Abstract
Strategies, such as the biological denitrification process, must be adopted to treat rich-nitrate water from biofloc systems, making it suitable for culture or proper disposal. This study evaluated the effect of water reuse from a BFT system after being subjected to a biological anaerobic denitrification process on shrimp performance and water quality in the rearing of Litopenaeus vannamei. A 63-day experiment was carried out at the Marine Station of Aquaculture of the Federal University of Rio Grande. L. vannamei juveniles (1.30 ± 0.48 g) were stocked in 150-L tanks at a density of 500 shrimp/m3. Four treatments (with three replicas each) were tested: natural seawater and denitrified seawater, both with and without biofloc inoculum. Temperature, salinity, dissolved oxygen, pH, ammonia, nitrite, nitrate, alkalinity, and total suspended solids (TSS) in the water were monitored. Ammonia and nitrite levels were higher without biofloc inoculum, while nitrate levels increased with inoculum use. Alkalinity and TSS were significantly higher in denitrified water with inoculum, driven mainly by the initial higher concentrations. A 100% mortality was found in the treatment using denitrified water without inoculum, probably due to the presence of byproducts after the denitrification process, but no differences in survival (>89%) were found in the other treatments. The biofloc inoculum helped with higher productivity (3.03 ± 0.08 kg/m3) and lower FCR (1.48 ± 0.05) in the treatment using seawater compared to the treatment in which no inoculum was used (2.62 ± 0.18 kg/m3 and 1.79 ± 0.16), in addition to serving as a biological treatment in denitrified seawater, readjusting the water for shrimp farming. As the growth performance of shrimp raised in denitrified water showed no differences compared to natural seawater, utilizing denitrified seawater from a biofloc system seems feasible for shrimp cultivation. However, alternatives for water treatment after the denitrification process need to be investigated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Due to the global population growth, FAO (2022) estimates that a 50% increase in food production would be required by 2030 to meet the world’s food demands. Aquaculture emerges as a relevant alternative to meet this demand for animal protein since it has shown constant growth over the years. Nevertheless, to help achieve these goals, aquaculture needs to focus on intensifying aquaculture activity, i.e., increasing its production while maximizing the use of crucial resources such as water and land (Piedrahita 2003).
A super-intensive production system has been gaining quite a reputation in aquaculture production, especially in shrimp farming: the biofloc system. The biofloc system is a closed aquaculture system with minimal or no water exchange that relies on a microbial community to control nitrogen compounds in the water and serve as supplemental food (Wasielesky et al. 2006; Ballester et al. 2010; Lara et al. 2017). This effective utilization of microbial communities for water maintenance and supplementary nutrition paves the way for increasing stocking densities in cultivation, aiding in reducing the spatial demands for production but also significantly enhancing productivity in shrimp farming endeavors.
The control of nitrogen compounds in biofloc system can be carried out by favoring different types of bacteria, heterotrophic and chemoautotrophic bacteria, and the predominance of one of these groups defines the primary path for removing ammonia from the system (Brandão et al. 2021). In the first group, the ammonia produced in the system is converted to microbial biomass by adding an external organic carbon source for bacterial growth. In the second group, chemoautotrophic bacteria oxidize ammonia into nitrate, through the nitrification process (Ebeling et al. 2006; Hostins et al. 2019).
Thus, in nitrification-dominated bioflocs, nitrate production is constant and accumulates in the system (Luo et al. 2020). Nitrate is a less toxic form of nitrogen to farmed animals compared to ammonia and nitrite (Ahmad et al. 2021), with the safety levels varying from 5.6 mg L−1 on salinity 1, 60.05 mg L−1 on salinity 5, and up to 278.9 mg L−1 on salinity 25 (Valencia-Castañeda et al. 2018; Alves Neto et al. 2019; Prates et al. 2023a). Depending on the salinity, the same water could be reused for several culture cycles. For example, Krummenauer et al. (2014) tested different levels of biofloc water reuse in the rearing of Litopenaeus vannamei and found that reusing 100% of the water resulted in no difference in water quality and shrimp growth compared to other levels of reuse. Besides that, the authors observed that nitrate concentration increased with a higher percentage of water reused.
However, after several cycles, the levels of nitrate accumulated in the system can become harmful to farmed animals, causing reduced growth and survival (Kuhn et al. 2010; Furtado et al. 2015a), resulting in losses in production performance, especially in low salinity aquaculture systems, due to the higher nitrate toxicity at lower salinities. As water becomes unsuitable for growing animals, it may become necessary to dispose of it, which can lead to several impacts on the environment if not properly discarded, including organic pollution, eutrophication, excessive nutrients, and chemical pollution (Ahmad et al. 2021).
To overcome this production bottleneck and to meet the requirements towards more sustainability, strategies must be adopted to treat the water and make it suitable for culture or proper disposal. One of those strategies is the biological denitrification process, which utilizes a microbial community to reduce nitrate to nitrogen gas. Denitrifying reactors are used in aquaculture recirculation systems (Aich et al. 2021; Letelier-Gordo et al. 2020; Preena et al. 2021), but their use is more limited in biofloc systems due to a larger amount of suspended solids in the water. A simple and low-cost protocol for the denitrification process is treating the water from a biofloc system right after harvest, in the culture tanks or sedimentation basins, by adding an organic carbon source and promoting an anoxic environment, which results in an effluent with a low concentration of nitrogen compounds. However, it is still necessary to evaluate if the water subjected to this process is suitable for shrimp farming again.
Thus, this study was designed to evaluate the effect of water reuse from a biofloc system after being subjected to a biological anaerobic denitrification process on shrimp performance and water quality in the rearing of L. vannamei.
Material and methods
Experimental design
A 63-day trial was conducted at the Marine Station of Aquaculture of the Federal University of Rio Grande, Rio Grande do Sul, Brazil (32° 12′ 14.1″ S 52° 10′ 39.8″ W), from May 8 to July 9, 2020. Nauplii of Pacific white shrimp L. vannamei was obtained from Aquatec LTDA (Canguaretama, RN, Brazil) and went through larvae rearing and nursery phases at the Marine Shrimp Culture Laboratory before being used in the experiment.
The experiment compared denitrified water and natural seawater (never used to farm shrimp) under different biofloc formation strategies: with or with no inoculum of mature rich-biofloc water. Thus, the treatments consisted of denitrified water with inoculum (DWI); denitrified water with no inoculum (DWN); seawater with inoculum (SWI); and seawater with no inoculum (SWN), with three replicates each.
Denitrification process
To obtain denitrified water for the experiment, three 1000-L rounded flat-bottom polyethylene tanks were filled with water containing bioflocs pumped from a Litopenaeus vannamei mature chemoautotrophic BFT system. To simulate a buildup of nitrate in the water after numerous BFT farming cycles, nitrate (N-NO3) levels were increased using sodium nitrate (NaNO3, Synth™) to approximately 200 mg/L (from an organically accumulated concentration of around 150 mg/L). The tanks were placed indoors with diffused natural sunlight (1071 ± 71.93 lux), and no aeration or mixing systems were provided. Submerged water heaters (Roxin™, 150 W) were used to regulate the water’s temperature, which was kept at about 29 °C. The water quality parameters before and after the denitrification process are shown in Table 1.
A source of organic carbon (regular table sugar cane, 42.92% of organic carbon) was added once to the tanks to start the denitrification process. The amount of organic carbon source required was determined from a modified version of the formula described by Brandão et al. (2021), shifting the variable “ammonia concentration” by the sum of the concentrations of inorganic nitrogen compounds measured (ammonia + nitrite + nitrate). A carbon/nitrogen ratio of 2/1 was used in the process.
Temperature, dissolved oxygen, and pH were monitored hourly with the aid of a multiparameter probe (Hanna™ HI 98194) until pH levels stabilized, and then, measurements were performed every 12 h. Ammonia, nitrite, nitrate, and alkalinity concentrations were assessed every 12 h, following the methodologies described in the “Water quality” section. During the period before pH stabilization, a solution of sodium hydroxide (NaOH, 1M) was added to the water whenever the measured pH levels were lower than 8. The denitrification process was considered completed when the measured nitrite and nitrate concentrations were close to 0 mg/L. After low levels of nitrite and nitrate were verified, the water was allowed to settle for 12 h, and then, the supernatant was transferred to the experimental culture units. Because the denitrification process returned ammonia to the water, the tanks were previously treated to reduce ammonia concentrations by performing organic fertilization before stocking the animals, considering a ratio of 6 g of carbon for each gram of ammonia (Ebeling et al. 2006). The tanks were kept under constant strong aeration until ammonia levels reached levels close to 0 mg/L.
Culture conditions
Rectangular polyethylene tanks with 180 L of volume (150 L of working volume, 0.79 × 0.79 × 0.40 m) were placed indoors in a greenhouse with diffused sunlight (1071 ± 71.93 lux) and natural photoperiod. Every tank was equipped with continuous aeration provided by a blower (Ibram™, 2 CV) and distributed by a microperforated hoses system (Aerotube™, 20 cm for each square meter). Submerged water heaters (Roxin™, 150 W) were used to regulate the water’s temperature, which was kept at about 29 °C.
The tanks were filled with natural seawater (salinity = 32) pumped from Cassino Beach or denitrified water pumped from the tanks used for denitrification, depending on the treatments. The water was treated with a chlorine solution (12% sodium hypochlorite—NaClO) and dechlorinated by aeration. The tanks were stocked with 75 juveniles of L. vannamei (1.30 g ± 0.48) at a stocking density of 500 shrimp/m3.
Biofloc formation
In the treatments using biofloc inoculum, 15 L (10% of the working volume) of water from a mature biofloc system (nitrification already established with undetectable levels of ammonia and nitrite in the water) was added to the tanks (Krummenauer et al. 2014). The water employed for the biofloc inoculum was the same used in the denitrification process, with corresponding water quality parameters detailed in Table 1.
In the treatments where bioflocs were stimulated from zero, organic fertilization was performed every time total ammonia nitrogen (TAN) levels exceeded 1.0 mg/L by adding liquid sugarcane molasses (37% of organic carbon), calculated as described in Brandão et al. (2021).
Water quality
Temperature, dissolved oxygen, and pH were monitored twice daily (8:00 and 17:00) using a digital multiparameter (Hanna HI 98194). Water samples were collected daily to determine total ammonia nitrogen (TAN = (NH4+ + NH3)-N) and nitrite (NO2−-N) concentrations according to UNESCO (1983) and Strickland and Parsons (1972), respectively. Water exchange (20% of total volume) was performed every time total ammonia levels exceeded 7.0 mg/L (approximately twice the safe level by Lin and Chen (2001)) or nitrite levels exceeded 26 mg/L (approximately the safe level by Lin and Chen (2003)). Nitrate (NO3−-N) concentrations were quantified weekly (Aminot and Chaussepied 1983). Salinity was measured weekly using a portable optical refractometer. When necessary, fresh water from the local supply company was added to the tanks to adjust the salinity, compensating for water losses by evaporation. Alkalinity was measured every 3 days following the methodology recommended by APHA-AWWA-WEF (2005). Every time pH reached values below 7.3 and/or alkalinity reached values below 150 mg/L, adjustments were made to correct it (Furtado et al. 2014) by adding calcium hydroxide or sodium bicarbonate to the tanks. Total suspended solids were measured twice a week (AOAC 1990). Samples were filtered using a vacuum pump through GF50-A glass fiber filters (Prismatec™). Suspended solid levels were maintained at 500 mg/L as recommended by Gaona et al. (2011) with the aid of sedimentation tanks. Commercial probiotics (INVE™ Sanolife PRO-W) were applied once a week (0.5 g/L) to help with water quality and microbial settlement.
Shrimp feeding and monitoring
Shrimp were fed twice a day (08:30 and 17:30) with a commercial diet containing 38% crude protein (Poty Active 38, 1.6 mm, Guabi™). The feed was spread in the tank, and 10% of the feed was placed in feeding trays to check consumption. The feed rate was adjusted based on the expected feed conversion rate, weekly growth, and estimated survival (Garza de Yta et al. 2004). To assess growth and health conditions, 30 animals were randomly collected weekly from each unit, individually weighed, and then returned to tanks. At the end of the study, all animals in each tank were sampled and weighed for final weight. The survival rate was obtained by dividing the number of animals at the end of the experiment in each tank by the number of stocked animals multiplied by 100. The feed conversion rate was calculated by dividing the total feed provided by the biomass gain. Finally, productivity was calculated by dividing the total biomass per tank volume.
Statistical analysis
Data are presented as mean ± standard deviation. Data were submitted to normality (Shapiro-Wilk test) and homoscedasticity (Levene’s test) tests. After checking these assumptions, a two-way repeated measures analysis of variance (ANOVA) was utilized to assess water quality parameters, using treatments as the primary factor and time (days or weeks) as the recurring measure. Additionally, a one-way ANOVA was applied to assess water quality parameters measured only one time at the experiment’s conclusion, as well as the zootechnical performance indices. When significant differences were detected (p < 0.05), the post hoc Tukey test was applied at a 95% confidence level (α = 0.05). The non-parametric Kruskal-Wallis test was employed to assess the parameters of water exchange and organic carbon usage. Percent values were transformed (arcsine of the square root) before being analyzed (Zar 2010). All statistical analysis was performed using R Statistical Software (version 4.1.1) within R Studio Software (version 2021.09.0).
Results
All the animals exposed to the treatment of denitrified water without biofloc inoculum (DWN) died suddenly after 1 week of culture, reaching 100% mortality in all replicates. For this reason, the values of the water quality parameters referring to a week of cultivation of the DWN treatment will be presented but were not considered in the statistical analyses.
Water quality
The mean values (± standard deviation) of the water’s chemical and physical parameters during the study are displayed in Table 2. No significant differences (p > 0.05) were found between the treatments in the mean concentrations of dissolved oxygen, temperature, and salinity among treatments. However, significant differences (p < 0.05) were found in pH, ammonia, nitrite, nitrate, nitrate accumulation, alkalinity, total suspended solids, water exchange, organic carbon, and alkalinizing agents among the treatments. Moreover, significant differences were found in time, indicating variation in these parameters over time.
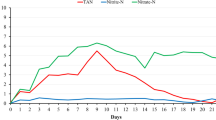
Significant differences in total ammonia concentrations were found between the treatments, in the time, and in the interaction between factors (time × treatment). A higher mean concentration of TAN was found in the treatment without biofloc inoculum compared to the others (Table 1). In the treatment without inoculum, ammonia started to accumulate in the system from the first day onwards, peaking at 9.5 mg/L on day 13 and reaching values lower than 1.0 mg/L from day 22, and differed from the other treatments from day 2 to day 17 (Fig. 1a). No differences were found in ammonia concentration over time in the treatments with inoculum, and they showed a similar pattern in ammonia levels throughout the experiment, keeping ammonia concentrations below 1.0 mg/L throughout the period.
Significant differences were found in the mean concentrations of nitrite among treatments, with higher values found in the treatment without using biofloc inoculum compared to the others. In the treatment without inoculum, nitrite concentration started to increase from day 10, reaching a peak of 46.00 mg/L on day 30 and decreasing from day 47. It was possible to observe a second increase in the last days, of a smaller peak, with a consequent decrease. In this treatment, nitrite levels were different from the other treatments from day 20 until day 47. For the rest of the time, no differences were detected among the treatments. In the seawater treatment with inoculum, nitrite concentrations increased in the first days, reaching a peak of 14.0 mg/L on day 14. Values below 1.0 mg/L were registered from day 19 until the end of the experiment. In the treatment using denitrified water, nitrite accumulation in the tanks was not observed, with the levels remaining below 1.0 mg/L for most of the time and the highest concentration recorded being 3.0 mg/L 56 days after the animals were stocked (Fig. 1b).
Significant differences in mean nitrate concentrations were found between the treatments, with the highest concentration found in the treatments using inoculum, while the lowest was found using seawater without inoculum. The same result was also found in nitrate final concentrations (nitrate buildup). Significant differences were found in time but not in the interaction of time and treatments, indicating that nitrate levels changed over time but in the same way in all treatments (Fig. 1c). Final nitrate concentrations (nitrate buildup) were lower in the treatment without inoculum compared to the others, and no differences were found between the treatments with biofloc inoculum.
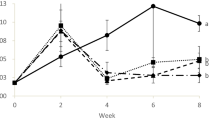
The treatment using denitrified water presented higher mean pH levels compared to the others. Significant differences were also found in time, indicating that pH fluctuated in the period. In the same way, significant differences were found in mean alkalinity concentration between treatments, with higher values found in the treatment with denitrified water compared to the others. Time and interaction were also significant, indicating that changes occurred and alkalinity levels were different over time between the treatments. The alkalinity concentrations in the treatment with denitrified water were different from the other treatments from day 1 until day 31; after that, alkalinity did not differ among the treatments. Figure 2 shows the alkalinity levels during the experiment, revealing a drop in the alkalinity concentration in the treatments, more pronounced in the treatment with denitrified water, followed by a stabilization close to 150 mg/L.
Corrections in the alkalinity concentration were performed in all treatments. However, the amount of alkalizing agent was 465.28% and 663.89% higher in the treatments using seawater without and with inoculum, respectively, compared to the treatment with denitrified water, which represented from 15 to 20% of the amount used in the other treatments. In addition, alkalinity correction in the treatment with denitrified water was performed 2 to 2.4 times less than in treatments using seawater.
Water exchanges were not performed in the treatments using inoculum, while without inoculum, water exchanges represented 173.33 ± 83.27% of the water volume in the tank. In addition, the organic carbon supplementation was performed only in the seawater treatment without inoculum, totaling 79.64 ± 24.61 g of table sugar per tank.
Growth performance
No significant differences were found for final weight, survival rates, and weekly growth rates among treatments. On the other hand, significant differences (p < 0.05) were found for final biomass, biomass gain, yield, and FCR between treatments using seawater (SWN and SWI), but the treatment using denitrified water with biofloc inoculum (DWI) did not differ from either (Table 3).
Discussion
This study assessed the effects of reusing water subjected to a biological anaerobic denitrification process on water quality and shrimp performance reared in a biofloc system. Physical parameters of the water, such as dissolved oxygen and temperature, were controlled with the aid of water heaters and aeration systems, seeming to have been efficient in maintaining levels within the recommended range for the species L. vannamei (Ponce-Palafox et al. 1997; Jiang et al. 2005) and probably did not affect the performance of the animals nor the establishment of the microbial community in the water.
In general, nitrogen compounds’ behavior was affected by the biofloc formation strategy adopted. In the biofloc inoculum treatments, ammonia and nitrate levels remained low throughout the experimental period and below the safety levels recommended for the species (Lin and Chen 2001, 2003). The use of biofloc inoculum consists of adding microbial biomass from a mature biofloc system to serve as a seed of microorganisms and accelerate microbial development and biofloc formation. The efficiency of using biofloc inoculum to keep ammonia and nitrite levels low can be contemplated in several studies (Krummenauer et al. 2014; Martins et al. 2020; Brol et al. 2021; Prates et al. 2023b).
On the other hand, ammonia and nitrite levels were higher in the treatment where biofloc inoculum was not used, which is also a well-known characteristic when floc formation is started from scratch. Chemoautotrophic bacteria, responsible for oxidizing ammonia to nitrite and then nitrate and controlling nitrogen compounds in the long term, grow slower and take longer to establish in the system (Ebeling et al. 2006). For this reason, it is common to observe an accumulation of both ammonia and nitrite in the system during the establishment of these bacteria, as demonstrated by Reis et al. (2023). In this way, different strategies are used to deal with nitrogen accumulation during the formation of bioflocs. In the case of ammonia, supplementation with organic carbon is used to stimulate the capture of ammonia produced by heterotrophic bacteria, while nitrite levels must be controlled with water exchange. For this reason, the treatment without inoculum showed higher values of organic carbon used and volume of water exchanged. Despite this, the use of organic fertilization to control ammonia levels in the first days and water exchange in case nitrite levels exceed the determined limits are effective strategies for the first establishment of biofloc systems, as shown in this study.
Alkalinity and pH levels decreased during the experimental period due to nitrifying organisms’ consumption of inorganic carbon. Alkalinity levels are strictly linked to neutralization, buffer capacity, and inorganic carbon requirement for cellular synthesis and growth (Biesterfeld et al. 2001; Shanahan and Semmens 2015). According to Ebeling, 7.05 g of alkalinity is needed to oxidize 1.0 g of ammoniacal nitrogen into nitrate, while heterotrophic bacteria consume alkalinity (3.57 g) to metabolize the same amount of ammonia. Therefore, as ammonia production is constant in the system, a gradual reduction in water alkalinity levels in biofloc systems is expected to occur due to consumption in the metabolization of this compound. Furthermore, alkalinity and pH also tend to decrease during biofloc culture due to water acidification caused by the release of carbon dioxide from the respiration of microbial and animal biomass in the water, which increases over time (Furtado et al. 2015b). Either way, alkalinity and pH levels remained near the levels recommended by Furtado et al. (2014), mainly because corrections were made with the addition of sodium bicarbonate and seemed not to be affecting the nitrification process or growth performance in this study.
Despite this, fewer alkalinity and/or pH corrections were performed in the treatment using denitrified water. This can be explained by the initial conditions of the culture water used since the denitrified water treatment had a higher alkalinity level at the beginning of the culture compared to seawater treatments. During the denitrification process, especially during the reduction step of nitrite to nitric oxide, hydroxyl ions (OH−) are produced. In addition, part of the OH− ions can react with the released CO2, resulting in bicarbonate (HCO3) and carbonate (CO32) production, further contributing to alkalinity increase (Albina et al. 2019). In this way, the high initial alkalinity levels in the denitrified water helped compensate for the consumption of alkalinity by the nitrification and respiration processes in the biofloc system, which delayed the alkalinity reduction to below-recommended levels and the need for corrective practices. This benefit was also observed in a study by Melo Filho et al. (2020), using a denitrification reactor coupled to a biofloc system. Thus, using denitrified water can represent an economy of resources and labor, such as alkalizing products and management activities during culture, since the amount of alkalizing compounds used in the treatment with denitrified water represented 15 to 20% of the total used in the other treatments, and corrections were performed from 2 to 2.4 times less.
The use of biofloc inoculum has played another role of paramount importance besides the control of nitrogen compounds in the system, as the choice of biofloc formation strategy contributed to the differences found in survival rates between treatments. As described in the “Results” section, a 100% mortality rate was found in the treatment using denitrified water without biofloc inoculum. On the other hand, a lower mortality rate (~10%) was found in the treatment using denitrified water with inoculum. This mortality may be related to compounds produced during or after the denitrification process or to opportunistic microorganism colonization after the process.
The anaerobic denitrification process is a biological process with several steps occurring sequentially, in which the nitrate present in the water is reduced into nitrogen gas with intermediate reduction steps to nitrite, nitric oxide, and nitrous oxide (Philippot 2002). Although these are the main products generated during the denitrification process, other biological processes co-occur in the anoxic environment, such as the degradation of organic matter, fermentation, reduction, and oxidation processes (Kirchman 2018), generating a diverse range of natural byproducts that microorganisms can use.
Soluble microbial products (SMP), organic substances originating from substrate metabolism, highly associated with biomass development and decay (Jarusutthirak and Amy 2006), can be produced during biological wastewater treatments. Among these are dissolved organic nitrogen (DON) products, such as urea, proteins, amino acids, nucleic acids, and humic-like substances (Zheng et al. 2021), produced more in the denitrification process than in nitrification (Wang et al. 2022a). DON compounds are important precursors in the formation of nitrogenous disinfection byproducts (DBP), defined as compounds formed by the reaction of natural organic matter with a disinfectant, such as chlorine and ozone (Noguera-Oviedo and Aga 2016; Wang et al. 2022b), and which may have cytotoxic, mutagenic, genotoxic, and carcinogenic characteristics (Richardson et al. 2007).
In this study, the water was subjected to a disinfection process using chlorine after the denitrification process and before the animals were stocked, which may have produced DBPs. Furthermore, keeping the aerobic biomass (sludge) from the biofloc system during the denitrification process may have contributed to the higher availability of substrate for forming precursor compounds of disinfection byproducts. Thus, the exposition of animals to lethal concentrations of toxic compounds produced during the disinfection process may be the cause of the mortality found since aquatic ecological risk and toxicity of these compounds to aquatic organisms have been assessed (da Costa et al. 2014; Domínguez Henao et al. 2018; Romanucci et al. 2019; Wang et al. 2022b).
Mortality caused by the denitrification process is not commonly reported in studies evaluating the integration of the denitrification process in aquaculture systems. The denitrification process in aquaculture is generally applied using reactors, in which the water is continuously subjected to the process and returns in smaller volumes to the tanks instead of treating the entire volume of water and reusing it at once. Possible pollutants, contaminants, and pathogenic organisms are then diluted in the larger volume of water when they return to the tanks, which already have a stable and active microbial community in the biofilters. Moreover, anaerobic processes are followed by treatment processes, including physical and biological filtration, activated sludge, submerged aerated biofilters, stabilization ponds, and soil application, among others (Von Sperling 2007), which help to reduce the presence of undesired compounds.
This way, using biofloc inoculum proved to be a good strategy for water treatment after the denitrification process since no high mortality was observed in the inoculum treatment. As stated, bioflocs have a very diverse community of microorganisms, formed by bacteria, archaea, ciliates, flagellates, amoebas, and other microorganisms, which confer several probiotic and nutritional benefits to cultivated organisms (Reis et al. 2019) and could have acted as a biofilter for toxic compounds. Biofiltration was found to remove a fraction of halogenated DBP precursors and to decrease concentrations of formed DBPs (Liu et al. 2017). Moreover, the biofloc inoculum may also have helped overcome pathogens microorganism colonization, thus allowing denitrified water for shrimp farming.
Survival rates were similar in all treatments except for the denitrified water without inoculum treatment, indicating that it could be possible to farm shrimp using denitrified water without affecting survival rates if a water treatment is carried out after the denitrification process and before the animals are stocked. Furthermore, survival rates found in this study are similar to those reported by Krummenauer et al. (2014) when testing different percentages of water reuse to grow L. vannamei in bioflocs and those reported by Xavier et al. (2022) with similar strategies of biofloc formation studied. No significant differences were found among treatments in growth rate, which indicates that this parameter was not affected by the treatments. Still, weekly growth rates were lower than expected for biofloc systems at similar stocking density, as growth rates of 0.8 to 1.2 g/week are more commonly found in the literature (Reis et al. 2019; Silveira et al. 2020; Xu et al. 2021).
Although there were no statistical differences between the treatments in the parameters of survival, final weight, and weekly growth, seawater without inoculum treatment presented lower values of production parameters such as productivity and food conversion rate, significantly differing from the treatment with seawater and biofloc inoculum, indicating that these parameters were influenced by the biofloc formation strategy and not by the water source. This lower performance may be related to the fact that the treatment without inoculum went through periods with higher levels of ammonia and nitrite during the culture due to the establishment of the nitrification process. It is known that the process of establishing nitrification in biofloc systems occurs gradually, with ammonia accumulation in the first days of culture until the establishment of oxidizing ammonia bacteria, which begin to oxidize ammonia into nitrite. Then, nitrite accumulates in the tanks until the establishment of oxidizing nitrite bacteria, which oxidize the nitrite to nitrate. Ammonia and nitrite are toxic to farmed organisms and can cause mortality and reduced growth (Lin and Chen 2001, 2003; Schuler et al. 2010). In this case, exposure to these compounds caused a reduction in growth and survival, which, despite not being sufficient to present significant differences in growth and survival rates, were sufficient to affect the biomass gain in this treatment. The opposite can be observed in the treatment with denitrified water and inoculum, which, despite having shown mortality similar to the treatment without inoculum, was not exposed to high levels of ammonia or nitrite and may compensate for the small mortality observed by obtaining a higher growth, reflected in the biomass gain.
This study’s feed conversion rate values were close to those expected for culture in biofloc systems. The values found in this study were lower than those reported by other authors when compared to treatments with similar biofloc formation strategies (with and without biofloc inoculum) (Xavier et al. 2022) or similar inoculum volume (Silveira et al. 2020), indicating good feed management during the farming cycle. Despite this, the treatment of seawater without inoculum presented higher FCR when compared to the treatment with inoculum. The use of the inoculum provided a nutritional contribution to the system since the microbial community present in the inoculum of bioflocs used was already mature, composed of a microbial loop with different trophic levels and microorganisms with different nutritional values (Reis et al. 2019), which may have provided the shrimp with a supplemental food source with different nutrients, helping to increase growth and reduce FCR.
Conclusion
The outcomes indicate that it is possible to reuse the water from a biofloc system after being subjected to an anaerobic biological denitrification process. Furthermore, a simpler and low-cost denitrification process can be carried out in sedimentation basins or the culture tanks after the animals are harvested, reducing the need for equipment and operational procedures. Despite this, it is still necessary to seek alternatives for the further treatment of the denitrified water to make it suitable to grow L. vannamei, regardless of the use of biofloc inoculum.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Ahmad A, Sheikh Abdullah SR, Hasan HA, Othman AR, Izzati IN (2021) Aquaculture industry: supply and demand, best practices, effluent and its current issues and treatment technology. J Environ Manage 287(March):112271. https://doi.org/10.1016/j.jenvman.2021.112271
Aich N, Nama S, Biswal A, Paul T (2021) A review on recirculating aquaculture systems: challenges and opportunities for sustainable aquaculture. Innov Farm 5(1):17–24
Albina P, Durban N, Bertron A, Albrecht A, Robinet J-C, Erable B (2019) Influence of hydrogen electron donor, alkaline pH, and high nitrate concentrations on microbial denitrification: a review. Int J Mol Sci 20(20):5163. https://doi.org/10.3390/ijms20205163
Alves Neto I, Brandão H, Furtado PS, Wasielesky W Jr (2019) Acute toxicity of nitrate in Litopenaeus vannamei juveniles at low salinity levels. Ciência Rural 49(1). https://doi.org/10.1590/0103-8478cr20180439
Aminot A, Chaussepied M (1983) Manuel des analyses chimiques en milieu marin. Brest: CNEXO, Paris
AOAC (1990) Official methods of analysis of the association of official analytical chemists, 15th edn. Association of Official Analytical Chemists
APHA-AWWA-WEF (2005) Standard methods for the examination of water and wastewater, 20th edn. American Public Health Association, American Water Works Association, Water Environment Federation, Washington, DC
Ballester ELC, Abreu PC, Cavalli RO, Emerenciano M, de Abreu L, Wasielesky W (2010) Effect of practical diets with different protein levels on the performance of Farfantepenaeus paulensis juveniles nursed in a zero exchange suspended microbial flocs intensive system. Aquac Nutr 16(2):163–172. https://doi.org/10.1111/j.1365-2095.2009.00648.x
Biesterfeld S, Farmer G, Russell P, Figueroa L (2001) Effect of alkalinity type and concentration on nitrifying biofilm activity. Proc Water Environ Fed 16:277–291. https://doi.org/10.2175/193864701790901951
Brandão H, Xavier ÍV, Santana GKK, Santana HJK, Krummenauer D, Wasielesky W (2021) Heterotrophic versus mixed BFT system: impacts on water use, suspended solids production and growth performance of Litopenaeus vannamei. Aquac Eng 95:102194. https://doi.org/10.1016/j.aquaeng.2021.102194
Brol J, Müller L, Prates ECA, de Farias BS, Pedrosa VF, de Almeida Pinto LA, Sant’anna Cadaval TR, Tesser MB, Wasielesky W, Ventura-Lima J (2021) Dietary chitosan supplementation in Litopenaeus vannamei reared in a biofloc system: effect on antioxidant status facing saline stress. Aquaculture 544(May):737034. https://doi.org/10.1016/j.aquaculture.2021.737034
da Costa JB, Rodgher S, Daniel LA, Espíndola ELG (2014) Toxicity on aquatic organisms exposed to secondary effluent disinfected with chlorine, peracetic acid, ozone and UV radiation. Ecotoxicology 23(9):1803–1813. https://doi.org/10.1007/s10646-014-1346-z
da SLGP, Krummenauer D, Poersch LH, Rosas VT, Wasielesky W (2020) Hyperintensive stocking densities for Litopenaeus vannamei grow-out in biofloc technology culture system. J World Aquac Soc 51(6):1290–1300. https://doi.org/10.1111/jwas.12718
Domínguez Henao L, Turolla A, Antonelli M (2018) Disinfection by-products formation and ecotoxicological effects of effluents treated with peracetic acid: a review. Chemosphere 213:25–40. https://doi.org/10.1016/j.chemosphere.2018.09.005
Ebeling JM, Timmons MB, Bisogni JJ (2006) Engineering analysis of the stoichiometry of photoautotrophic, autotrophic, and heterotrophic removal of ammonia–nitrogen in aquaculture systems. Aquaculture 257(1–4):346–358. https://doi.org/10.1016/j.aquaculture.2006.03.019
FAO (2022) The State of World Fisheries and Aquaculture 2022. FAO, Rome, Italy
Furtado PS, Campos BR, Serra FP, Klosterhoff M, Romano LA, Wasielesky W (2015a) Effects of nitrate toxicity in the Pacific white shrimp, Litopenaeus vannamei, reared with biofloc technology (BFT). Aquac Int 23(1):315–327. https://doi.org/10.1007/s10499-014-9817-z
Furtado PS, Fugimura MMS, Monserrat JM, Souza DM, Garcia LDO, Wasielesky W (2015b) Acute effects of extreme pH and its influences on the survival and biochemical biomarkers of juvenile White Shrimp, Litopenaeus vannamei. Mar Freshw Behav Physiol 48(6):417–429. https://doi.org/10.1080/10236244.2015.1086539
Furtado PS, Gaona CAP, Poersch LH, Wasielesky W (2014) Application of different doses of calcium hydroxide in the farming shrimp Litopenaeus vannamei with the biofloc technology (BFT). Aquac Int 22(3):1009–1023. https://doi.org/10.1007/s10499-013-9723-9
Gaona CAP, Poersch LH, Krummenauer D, Foes GK, Wasielesky WJ (2011) The effect of solids removal on water quality, growth and survival of Litopenaeus vannamei in a biofloc technology culture system. Int J Recirc Aquac 12(1):54–73. https://doi.org/10.21061/ijra.v12i1.1354
Garza de Yta A, Rouse DB, Davis DA (2004) Influence of nursery period on the growth and survival of Litopenaeus vannamei under pond production conditions. J World Aquac Soc 35(3):357–365. https://doi.org/10.1111/j.1749-7345.2004.tb00099.x
Hostins B, Wasielesky W, Decamp O, Bossier P, De Schryver P (2019) Managing input C/N ratio to reduce the risk of acute hepatopancreatic necrosis disease (AHPND) outbreaks in biofloc systems – a laboratory study. Aquaculture 508(2018):60–65. https://doi.org/10.1016/j.aquaculture.2019.04.055
Jarusutthirak C, Amy G (2006) Role of soluble microbial products (SMP) in membrane fouling and flux decline. Environ Sci Technol 40(3):969–974. https://doi.org/10.1021/es050987a
Jiang L, Pan L, Fang-Bo (2005) Effect of dissolved oxygen on immune parameters of the white shrimp Litopenaeus vannamei. Fish Shellfish Immunol 18(2):185–188. https://doi.org/10.1016/j.fsi.2004.07.001
Kirchman DL (2018) Processes in anoxic environments. In: Processes in microbial ecology, 2nd edn. Oxford University Press, pp 195–216
Krummenauer D, Samocha T, Poersch L, Lara G, Wasielesky W (2014) The reuse of water on the culture of pacific white shrimp, Litopenaeus vannamei in BFT system. J World Aquac Soc 45(1):3–14. https://doi.org/10.1111/jwas.12093
Kuhn DD, Smith SA, Boardman GD, Angier MW, Marsh L, Flick GJ (2010) Chronic toxicity of nitrate to Pacific white shrimp, Litopenaeus vannamei: impacts on survival, growth, antennae length, and pathology. Aquaculture 309(1–4):109–114. https://doi.org/10.1016/j.aquaculture.2010.09.014
Lara G, Honda M, Poersch L, Wasielesky W (2017) The use of biofilm and different feeding rates in biofloc culture system: the effects in shrimp growth parameters. Aquac Int 25(5):1959–1970. https://doi.org/10.1007/s10499-017-0151-0
Letelier-Gordo CO, Huang X, Aalto SL, Pedersen PB (2020) Activated sludge denitrification in marine recirculating aquaculture system effluent using external and internal carbon sources. Aquac Eng 90(May):102096. https://doi.org/10.1016/j.aquaeng.2020.102096
Lin Y-C, Chen J-C (2001) Acute toxicity of ammonia on Litopenaeus vannamei Boone juveniles at different salinity levels. J Exp Mar Bio Ecol 259(1):109–119. https://doi.org/10.1016/s0022-0981(01)00227-1
Lin Y-C, Chen J-C (2003) Acute toxicity of nitrite on Litopenaeus vannamei (Boone) juveniles at different salinity levels. Aquaculture 224(1–4):193–201. https://doi.org/10.1016/S0044-8486(03)00220-5
Liu C, Olivares CI, Pinto AJ, Lauderdale CV, Brown J, Selbes M, Karanfil T (2017) The control of disinfection byproducts and their precursors in biologically active filtration processes. Water Res 124:630–653. https://doi.org/10.1016/j.watres.2017.07.080
Luo G, Xu J, Meng H (2020) Nitrate accumulation in biofloc aquaculture systems. Aquaculture 520:734675. https://doi.org/10.1016/j.aquaculture.2019.734675
Martins MA, Poli MA, Legarda EC, Pinheiro IC, Carneiro RFS, Pereira SA, Martins ML, Gonçalves P, Schleder DD, do Nascimento Vieira F (2020) Heterotrophic and mature biofloc systems in the integrated culture of Pacific white shrimp and Nile tilapia. Aquaculture 514(August 2019):734517. https://doi.org/10.1016/j.aquaculture.2019.734517
Melo Filho MES, Owatari MS, Mouriño JLP, Lapa KR, Soares HM (2020) Application of nitrification and denitrification processes in a direct water reuse system for pacific white shrimp farmed in biofloc system. Aquac Eng 88(August 2019):102043. https://doi.org/10.1016/j.aquaeng.2020.102043
Noguera-Oviedo K, Aga DS (2016) Lessons learned from more than two decades of research on emerging contaminants in the environment. J Hazard Mater 316:242–251. https://doi.org/10.1016/j.jhazmat.2016.04.058
Philippot L (2002) Denitrifying genes in bacterial and Archaeal genomes. Biochim Biophys Acta - Gene Struct Expr 1577(3):355–376. https://doi.org/10.1016/S0167-4781(02)00420-7
Piedrahita RH (2003) Reducing the potential environmental impact of tank aquaculture effluents through intensification and recirculation. Aquaculture 226:35–44. https://doi.org/10.1016/S0044-8486(03)00465-4
Ponce-Palafox J, Martinez-Palacios CA, Ross LG (1997) The effects of salinity and temperature on the growth and survival rates of juvenile white shrimp, Penaeus vannamei, Boone, 1931. Aquaculture 157(1–2):107–115. https://doi.org/10.1016/S0044-8486(97)00148-8
Prates E, Holanda M, Pedrosa VF, Monserrat JM, Wasielesky W (2023a) Compensatory growth and energy reserves changes in the Pacific white shrimp (Litopenaeus vannamei) reared in different temperatures and under feed restriction in biofloc technology system (BFT). Aquaculture 562(September 2022):738821. https://doi.org/10.1016/j.aquaculture.2022.738821
Prates ECA, Damasceno JN, Okamoto M, Holanda M, Costa M, Monserrat JM, Wasielesky W (2023b) Determinação da toxicidade aguda de nitrato para o camarão branco do Pacífico Litopenaeus vannamei. In: Anais Eletrônicos do X Aquaciência. AQUABIO, Florianópolis, SC
Preena PG, Rejish Kumar VJ, Singh ISB (2021) Nitrification and denitrification in recirculating aquaculture systems: the processes and players. Rev Aquac 13(4):2053–2075. https://doi.org/10.1111/raq.12558
Reis WG, Wasielesky W Jr, Abreu PC, Brandão H, Krummenauer D (2023) The influence of different light wavelengths in the culture of the Pacific white shrimp Litopenaeus vannamei reared in BFT using LED lights. Aquaculture 563:738924. https://doi.org/10.1016/j.aquaculture.2022.738924
Reis WG, Wasielesky W, Abreu PC, Brandão H, Krummenauer D (2019) Rearing of the Pacific white shrimp Litopenaeus vannamei (Boone, 1931) in BFT system with different photoperiods: effects on the microbial community, water quality and zootechnical performance. Aquaculture 508(April):19–29. https://doi.org/10.1016/j.aquaculture.2019.04.067
Richardson S, Plewa M, Wagner E, Schoeny R, Demarini D (2007) Occurrence, genotoxicity, and carcinogenicity of regulated and emerging disinfection by-products in drinking water: a review and roadmap for research. Mutat Res Mutat Res 636(1–3):178–242. https://doi.org/10.1016/j.mrrev.2007.09.001
Romanucci V, Siciliano A, Galdiero E, Guida M, Luongo G, Liguori R, Di Fabio G, Previtera L, Zarrelli A (2019) Disinfection by-products and ecotoxic risk associated with hypochlorite treatment of tramadol. Molecules 24(4):693. https://doi.org/10.3390/molecules24040693
Schuler DJ, Boardman GD, Kuhn DD, Flick GJ (2010) Acute toxicity of ammonia and nitrite to pacific white shrimp, Litopenaeus vannamei, at low salinities. J World Aquac Soc 41(3):438–446. https://doi.org/10.1111/j.1749-7345.2010.00385.x
Shanahan JW, Semmens MJ (2015) Alkalinity and pH effects on nitrification in a membrane aerated bioreactor: an experimental and model analysis. Water Res 74:10–22. https://doi.org/10.1016/j.watres.2014.12.055
Silveira LGP, Rosas VT, Krummenauer D, Poersch LH, Wasielesky W Jr (2020) Comparison between horizontal and vertical substrate in shrimp super-intensive culture in bioflocs system. Aquac Eng 96(November 2021):102218. https://doi.org/10.1016/j.aquaeng.2021.102218
Strickland JDH, Parsons TR (1972) A practical handbook of seawater analysis, 2nd edn. Fisheries Research Board Of Canada, Ottawa
UNESCO (1983) Chemical methods for use in marine environmental monitoring. In: Manuals and guides 12. Intergovernmental Oceanographic Commission, Paris, France
Valencia-Castañeda G, Frías-Espericueta MG, Vanegas-Pérez RC et al (2018) Acute toxicity of Ammonia, Nitrite and Nitrate to shrimp litopenaeus vannamei postlarvae in low-salinity water. Bull Environ Contam Toxicol 101:229–234. https://doi.org/10.1007/s00128-018-2355-z
Von Sperling M (2007) Basic principles of wastewater treatment (Biological Wastewater Treatment Volume 2). IWA Publishing, London, UK
Wang J, Zheng F, Yu Z, Chen J, Lu H (2022a) Dissolved organic nitrogen derived from wastewater denitrification: composition and nitrogenous disinfection byproduct formation. J Hazard Mater 440(August):129775. https://doi.org/10.1016/j.jhazmat.2022.129775
Wang Y, Liu H, Yang X, Wang L (2022b) Aquatic toxicity and aquatic ecological risk assessment of wastewater-derived halogenated phenolic disinfection byproducts. Sci Total Environ 809:151089. https://doi.org/10.1016/j.scitotenv.2021.151089
Wasielesky W, Atwood H, Stokes A, Browdy CL (2006) Effect of natural production in a zero exchange suspended microbial floc based super-intensive culture system for white shrimp Litopenaeus vannamei. Aquaculture 258(1–4):396–403. https://doi.org/10.1016/j.aquaculture.2006.04.030
Xavier M, Wasielesky Júnior W, Hostins B, Bequé E, Krummenauer D (2022) The use of a flocculant additive and its effect on biofloc formation, nitrification, and zootechnical performance during the culture of Pacific white shrimp Penaeus vannamei (Boone, 1931) in a BFT system. Lat Am J Aquat Res 50(2):181–196. https://doi.org/10.3856/vol50-issue2-fulltext-2777
Xu W, Xu Y, Su H, Hu X, Xu Y, Li Z, Wen G, Cao Y (2021) Production performance, inorganic nitrogen control and bacterial community characteristics in a controlled biofloc-based system for indoor and outdoor super-intensive culture of Litopenaeus vannamei. Aquaculture 531(June 2020):735749. https://doi.org/10.1016/j.aquaculture.2020.735749
Zar JH (2010) Biostatistical analysis, 5th edn. Prentice Hall, Upper Saddle River
Zheng F, Wang J, Xiao R, Chai W, Xing D, Lu H (2021) Dissolved organic nitrogen in wastewater treatment processes: transformation, biosynthesis and ecological impacts. Environ Pollut 273:116436. https://doi.org/10.1016/j.envpol.2021.116436
Acknowledgements
Special thanks to GUABI Animal Health and Nutrition, AQUATEC, TREVISAN, and Al Aqua for donating the experimental diets, shrimp post-larvae, and aeration system respectively.
Funding
The authors are grateful for the financial support provided by the National Council for Scientific and Technological Development (CNPq), Coordination for the Improvement of Higher-Level Personnel (CAPES), and Foundation for Research Support of the State of Rio Grande do Sul (FAPERGS), grant number 23/2551-0000180-2. Wasielesky, W. is a research fellow of CNPq under process number PQ 307741/2022-2.
Author information
Authors and Affiliations
Contributions
HB: conceptualization; methodology; investigation; data curation; formal analysis; writing—original draft; writing—review and editing; visualization. WGR: conceptualization; methodology; investigation; data curation; formal analysis; writing—review and editing. DK: conceptualization; writing—review; supervision; funding acquisition; resources. WWJ: conceptualization; writing—review and editing; supervision; project administration; funding acquisition; resources.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Handling Editor: Mauricio G. C. Emerenciano
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Brandão, H., dos Reis, W.G., Krummenauer, D. et al. Growth performance of Litopenaeus vannamei under biofloc system using denitrified seawater. Aquacult Int 32, 3129–3145 (2024). https://doi.org/10.1007/s10499-023-01315-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-023-01315-0