrogen (TAN) (UNESCO 1983). Analyses of the nitrite (NO2-N), nitrate (NO3-N) and phosphate (PO4-P) concentrations were performed every 5 days following the methods described by Strickland and Parsons (1972). The alkalinity was determined according to the method described by APHA (1989). The turbidity was measured once a week using a turbidimeter (Hach 2100P, Hach Company; Loveland, CO, United States). The settled solids were measured using Imhoff cones and were based on the volume of the bioflocs in 1 L after 15 min of sedimentation (Avnimelech 2009). The total suspended solids (TSS) were determined according to Strickland and Parsons (1972).
Microbial community assessment
Water samples (50 mL) from the treatments were collected every 3 days and preserved with borate-buffered formalin (4 % v/v) for further analysis of the biofloc composition (Thompson et al. 2002). The abundance of ciliates and rotifers was determined in 2.1 mL samples poured into sedimentation chambers (Utermöhl 1958) using an Olympus inverted light microscope equipped with phase contrast. For bacterial and flagellate counts, 0.1 mL subsamples were concentrated on darkened polycarbonate membrane filters (Nuclepore, 0.2 μm), stained with the fluorochrome Acridine Orange (5 μg mL−1) and counted using a Zeiss Axioplan epifluorescence microscope (Thornwood, NY) equipped with a 487 701 light filter set (BP365/11; FT 395; LP 397) at a 1000× final magnification (Porter and Feig 1980).
Shrimp feeding and monitoring
The shrimp were fed three times per day with a commercial diet (Potimar Active 38 % CP, 2 mm). The feeding rate followed the method proposed by Jory et al. (2001).
Each week, 60 shrimp were randomly sampled from each tank and individually weighed. The weight was individually measured using a digital scale that is accurate to 0.01 g (Marte®científica AS2000; Santa Rita do Sapucaí, MG, Brazil). At the end of the culture cycle, 200 shrimp per tank were individually weighed, and the survival rate was estimated from the total harvest weight.
Proximate composition of bioflocs
At the end of the study, samples of the biofloc of each tank were collected for further proximate analysis (dry matter, crude protein, lipid, fiber and ash content). The suspended material was collected by filtering the water of tanks using a submerged pump with a flow rate of 4000 L h−1 with a mesh size of 50 μm. The analysis were performed in the Animal Nutrition Laboratory (LNA) from Federal University of Pelotas (UFPEL), Rio Grande do Sul, Brazil. The methodology follows the AOAC (2000) protocols.
Statistical analysis
Significant differences (p < 0.05) in the zootechnical performance and water parameters were analyzed using a one-way ANOVA. Tukey’s multiple-range test was applied when significant differences were detected. All of the tests were performed after confirmation of the homogeneity of variance (Levene’s test) and normality of the data distribution (Kolmogorov–Smirnov test). To satisfy the ANOVA assumptions, the survival data were arcsine/square-root transformed using a constant exponent (arcsine × 0.5) (Zar 1996).
Results
The mean values (±SD) of the physical and chemical parameters during the experiment in the blower (BL), vertical pump (VP) and propeller (PR) treatments are presented in Table 1.
The temperature throughout the experimental period did not significantly differ among the three treatments (p > 0.05). The minimum and maximum temperature values in the BL, VP and PR treatments were 26.3 and 31.6, 26.55 and 30.75, and 28.5 and 34.4 °C, respectively.
Similarly, no significant differences were observed between the mean dissolved oxygen concentrations in all treatments. The minimum and maximum DO values in the BL, VP and PR treatments were 3.07 and 6.4 mg L−1, 2.13 and 6.7 mg L−1, and 0.82 and 6.4 mg L−1, respectively.
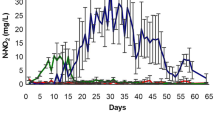
The total ammonia concentrations were lower in the BL and VP treatments; however, both treatments differed statistically (p < 0.05) from the PR treatment, which had the high ammonia concentration during the rearing period (Table 1; Fig. 1).
The nitrite levels were significantly higher (p < 0.05) in the BL and VP treatments compared to the PR treatment (Fig. 2). The nitrate concentrations were significantly higher in the BL treatment (Fig. 3). This treatment differed statistically (p < 0.05) from the VP and PR treatments.
The salinity, pH and orthophosphate did not differed significantly between the treatments (p > 0.05). The alkalinity levels were significantly lower in BL treatment (p < 0.05), the VP treatment showed intermediary values, and PR presented higher concentrations of alkalinity. All treatments showed statistical differences between them (p < 0.05).
The results of the settleable solids (SS) were significantly higher in the BL than in the other treatments (p < 0.05). Similarly, for the total suspended solids, the BL treatment showed significantly higher values compared to the VP and PR treatments, which were not significantly different (Fig. 4).
Table 2 summarizes the ciliate, flagellate and diatom abundances observed with different treatments and on different days. The densities of the ciliates and flagellates were constant throughout the culture in the BL and PR treatments; however, the density was lower in the PR treatment. In the VP treatment, the densities of the ciliates and flagellates had a temporal variation, with a strong decrease after the 8th day. However, beginning on the 18th day, there was an increase in the abundance of ciliates and flagellates; the concentration was tenfold greater than in the other treatments on the 23rd day. The rotifers were only observed in the BL treatment in the last few days of the culture (8.80 ± 8.23 organisms × 103 mL−1).
The bacteria abundance observed by epifluorescence is presented in Table 3. The abundance of coccoid bacteria did not differ significantly among the treatments until day 23. On the 28th day, the PR treatment had significantly higher densities than the BL and VP treatments. For bacilli, no significant differences were observed until the 23rd day, which is when the BL treatment became significantly different from the PR treatment; however, the VP treatment showed no significant differences compared to the other two treatments. There were high numbers of amoeboid organisms in the treatments using the blower and vertical pump. For filamentous cyanobacteria, there were no significant differences among the treatments until the 28th day of the experiment.
The growth performance of the shrimp is presented in Table 4. The final weights did not differ statistically (p > 0.05) between the BL and PR treatments; however, the weights in both of those treatments were significantly higher than the final weight observed in the VP treatment. The survival and final biomass were significantly lower (p < 0.05) in the PR treatment.
The centesimal composition of the bioflocs formed in each treatment is presented in Table 5. The dry matter was lower in BL (p < 0.05) and equal for VP and PR treatments (p > 0.05). The crude protein was higher in BL (p < 0.05) and did not differ significantly between VP and PR (p > 0.05). Lipid content was lower in BL and higher in the VP treatments (p < 0.05); both these treatments did not differ significantly from PR treatment (p > 0.05). The ash content was higher in BL (p < 0.05) and statistically equal in VP and PR (p > 0.05). The fiber was lower in BL (p < 0.05) and did not differ between VP and PR (p > 0.05).
Discussion
The management of the water quality is of great importance in aquaculture systems. In BFT cultures with minimal or no water exchange, management of the water quality is necessary because it is influenced by the rates of respiration and excretion of the reared animals at high stocking densities as well as by the respiratory processes of the microbial community in the water (Vinatea et al. 2010; Silva et al. 2013a). However, the microbial community has a positive effect in these culture systems, such as improving the water quality and enhancing the productivity by serving as a complementary food source (Silva et al. 2013a; Xu et al. 2012; Wasielesky et al. 2006).
In this study, the temperature was maintained between 28 and 30 °C in all treatments, which was within the optimal ranges for the growth of shrimp (Ponce-Palafox et al. 1997). Usually, the recommended dissolved oxygen concentrations in shrimp farms should be higher than 5 mg DO L−1. Prolonged periods of exposure to concentrations below 1.5 mg DO L−1 can be lethal for shrimp; however, shrimp can survive when exposed to these low oxygen concentrations for short periods (Van Wyk and Scarpa 1999). In this study, the mean oxygen concentrations recorded in all treatments remained below those recommended, which might have affected the growth of the shrimp. In the PR treatment, low DO concentrations (0.82 mg DO L−1) coupled with a high temperature (34.4 °C) in one of the experimental tanks likely contributed to the low shrimp survival observed. The pH and alkalinity were closely related parameters, and in BFT systems, there is a decrease in both throughout the culture, mainly because of the consumption of inorganic carbon by the heterotrophic and nitrifying bacteria and the growth of shrimp (Ebeling et al. 2006; Furtado et al. 2015a). The lower alkalinity levels in BL treatment indicate there was an increase in heterotrophic and nitrifying bacterial growth and thereby a greater alkalinity consumption in this treatment. Furthermore, the values of pH and alkalinity were within the recommended range to L. vannamei (Furtado et al. 2011, 2015a).
According to Lin and Chen (2001), the safe level of ammonia for L. vannamei juveniles in the salinity range used in this study is 3.55–3.95 mg L−1. In this trial, the TAN concentrations for the BL and VP treatments remained within this range, whereas the mean concentrations reported for the PR treatment exceeded these values, which indicated that the growth and survival of the shrimp were affected by the high concentrations of TAN. Nitrite is an intermediate product of nitrification, and its accumulation in BFT systems could be toxic to animals because of the minimal water exchange (Vinatea et al. 2010; Silva et al. 2013a). The mean nitrite concentrations did not exceed the recommended values by Lin and Chen (2003). The higher nitrite concentrations in BL and VP showed a faster nitrification process in these treatments, which may be caused by increased bacterial growth. In culture systems with no water renewal, nitrate is the nitrogen form that tends to accumulate in greater amounts. In the BL treatment, the nitrate concentrations were higher than in the other treatments, indicating again that the nitrification process was taking place more quickly in this treatment. In this study, although the average concentrations of nitrite have been higher in the BL treatment, it is important to highlight that due to the duration of the rearing (33 days), it cannot follow a complete nitrification process that normally occurs in the BFT systems, leading only to a final high concentration of nitrate. Furtado et al. (2015b) indicate acceptable concentrations of nitrate up to 177 mg L−1 in BFT culture. The values observed in this study did not reach this concentration, which do not affect the shrimp growth. Relating the nitrification process with the alkalinity consumption, it can be observed that in BL treatment, where the alkalinity levels were lower, the nitrification occurred more efficiently, confirming the consumption of inorganic carbon by the nitrifying bacteria that form the bioflocs and metabolize these compounds. According to Ebeling et al. (2006), to each gram of TAN converted in microbial biomass, there was a consumption of 4.71 g of dissolved oxygen, 3.57 g of alkalinity, 15.17 g of carbohydrates and a production of 8.07 g of microbial biomass and 9.65 g of CO2.
The total suspended solid levels (TSS) recommended for BFT systems are in the range of 200–500 mg L−1 (Samocha et al. 2007; Gaona et al. 2012). The total level of suspended solids recorded in this study was close to the recommended range and showed the same pattern as that found for the settleable solids, where high biofloc formation was observed in the BL treatment. These results indicate that there was a higher aggregation rate when diffused air was used, whereas the propeller and vertical pump aerators ruptured or broke apart the bioflocs.
The lower abundances of ciliate and flagellates found in the PR treatment showed an inhibition of the development of microorganisms that form bioflocs when using this type of aerator. Furthermore, the oscillations in the abundance of ciliates observed in other treatments suggested the possibility of shrimp predation on protozoa. As demonstrated by Thompson et al. (1999), ciliates have a significant role in the diet of shrimp larvae and potentially control pathogenic organisms through grazing. In other treatments, the densities of ciliates and flagellates had an inverse relationship throughout the experiment, which is indicative of a trophic interaction that is typical of the “microbial loop” reported by Azam and Fuhrman (1984). According to Decamp et al. (2001), ciliates and flagellates are sources of highly unsaturated fatty acids (HUFAs) and steroids and have a high intracellular concentration of free amino acids. In this study, these microorganisms likely had a stimulatory growth effect on the shrimp, which was mainly observed in the BL treatment.
Amoebas appeared in the VP treatment in higher numbers than in the BL treatment and were not observed in the PR treatment. These microorganisms are heterotrophic and feed on small organisms, such as bacteria, flagellates, diatoms, ciliates and small metazoans such as rotifers. Amoebas are typically associated with surfaces and can ingest bacteria at high rates in places where ciliates and metazoans are not present (Decamp et al. 2003). Therefore, these microorganisms may have influenced the microbial food chain, which potentially affected the growth performance of the reared shrimp.
Rotifers were detected only in the final stage of the study and only in the BL treatment. These organisms are efficient predators of ciliates (Decamp et al. 2003) and could influence the predation within the microbial chain. Furthermore, the presence of rotifers using the blower may indicate a mature state of the microbial community resulting from the use of this aeration device.
It is clear from the data presented that the ammonia-oxidizing bacteria were affected by the smaller amount and likely the smaller size of particles in the PR treatment. It is known that ammonium- and nitrite-oxidizing bacteria are normally present on aggregates and form colonies (Delatolla et al. 2009; Vlaeminck et al. 2010), and the establishment of these colonies may be influenced by the particle size, which is determined by the water shear rate. Larsen et al. (2008) showed that increased shear rates in activated sludge systems formed particles smaller than the medium size of a colony (13–22.5 µm) of the ammonia-oxidizing bacteria Nitrosomonas oligotropha, which led to a low rate of ammonia oxidation that was similar to the rate observed in our study. The bacterial counting analysis showed a high quantity of bacillus and coccoid bacteria in the PR treatment when compared to other treatments. Despite the large number of bacteria, the bacteria present in tanks that used the propeller aerators were not efficient at metabolizing nitrogen compounds, which confirmed that the nitrifying bacteria need a substrate for adherence (small and medium particles) to perform nitrogen cycling in the system. The smaller values of nitrate in the propeller and vertical pump treatments indicate that the nitrite-oxidizing bacteria were affected by the reduction in particle size and quantity. The water turbulence and propeller action may have contributed to the decrease in particle size in the biofloc system, which would have hampered the establishment of nitrite-oxidizing bacteria.
The numbers of bacteria in the BL and VP treatments were lower when compared to the PR treatment, which was likely because of the grazing that ciliates and flagellates exert on the bacterial community in these treatments. In the PR treatment, there was a small development of protozoa, which resulted in an increase in the number of bacteria because of the absence of predators in the microbial food chain.
The presence of cyanobacteria (filamentous bacteria) was influenced by the low dissolved oxygen concentrations that were observed in some periods of the culture (Ray et al. 2010). The presence of these organisms in the culture may have affected shrimp performance because some cyanobacteria produce substances that may be toxic or contribute to “off” flavors of cultured animals (Alonso-Rodriguez and Paez-Osuna 2003; Zimba et al. 2006). The presence of diatoms was lower in the PR and BL treatments during the experimental period. In the VP treatment, there were a higher number of diatoms at the beginning of the study; however, the diatom count decreased throughout the experiment. The addition of molasses to the water contributed to the reduction in water transparency, which most likely reduced the growth of diatoms in the tanks.
The final weight did not differ significantly between the BL and PR treatments; nevertheless, the lower survival rate reported in the PR treatment might have influenced this result. The decrease in stocking density because of mortalities promoted the growth of the shrimp that survived in tanks, and several authors have reported an inverse relationship between stocking densities and the performance of shrimp (Krummenauer et al. 2011; Moss and Moss 2004; Otoshi et al. 2007; Williams et al. 1996).
L. vannamei uses bioflocs as a source of supplementary food, which improves feed utilization and reduces the feed conversion rate (FCR) (Burford et al. 2003; Wasielesky et al. 2006). Lower feed conversion rates were observed in the BL and VP treatments, which are the same treatments where more diversity was recorded in the dominant microbes, and the densities of these microorganisms were lower, potentially indicating the greater use of such organisms as a dietary supplement for shrimp. The shrimp in the BL treatment had better growth results because of the increased formation of microbial aggregates, which was reflected in the improved water quality in this treatment. The growth results were similar to those reported by Krummenauer et al. (2011) and Gaona et al. (2012) that performed studies in the same culture units. Furthermore, the results obtained were similar and/or higher than other trials carried out in greenhouses, evidencing the potential of producing high shrimp biomasses in smaller areas (Samocha et al. 2004; Ray et al. 2010).
Proximal composition (%DW) of the bioflocs of tanks was higher than observed by Xu et al. (2012) that noticed 25.61 %CP using brown sugar as carbon source, similar to the results observed by Maicá et al. (2012) that founded 28.76 %CP in a salinity of 25 and slightly lower than the results obtained by Wasielesky et al. (2006) that observed 31.07 % CP. The lipid content was lower than the observed by Maicá et al. (2012) and higher than the results obtained by Wasielesky et al. (2006). The lipid seems to be the component that shows more variations in their values according to the studies published in this regard (Crab et al. 2010; Silva et al. 2013b). Nutritional studies with L. vannamei indicated that the lipid requirement ranges from 10–12 % (Zhang et al. 2013), and these levels significantly tune the growth and enhance the immune abilities. This implies that a commercial feed is still necessary to provide adequate lipid levels to shrimp. The higher ash content in BL treatment could be related to the higher amount of shrimp fecal matter in suspension in tanks with this type of aerator. Similar values were recorder by other authors using the same diffused aeration system (Wasielesky et al. 2006; Maicá et al. 2012; Silva et al.; 2013b).
In conclusion, the results indicate that diffused air systems (blower) are more efficient in particle aggregation and in biofloc formation in culture, contributing to the shrimp growth. In contrast, the propeller aeration system does not contribute to biofloc aggregation and destroys the bioflocs, and the development of a “healthy” microbial community is compromised. The vertical pump, despite not showing high values of total suspended solids (bioflocs), was able to provide a microbial community that contributed to the growth and survival of the shrimp.
References
Alonso-Rodriguez R, Paez-Osuna F (2003) Nutrients, phytoplankton and harmful algal blooms in shrimp ponds: a review with special reference to the situation in the Gulf of California. Aquaculture 219:317–336
American Public Health Association (APHA) (1989) Standard methods for the examination of water and waste water, sixteenth edn. American Public Health Association, AWWA, WPFC, New York
Association of Official Analytical Chemists (AOAC) (2000) Official methods of analysis, 17th edn. AOAC, Gaithers-burg
Avnimelech Y (2009) Biofloc technology—a practical guide book, 1st edn. The World Aquaculture Society, Baton Rouge
Azam F, Fuhrman JA (1984) Measurement of bacterioplankton growth in the sea and its regulation by environmental conditions. In: Hobbie JE, Williams PJ leB (eds) Heterotrophic activity in the sea. Springer, New York, pp 179–196
Biddanda BA (1985) Microbial synthesis of macroparticulate matter. Mar Ecol Prog Ser 20:241–251
Biddanda BA, Pomeroy LR (1988) Microbial aggregation and degradation of phytoplankton-derived detritus in seawater. I. Microbial succession. Mar Ecol Prog Ser 42:79–88
Burford MA, Thompson PJ, Bauman RH, Pearson DC (2003) Nutrient and microbial dynamics in high-intensive, zero-exchange shrimp ponds in Belize. Aquaculture 219:393–411
Crab R, Avnimelech Y, Defoirdt T, Bossier P, Verstraete W (2007) Nitrogen removal techniques in aquaculture for a sustainable production. Aquaculture 270:1–14
Crab R, Chielens B, Wille M, Bossier P, Verstraete W (2010) The effects of different carbon sources on the nutritional value of bioflocs, a feed for Macrobrachium rosenberguii postlarvae. Aquac Res 41:559–567
Crab R, Defoirdt T, Bossier P, Verstraete W (2012) Biofloc technology in aquaculture: beneficial effects and future challenges. Aquaculture 356–357:351–356
De Schryver P, Crab R, Defroidt T, Boon N (2008) The basics of bio-flocs technology: the added value for aquaculture. Aquaculture 277:125–137
Decamp O, Moss S, Nagano N (2001) Live protozoa: suitable live food for larval fish and shrimp? Glob Aquac Advoc 4(5):28–29
Decamp OE, Cody J, Conquest L, Delanoy G, Tacon AGJ (2003) Effect of salinity on natural community and production of Litopenaeus vannamei (Boone) within experimental zero-water Exchange culture systems. Aquac Res 34:345–355
Delatolla R, Tufenkj N, Comeau Y, Lamarre D, Gadbois A, Berk D (2009) In situ characterization of nitrifying biofilm: minimizing biomass loss and preserving perspective. Water Res 43:1775–1787
Dilling L, Alldredge AL (2000) Fragmentation of marine snow by swimming macrozooplankton: a new process impacting carbon cycling in the sea. Deep Sea Res (1 Oceanogr Res Pap) 47:1227–1245
Ebeling JM, Timmons MB, Bisogni JJ (2006) Engineering analysis of the stoichiometry of photoautotrophic, autotrophic, and heterotrophic control of ammonia-nitrogen in aquaculture production systems. Aquaculture 257:346–358
Emerenciano M, Ballester ELC, Cavalli RO, Wasielesky W Jr (2012) Biofloc technology application as a food source in a limited water exchange nursery system for pink shrimp Farfantepenaeus brasiliensis (Latreille, 1817). Aquac Res 43(3):447–457
Furtado PS, Poersch LH, Wasielesky W Jr (2011) Effect of calcium hydroxide, carbonate and sodium bicarbonate on water quality and zootechnical performance of shrimp Litopenaeus vannamei reared in bio-flocs technology (BFT) systems. Aquaculture 321:130–135
Furtado PS, Poersch LH, Wasielesky W Jr (2015a) The effect of different alkalinity levels on Litopenaeus vannamei reared with biofloc technology (BFT). Aquac Int 23:345–358
Furtado PS, Campos BR, Serra FP, Klosterhoff M, Romano LA, Wasielesky W Jr (2015b) Effects of nitrate toxicity in the Pacific white shrimp, Litopenaeus vannamei, reared with biofloc technology (BFT). Aquac Int 23:315–327
Gaona CAP, Fóes G, Krummenauer D, Poersch L, Wasielesky W Jr (2012) The effect of solids removal on water quality, growth and survival of Litopenaeus vannamei in a biofloc technology culture system. Int J Recirc Aquac 12:54–73
Hargreaves JA (2006) Photosynthetic suspended-growth systems in aquaculture. Aquac Eng 34:344–363
Jory DE, Cabrera TR, Dugger DM, Fegan D, Lee PG, Lawrence AL, Jackson CJ, McIntosh RP, Castañeda J (2001) A global review of shrimp feed management: status and perspectives. In: Browdy CL, Jory DE (eds) The new wave: proceedings of the special session on sustainable shrimp culture. The World Aquaculture Society, Baton Rouge, pp 104–152
Krummenauer D, Peixoto S, Cavalli RO, Poersch LH, Wasielesky W Jr (2011) Superintensive culture of white shrimp, Litopenaeus vannamei, in a biofloc technology system in southern Brazil at different stocking densities. J World Aquac Soc 42:726–733
Krummenauer D, Samocha T, Poersch LH, Lara G, Wasielesky W Jr (2014) The reuse of water on the culture of Pacific white shrimp, Litopenaeus vannamei, in BFT system. J World Aquac Soc 45(1):3–14
Larsen P, Nielsen J, Svendsen T, Hielsen P (2008) Adhesion characteristics of nitrifying bacteria in activated sludge. Water Res 42:2814–2826
Lin YC, Chen JC (2001) Acute toxicity of ammonia on Litopenaeus vannamei Boone juveniles at different salinity levels. J Exp Mar Biol Ecol 259:109–119
Lin YC, Chen JC (2003) Acute toxicity of nitrite on L. vannamei (Boone) juveniles at different salinity levels. Aquaculture 224:193–201
Maicá PF, Borba MR, Wasielesky W Jr (2012) Effect of low salinity on microbial floc composition and performance of Litopenaeus vannamei (Boone) juveniles reared in a zero-water-exchange super-intensive system. Aquac Res 43:361–370
Moss KK, Moss SM (2004) Effects of artificial substrate and stocking density on the nursery production of pacific white shrimp Litopenaeus vannamei. J World Aquac Soc 35:536–542
Otoshi CA, Naguwa SS, Falesch FC, Moss SM (2007) Shrimp behavior may affect culture performance at super intensive stocking densities. Glob Aquac Advoc 10(2):67–69
Otoshi CA, Moss DR, Moss SM (2011) Growth-enhancing effect of pond water on four size classes of Pacific white shrimp, Litopenaeus vannamei. J World Aquac Soc 42(3):417–422
Ponce-Palafox J, Martinez-Palacios CA, Ross LG (1997) The effects of salinity and temperature on the growth and survival rates of juvenile white shrimp Penaeus vannamei, Boone, 1931. Aquaculture 157:107–115
Porter KG, Feig YS (1980) The use of DAPI for identifying and counting aquatic microflora. Limnol Oceanogr 25:943–948
Ray AJ, Seaborn G, Leffler JW, Wilde SB, Lawson A, Browdy CL (2010) Characterization of microbial communities in minimal-exchange, intensive aquaculture systems and the effects of suspended solids management. Aquaculture 310:130–138
Samocha TM, Lawrence AL, Collins CA, Castille FL, Bray WA, Davies CJ, Lee PG, Wood GF (2004) Production of the Pacific withe shrimp, Litopenaeus vannamei, in high density greenhouse-enclosed raceways using low salinity growndwater. J Appl Aquac 15(3/4):1–19
Samocha TM, Patnaik S, Speed M, Ali AM, Burger JM, Almeida RV, Ayub Z, Harisanto M, Horowitz A, Brock DL (2007) Use of molasses as carbon source in limited discharge nursery and grow-out systems for Litopenaeus vannamei. Aquac Eng 36:184–191
Silva KR, Wasielesky W Jr, Abreu PC (2013a) Nitrogen and phosphorus dynamics in the biofloc production of the Pacific White shrimp, Litopenaeus vannamei. J World Aquac Soc 44:30–41
Silva AF, Lara G, Ballester ELC, Krummenauer D, Abreu C, Wasielesky W Jr (2013b) Efeito das altas densidades de estocagem no crescimento e sobrevivência de Litopenaeus vannamei na fase final de engorda, cultivados em sistema de bioflocos (BFT). Ciênc Anim Bras 14(3):279–287
Strickland JDH, Parsons TR (1972) A practical handbook of seawater analysis, 2nd edn. Fisheries Research Board of Canada, Ottawa
Thompson FL, Abreu PC, Cavalli RO (1999) The use of microorganisms for water quality and nourishment in intensive shrimp culture. Aquaculture 174:139–153
Thompson FL, Abreu PC, Wasielesky W Jr (2002) Importance of biofilm for water quality and nourishment in intensive shrimp culture. Aquaculture 203:263–278
UNESCO (United Nations Educational, Scientific and Cultural Organization) (1983) Chemical methods for use in marine environmental monitoring. Intergovernmental Oceanographic Commission, Manual and Guides, Paris
Utermöhl H (1958) Zur vervolkommnurg der quantitativen phytoplankton mettthodik. Int Ver Theor Angew Limnol 9:1–38
Van Wyk P, Scarpa J (1999) Water quality requirements and management. In: Van Wyk P, Davis-Hodgkins M, Laramore R, Main K, Mountain J, Scarpa J (eds) Farming marine shrimp in recirculating freshwater systems. Florida Department of Agriculture and Consumer Services, Tallahassee, p 128
Vinatea L, Gálvez AO, Browdy CL, Stokes A, Venero J, Haveman J, Lewis BL, Lawson A, Schuler A, Leffler JW (2010) Photosynthesis, water respiration and growth performance of Litopenaeus vannamei in a super-intensive raceway culture with zero water exchange: interaction of water quality variables. Aquac Eng 42:17–24
Vlaeminck SE, Terada A, Smets BF, De Clippeleir H, Schaubroeck T, Bolca S, Demeestere L, Mast J, Boon N, Carballa M, Verstraete W (2010) Aggregate size and architecture determine microbial activity balance for one-stage partial nitritation and anammox. Appl Environ Microbiol 76(3):900–909
Wasielesky W Jr, Atwood HI, Stokes A, Browdy CL (2006) Effect of natural production in brown water super-intensive culture system for white shrimp Litopenaeus vannamei. Aquaculture 258:396–403
Wheaton FW (1977) Aquacultural engineering. Krieger Publishing, Malabar, pp 643–679
Williams AS, Davis DA, Arnold CR (1996) Density-dependent growth and survival of Penaeus setiferus and Penaeus vannamei in a semi-closed recirculating system. J World Aquac Soc 27:107–112
Xu W-J, Pan L-Q, Zhao D-H, Huang J (2012) Preliminary investigation into the contribution of bioflocs on protein nutrition of Litopenaeus vannamei fed with different dietary protein levels in zero-water exchange culture tanks. Aquaculture 350–353:147–153
Zar JH (1996) Biostatistical analysis, 3rd edn. Prentice Hall, New Jersey
Zhang S, Li J, Wu X, Zhong W, Xian J, Liao S, Miao Y, Wang A (2013) Effects of different dietary lipid level on the growth, survival and immune-relating genes expression in Pacific white shrimp, Litopenaeus vannamei. Fish Shellfish Immun 34:1131–1138
Zimba PV, Camus A, Allen EH, Burkholder JM (2006) Co-occurrence of white shrimp, Litopenaeus vannamei, mortalities and microcystin toxin in a southeastern USA shrimp facility. Aquaculture 261:1048–1055
Acknowledgments
The authors are grateful for the financial support provided by the National Council for Scientific and Technological Development (CNPq), the Ministry of Fishery and Aquaculture (MPA) and Coordination for the Improvement of Higher Level Personnel (CAPES). Wasielesky, W.J., Abreu, P.C. and Poersch, L.H. are research fellows of CNPq.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lara, G., Krummenauer, D., Abreu, P.C. et al. The use of different aerators on Litopenaeus vannamei biofloc culture system: effects on water quality, shrimp growth and biofloc composition. Aquacult Int 25, 147–162 (2017). https://doi.org/10.1007/s10499-016-0019-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-016-0019-8