Abstract
The study aimed to characterize the dynamics involved in the water quality parameters in a biofloc system (BFT) during the super-intensive cultivation of Litopenaeus vannamei using two levels of artificial brackish water. The test was designed with two salinity levels, T16 (16‰) and T8 (8‰), with 4 replicates, and 250 shrimps/m2 by 60 days, water analysis and animal performance were studied in each treatment. No significant differences were observed between the two salinities for biochemical oxygen demand (BOD5), total chemical oxygen demand (CODt), and fixed suspended solids (FSS). Using CODt/BOD5, it was possible to identify the water biodegradability and manage the concentration of organic and inorganic matter in the medium. Filtered chemical oxygen demand (CODf) was used to monitor the dissolved organic matter, which was higher in T16. Carbohydrate (molasses) did not control total ammonia reaching in T8 = 1.16±0.64 mg/L. This organic matter addition reduced the growth of chemoautotrophic nitrifying bacteria and interfered in the nitrogen dynamics. Regarding total solids and suspended solids, there was a significant difference between treatments, except for FSS. Dissolved oxygen (DO) and oxygen saturation (sO2) were significantly different between the treatments. The maintenance of a more neutral pH and greater alkalinity were observed, with significant differences between the treatments throughout the whole cultivation. Regarding the shrimp growth performance, the high salinity presented more weight gain, specific growth rate, feed conversion and final biomass, and lower mortality then lower salinity. These results showed that shrimps presented a higher performance in salinity 16‰.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The intensification of aquaculture, which invariably leads to generation of considerable waste formation, aims to increase food production to meet the increased demand for a growing global population (Muhlert et al. 2013).
According to Bossier and Ekasari (2017), nutrient waste can be recycled by natural biogeochemical processes involving microorganisms, that may be consumed by cultured animals. This is the principle of the Biofloc Technology (BFT), in which the nutrients and organic matter may be recycled, mainly by heterotrophic bacterial growth through the addition of extra carbon to the aquaculture system (Avnimelech 2015; Emerenciano et al. 2017; Abakari et al. 2020).
The BFT system is considered sustainable because the water renewal is zero or minimal, reducing costs of pumping water, minimizing the introduction of pathogens from external water (biosecurity), and avoiding environmental contamination with the disposal of large volumes of wastewater (Avnimelech 1999; Burford et al. 2003).
From the relationship between oxygen demand analysis (BOD5) and filtered chemical oxygen demand (CODf), it is possible to identify the biodegradability of culture water and the availability of organic matter to the heterotrophic bacterial community (von Sperling 1996); however in the literature, little is known about organic concentration added to the BFT system.
Thus, observing the behavior of organic parameters over time can contribute to the management of the BFT system and the consequent zootechnical performance of the animals (Kumar et al. 2018; Liu et al. 2018). With the increase in organic load, total solids in the system tend to increase, changing the biofloc composition, the water quality (dissolved oxygen, carbon dioxide, pH, alkalinity, etc.), which can affect animal health (Crab et al. 2007).
The control of total suspended solids (TSS) could be effective to control organic and inorganic matter, suspended microbial biomass, and food available to bacteria. According to Souza et al. (2019), smaller particles in the BFT system tend to negatively interfere with the nitrification process. The use of clarifiers is effective to maintain the TSS in the optimum level for shrimp culture (Gaona et al. 2016a, 2016b, 2017).
The salinity of the water is another point to be addressed because, despite having euryhaline characteristics, the Pacific white shrimp Litopenaeus vannamei may have lesser osmoregulatory expenditure when in brackish water (Decamp et al. 2003). Moreover, information about L. vannamei tolerance to low salinity is needed, once this is demanding to reduce expenses with salts on shrimp farming in inland areas far from the coast. However, low salinity stress can cause problems related to growth and mortalities of L. vannamei, as described in the literature (Zhao et al. 2015; Esparza-Leal et al. 2016; Fregoso-López et al. 2017).
The objective of this study was to characterize and evaluate the water quality parameters during the intensive cultivation of L. vannamei in brackish water (artificially produced), using the BFT system without water renewal. We also studied shrimp performance after 60 days under indoor conditions.
Materials and methods
The experiment was conducted for 60 days at the Mariculture and water quality Laboratory and the physical-chemical analyses were carried out at the Environmental Sanitation Laboratory and at the Mariculture and water quality Laboratory of the Federal University of Minas Gerais.
Experimental design
Post-larvae of L. vannamei were obtained from Aquatec® Ltd (RN-Brazil). The post-larvae were kept in nursery units in a BFT system until they reached a mean weight of 1.0 g. After that they were transferred to grow-out units where they were maintained at a density of 250 shrimp/m2 until the beginning of the study. During this period, they were fed using commercial feed with 38% crude protein (Potimar, Guabi, Brazil) composed of moisture (10%), crude protein (38%), etheric extract (7.5%), fibrous matter (5%), mineral matter (13%), calcium (3%), phosphorus (1.45%), vitamin a (13,000 IU), vitamin d3 (2,500 IU), vitamin e (200 IU), and vitamin c (500 mg).
The design was completely randomized with two treatments (salinity 16‰ = T16 and 8‰ = T8) and four replicates. Additionally, each treatment had a matrix tank (external to maintain water volume for the treatments), developed as described by Wasielesky et al. (2006, 2013), Emerenciano et al. (2011, 2012), and Lara et al. (2017). The polyethylene tanks used for shrimp production had 100 L (0.23 m2), and for matrix purposes had 500 L (0,71 m2). The volume used was 80 L, maintained by the matrix tank. There was no water renewal, only the replacement of evaporated water. Each tank had diffused aeration supplied by a blower to keep dissolved oxygen close to water saturation. The photoperiod was natural.
The flow of water between the tanks was continuous, i.e., water recirculated through the system without the obstruction of flux, through a pump with a flow rate of 21 L/min. The density of shrimps (4.05 g ± 0.62 g) in each tank with biofloc was 250 animals/m2, totalizing 60 shrimps per experimental unity. The feed was given twice daily (8:00 a.m. and 4:00 p.m.) with a commercial feed 38% CP (Potimar, Guabi) at 3% of total biomass during the experimental period. Biometrics was performed weekly on 20 randomly sampled animals to determine growth and monitoring of feed use and adjustments.
To achieve the salinity in each treatment (8‰ and 16‰), we used mature biofloc from previous shrimp cultivation with 8‰ salinity. In T16 treatment, salinity was adjusted to 16‰ in 24 h. The brackish water was artificially produced according to ionic concentration described by Grasshoff et al. (1983) with brief modifications. Corrections were made to keep pH values above 7.8 and alkalinity above 150 mg/L of CaCO3, by adding hydrated lime and dolomitic lime (Rural MF, Brazil). When ammonia concentrations were greater than 0.5 mg/L, a source of carbon, molasses (Rural MF, Brazil) was added in sufficient quantity to maintain the C:N ratio 6:1, as previously reported (Avnimelech 1999; Ebeling et al. 2006).
Clarification was performed in treatments where settleable solids (SetS) concentrations were higher than 15 mL/L by the use of polystyrene Imhoff graduated settling cone (1000 mL), based on the methodology described by Gaona et al. (2011) and was built in PVC with a volume of 53 L. The operating principle considered gravitational action for solid sedimentation in the clarifier bottom and lighter water returned to the system. A submerged pump (19 W, BOYU) was used to capture the medium to be clarified and was maintained for 3 to 5 h.
Water quality monitoring
To characterize water quality of the shrimp cultivation, the following physicochemical variables were analyzed: dissolved oxygen (DO) (mg/L) and oxygen saturation (sO2) (%) were monitored daily in the morning using a digital oximeter (Hanna, HI9146, Czech Republic); pH and temperature (T) with multiparameter apparatus (Hanna, HI98129, Czech Rep.); salinity using refractometer (Atago, ATC-S, Mill 2440, Japan); total ammonia (TA-N) and nitrite (NO2--N) determined by spectrophotometric method (UNESCO 1983); and settleable solids (SetS) with Imhoff cone (Avnimelech 2007).
The total chemical oxygen demand (CODt), filtered oxygen demand (CODf), total solid (TS), total fixed solid (TFS), total volatile solid (TVS), total suspended solid (TSS), volatile suspended solids (VSS), and fixed suspend solids (VSS) were analyzed twice weekly, while the 5-day biochemical oxygen demand (BOD5) was analyzed once a week using the APHA methods (APHA 1999).
To evaluate the organic contribution of molasses, BOD5 and CODt of 20 g of this fertilizer were analyzed and the values obtained were 8432 mg/L and 3,8974 mg/L, respectively.
Performance parameters
Every week a random sample of 20 shrimps from each experimental unit was individually dried in paper towel, weighed on a precision balance, and then returned to their home tanks. At the end of the experiment, all shrimps were quantified and total biomass estimated. The performance of L. vannamei juveniles subjected to different treatments of salinity was evaluated according to the following parameters: survival rate (%), weekly weight gain, specific growth rate, feed conversion factor, and final biomass.
-
Survival rate (%) = Number of surviving animals / Initial number of animals x 100
-
Weekly weight gain (g/week) = Weight gain / week of cultivation
-
Specific growth rate (SGR) (%/day) = 100 × (ln final weight (g) - ln initial weight (g) / time in days
-
Feed Conversion Factor (FCF) = Feed consumption / final biomass
-
Final biomass (g) = Final number of animals x final weigth (g)
Statistical analysis
The data were tested by Lilliefors and Cochran tests to verify normal distribution and homoscedasticity of variances respectively. Analysis of variance (ANOVA) and Tukey’s test were performed to compare the means of qualitative factors using the software INFOSTAT (2008). The type I error rate of 5% probability was assumed.
Results and discussion
Water quality monitoring
Table 1 shows the averages for BOD5, CODt, and CODf in the two treatments. It was observed that there was no significant difference between the treatments for BOD5 and CODt, possibly due to the amount of molasses added during the experimental period. From BOD5 and CODt values, it was possible to show that CODt/BOD5 ratio was higher than 3.5 in both treatments, showing high concentrations of inert or non-biodegradable material in the systems (von Sperling 1996). Therefore, little organic material available for bacteria was present in the medium. According to von Sperling (1996), this inert organic material is formed through the decay of bacterial biomass by endogenous metabolism, death, predation, and others.
Ray et al. (2011) also worked with mesohaline salinity (16‰) aimed inland shrimp cultivation, and comment on the importance of salinity in shrimp growth. According to Medeiros et al. (2005), salinity can cause adverse effects on the microbial flora, causing plasmolysis and loss of cell activity, which may explain the high concentration of inert material in both treatments.
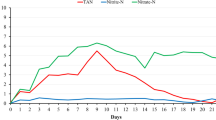
Besides, it was found that the amount of organic material dissolved (CODf) was different between the two treatments. In T16, the mean was 1,278.13 ± 599.09 mg/L, which represented 95.5% of soluble material and in T8, the average was 741.63 ± 330.19 mg/L, which represented 58.55% of soluble material. This difference was possibly due to the amount of feed provided during the experiment that varied according to the biometry, in T16 due to the gain of animals’ weight, the amount of feed was increased. When observing Fig. 1, it was noticed that the concentration of CODf in T16 increased in greater proportions than in T8.
The higher soluble matter in T16 cannot be attributed to the addition of molasses, since in T8, the total molasses added to control the ammonia concentration was higher than in T16. Thus, in addition to not being biodegradable and consequently not being available for bacterial consumption, it accumulates in the system. Despite a large amount of dissolved organic matter, it was not biodegradable and probably was originated from the decomposition of the bacterial community (von Sperling 1996). It justified the high CODf concentration throughout the experiment.
Although molasses was not directly related to CODf difference between the treatments, its participation in the increase of organic matter soluble was clear, since in 20 g of molasses diluted in 100 mL of water, about 73% was soluble fraction of organic material (CODf). The BOD5 and CODt values obtained for molasses showed lower concentrations of biodegradable material, which may also have an influence on inert organic matter in the water. Although Arantes et al. (2017) associated high values of biodegradable material, via BOD5, with the amount of feed and the continuous intake of carbohydrate added in the system throughout the culture, the two carbon fertilizations (inputs) not only presented biodegradable organic matter, but also contributed to the increase in inert material.
Zhao et al. (2014) verified low values of CODt in carp biofloc systems, they showed that addition of corn starch can increase the C:N ratio, maintaining low concentrations of CODt in the control tank, with C:N = 7:1. The CODt mean was 11.53 ± 0.74 mg/L, with no significant difference among treatments with higher C:N ratios. In this way, molasses may not be the best input for the maintenance of the C:N ratio, since it considerably increases CODt concentrations in the cultivation system.
In addition to inert organic material, the presence of inorganic material in the water was also observed through solids analysis. In relation to total solids, there was a significant difference between treatments, both in relation to organic solids (TVS) and inorganic solids (TFS) (Table 1). Among the two treatments, most of these solids corresponded to the inorganic fraction (TFS), being 86% in T16 and 77% in T8, which demonstrated high concentrations of inert material in the tanks. As salinity increases, the amount of inorganic salt in the water was increased, as demonstrated by the higher TFS value in the 16‰. Inputs used for pH correction, maintenance of the C:N ratio, and feeding of the animals may also contribute to this inorganic fraction.
From the values of total and suspended solids, it was possible to infer that there was a fraction of dissolved solids higher than the particulate fraction. However, most dissolved solids were inorganic and therefore not used by the bacterial community present in the medium. Only 17% of the dissolved solids were biodegradable and consumed immediately by the organisms as carbon source since there was no need for hydrolysis.
Concerning the suspended solids, there was a significant difference between the treatments for TSS and VSS (Table 2). By relating these parameters, it was possible to determine that most of the particulate matter had an organic origin, being 71% in T8 and 56% in T16. In Table 2 it is possible to observe that the increase of microbial biomass occurred rapidly. Also, in T8, microbial growth was favored, due to the higher values of VSS, demonstrating that in low salinities, growth of heterotrophic and chemoautotrophic bacteria was facilitated. A different response was observed by Maicá et al. (2012), when evaluating the effect of low salinities on biofloc composition and performance of L. vannamei in the BFT system. The authors described that with increasing salinity, there was an increase of suspended solids, and in the salinity of 25‰, the mean concentration of TSS was 256.0 ± 12.71 mg/L. Thus, we cannot attribute in isolation that salinity was responsible for the amount of TSS.
Values close to those found in the present study were also reported by Ray et al. (2010) working with diets based on fish oil and vegetable meal in BFT (without clarification), with a mean TSS concentration of 820 ± 135 mg/L and 745 ± 165 mg/L; and VSS of 509 ± 99 and 485 ± 114 mg/L, respectively. The use of a clarifier is advisable because it can reduce biofloc, BOD5 concentration, decrease animal stress, or alter the microorganism community, favoring the shrimp production (Ray et al. 2010, 2011). According to Schveitzer et al. (2013b), TSS between 400 and 600 mg/L prevents damage in L. vannamei during its superintensive cultivation.
The treatments were clarified by measuring SetS above 15 mL/L. However, the mean concentration of SetS exceeded this assumption, with mean values of 51.27 ± 21.34 mL/L for T8 and 46.78 ± 22.21 mL/L for T16. The clarification time was not sufficient to efficiently remove the particulate biofloc volume. Ray and Lotz (2017) presented mean values of 7 ± 1 mL/L in salinity 10‰ and 9 ± 1 mL/L in salinity 30‰ for SetS since they maintained the clarifier (settling chambers) with a continuous flow rate of 15 L/min, except once a week in which the water flow was interrupted for 1 hour to allow the complete sedimentation of the biofloc particles.
In the Fig. 1, it was possible to verify that nitrification was not the main route of ammonia removal throughout the shrimp cultivation, since the by-product of the nitrification reactions remained constant throughout the time, with few variations. In addition to slower growth of nitrifying bacteria, competition for space and oxygen occurs with heterotrophic bacteria, as mentioned by Hargreaves (2006).
Therefore, adding organic matter to the system eventually inhibited the growth of chemoautotrophic bacteria. In addition, salinity interferes with nitrogen dynamics in the system, since there was a significant difference between treatments for ammonia, nitrite, and nitrate, with higher means in the lower salinity, except for nitrate, whose average was higher in T16. Decamp et al. (2003) working with L. vannamei in BFT did not observe significant differences of these parameters in different salinities and associated the accumulation of nitrogen to the absence of water renewal and nitrification processes that occur in both salinities.
Regarding phosphate, there is an increase in its concentration over time (Table 2) since feed is the main source of phosphorus loading in aquaculture. Only about 13% of the phosphorus present in the food is usually incorporated into shrimp biomass, the rest could compromise water quality (Briggs and Fvnge-Smith 1994), but phosphate is considered to be slightly toxic to aquatic organisms and the concentrations found in the present study can be considered harmless according Kim et al. (2013).
During the experiment, the method of carbon addition as a form of ammonia control was not efficient, since the tendency of ammonia concentration was to increase in both treatments, as observed in Fig. 1 and in Table 2, with a significant difference between the treatments from the fourth week. This corroborates the previous finding regarding the amount of inert waste in the system, superior to active biomass (VSS) responsible for the biological degradation of organic matter. That is, the assimilation of ammonia in the biomass of heterotrophic bacteria was also low, perhaps due to the C:N ratio or to the removal of active biomass by the clarifier.
Ammonia toxicity is mainly dependent on pH levels, with ammonia (NH3) concentrations increasing in alkaline medium (Colt 2006). In this way, control of pH is extremely important. With the increase of salinity, the maintenance of a more neutral pH was observed (Table 2), with significant differences between treatments throughout whole shrimp cultivation. The highest pH may have contributed to animal mortality, mainly in T8, since L. vannamei are less tolerant to ammonia at lower salinities (Li et al. 2007). The alkalinity of T16 was higher than T8, showing that in higher salinities, alkalinity tends to be higher. This difference may be related to cellular plasmolysis at higher salinities, decreasing the number of active bacteria that consume alkalinity. However, Ray and Lotz (2017) did not find a significant difference in alkalinity between salinity of 10‰, 20‰, and 30‰ in L. vannamei production using BFT. The alkalinity values in the present study did not negatively affect the shrimp, because, in both treatments, they were higher than 75 mg/L, as described by Furtado et al. (2015).
It was possible to observe that DO and sO2 were significantly different between treatments, with the increase of salinity. The oxygen solubility in the water decreases, as observed by Maicá et al. (2012), who verified DO reduction in higher salinities in BFT. However, it is noteworthy that in both treatments, DO concentration was considered ideal for shrimps, that is, above 3 mg/L (Boyd 2015). According to McGraw et al. (2001), DO levels close to saturation result in maximum productivity and levels below 50% saturation should be avoided. In addition, oxygen is required in biological stabilization of the organic matter, so in the T16, whose BOD5 was higher, more oxygen was demanded for biological degradation of organic matter.
Zootechnical performance
The means and standard deviations of shrimp performance are presented in Tables 3 and 4. It was possible to verify that the weight gain was higher in T16 with averages of 5.99 ± 0.54 g and 2.91 ± 0.93 g in T8, as well as specific growth rate and final biomass which were also higher in T16. Higher mortality was observed in T8, showing that salinity affected shrimp performance. It is noteworthy that total ammonia peaks also contributed to mortality in both treatments.
As observed in Fig. 2, lower zootechnical performances at lower salinities in BFT systems have also been noted by Decamp et al. (2003), which attributed a lower growth and survival rate in salinity 9‰ due to the relationship between alkalinity greater than 150 mg/L of CaCO3 and high concentrations of chlorides (> 300 mg/L) for shrimp success at low salinities.
Maicá et al. (2012) also observed a positive influence of increased salinity on shrimp survival, weight gain and final biomass. Smaller growths at lower salinities may be related to the use of proteins not only for feeding, but also for the maintenance of osmotic equilibrium (Rosas et al. 2001).
Jatobá et al. (2014) in a comparative study between zootechnical indexes in conventional intensive system and BFT, with the same density of 250 animals/m2, observed that when feeding rations with 36.7% CP, the best performance was obtained in BFT system, with a weight gain of 9.18 ± 1.14 g and a weekly growth rate of 1.84 ± 0.23 g/week. This confirms the efficiency of BFT cultivation with high densities and that the reduction of the protein percentage in the feed to 30.3% did not significantly alter the weight gain and weekly growth rate, demonstrating the economic viability allied to the productivity.
The feed conversion rates did not show a significant difference between treatments, since in both there was formation of bacterial communities, which help to reduce feed intake and feed conversion of shrimps when compared to animals raised in clear water (Wasielesky et al. 2006). However, feed conversion rates were relatively high when compared to other works in BFT system. Maicá et al. (2012) verified rates of 0.81 to 0.87 at low salinities with density of 300 shrimp/m2; Krummenauer et al. (2011) with density of 300 shrimp/m2 observed a rate of 1.29 ± 0.05 in BFT system.
Also, biofloc can improve weight gain of shrimps by providing nutrients that would be found only in the feed. Ekasari et al. (2014) evaluated the nutritional composition of biofloc and observed that particles greater than 100 μm and less than 48 μm present high nutritional value for shrimp, with high concentrations of lipids and proteins in larger biofloc and essential amino acids in smaller biofloc.
Souza et al. (2019) described that particle size of the biofloc did not interfere in the nitrification process and when the TSS > 500 mg/L negatively affect the productivity of L. vannamei. Several authors observed that artificial substrates could increase the nitrification (Santos et al. 2019) and area for the shrimps (Schveitzer et al. 2013a) in BFT systems. However, more studies are needed relating the biofloc and the nitrification process, taking into account the concern with water quality.
Conclusion
Assessing the water quality of the BFT system allows management of the non-biodegradable material that accumulates in the system. The higher salinity (16‰) was better for the development of L. vannamei. Besides, use of molasses as a form of ammonia control was not so efficient and contributed to the increase of organic matter in the environment. It was observed that water quality changes during shrimp cultivation in biofloc can interfere in shrimp performance.
References
Abakari G, Luo G, Kombat EO (2020) Dynamics of nitrogenous compounds and their control in biofloc technology (BFT) systems: A review. Aquacult Fish (in press) Available online 9 July 2020. https://doi.org/10.1016/j.aaf.2020.05.005
APHA - American Public Health Association (1999) Standard Methods for the Examination of Water and Wastewater. Stand. Methods
Arantes R, Schveitzer R, Quadros W, Regina K, Vinatea L (2017) Aquacultural engineering nutrient discharge, sludge quantity and characteristics in biofloc shrimp culture using two methods of carbohydrate fertilization. Aquacult Eng 76:1–8. https://doi.org/10.1016/j.aquaeng.2016.11.002
Avnimelech Y (1999) Carbon/nitrogen ratio as a control element in aquaculture systems. Aquaculture 176:227–235. https://doi.org/10.1016/S0044-8486(99)00085-X
Avnimelech Y (2007) Feeding with microbial flocs by tilapia in minimal discharge bio-flocs technology ponds. Aquaculture 264:140–147. https://doi.org/10.1016/j.aquaculture.2006.11.025
Avnimelech Y (2015) Biofloc Technology - A practical guide book, 3rd edn. The World Aquaculture Society, Baton Rouge
Bossier P, Ekasari J (2017) Biofloc technology application in aquaculture to support sustainable development goals. Microb Biotechnol 10:1012–1016. https://doi.org/10.1111/1751-7915.12836
Boyd CE (2015) Water quality an introduction, Second edn. Springer, New York
Briggs MRP, Fvnge-Smith SJ (1994) A nutrient budget of some intensive marine shrimp ponds in Thailand. Aquacult Res 25:789–811. https://doi.org/10.1111/j.1365-2109.1994.tb00744.x
Burford MA, Thompson PJ, McIntosh RP, Bauman RH, Pearson DC (2003) Nutrient and microbial dynamics in high-intensity, zero-exchange shrimp ponds in Belize. Aquaculture 219:393–411. https://doi.org/10.1016/S0044-8486(02)00575-6
Colt J (2006) Water quality requirements for reuse systems. Aquacult Eng 34:143–156. https://doi.org/10.1016/j.aquaeng.2005.08.011
Crab R, Avnimelech Y, Defoirdt T, Bossier P, Verstraete W (2007) Nitrogen removal techniques in aquaculture for a sustainable production. Aquaculture 270:1–14. https://doi.org/10.1016/j.aquaculture.2007.05.006
Decamp O, Cody J, Conquest L, Delanoy G, Tacon AGJ (2003) Effect of salinity on natural community and production of Litopenaeus vannamei (Boone), within experimental zero-water exchange culture systems. Aquacult Res 34:345–355. https://doi.org/10.1046/j.1365-2109.2003.00842.x
Ebeling JM, Timmons MB, Bisogni JJ (2006) Engineering analysis of the stoichiometry of photoautotrophic, autotrophic, and heterotrophic removal of ammonia-nitrogen in aquaculture systems. Aquaculture 257:346–358. https://doi.org/10.1016/j.aquaculture.2006.03.019
Ekasari J, Angela D, Waluyo SH, Bachtiar T, Surawidjaja EH, Bossier P, De Schryver P (2014) The size of biofloc determines the nutritional composition and the nitrogen recovery by aquaculture animals. Aquaculture 426-427:105–111. https://doi.org/10.1016/j.aquaculture.2014.01.023
Emerenciano M, Ballester ELC, Cavalli R, Wasielesky WJ (2011) Effect of biofloc technology (BFT) on the early postlarval stage of pink shrimp Farfantepenaeus paulensis: growth performance, floc composition and salinity stress tolerance. Aquacult Int 19:891–901. https://doi.org/10.1007/s10499-010-9408-6
Emerenciano M, Ballester ELC, Cavalli R, Wasielesky WJ (2012) Biofloc technology application as a food source in a limited water exchange nursery system for pink shrimp Farfantepenaeus brasiliensis (Latreille, 1817). Aquacult Res 43:447–457. https://doi.org/10.1111/j.1365-2109.2011.02848.x
Emerenciano MGC, Martinez-Cordova LR, Martinez-Porches M, Miranda-Baeza A (2017) Biofloc techology (BFT): A tool for water quality management in aquaculture. In H. Tutu (Ed.). Water quality (pp. 91-109). https://doi.org/10.5772/66416 IntechOpen.
Esparza-Leal HM, Xavier JAA, Wasielesky WJ (2016) Performance of Litopenaeus vannamei postlarvae reared in indoor nursery tanks under biofloc conditions at different salinities and zero-water exchange. Aquacult Int 24:1435–1447. https://doi.org/10.1007/s10499-016-0001-5
Fregoso-López MG, Morales-Covarrubias MS, Franco-Nava MA, Ramírez-Rochín J, Fierro-Sanudo JF, Ponce-Palafox JT, Páez-Osuna F (2017) Histological alterations in gills of shrimp Litopenaeus vannamei in low-salinity waters under different stocking densities: Potential relationship with nitrogen compounds. Aquac Res 48:58545863–58545863. https://doi.org/10.1111/are.13408
Furtado PS, Poersch LH, Wasielesky W (2015) The effect of different alkalinity levels on Litopenaeus vannamei reared with biofloc technology (BFT). Aquacult Int 23:345–358. https://doi.org/10.1007/s10499-014-9819-x
Gaona CAP, Poersch LH, Krummenauer D, Foes GK, Wasielesky WJ (2011) The Effect of Solids Removal on Water Quality, Growth and Survival of Litopenaeus vannamei in a Biofloc Technology Culture System. Internat J Recircul Aquac 12:54–73 http://hdl.handle.net/10919/90645
Gaona CAP, Serra FP, Furtado PS, Poersch LH, Wasielesky WJ (2016a) Biofloc management with different flow rates for solids removal in the Litopenaeus vannamei BFT culture system. Aquacult Int 24:1263–1275. https://doi.org/10.1007/s10499-016-9983-2
Gaona CAP, Serra FP, Furtado PS, Poersch LH, Wasielesky WJ (2016b) Effect of different total suspended solids concentrations on the growth performance of Litopenaeus vannamei in a BFT system. Aquac Eng 72-73:65–69. https://doi.org/10.1016/j.aquaeng.2016.03.004
Gaona CAP, Almeida MS, Viau V, Poersch LH, Wasielesky WJ (2017) Effect of different total suspended solids levels on a Litopenaeus vannamei (Boone, 1931) BFT culture system during biofloc formation. Aquac Res 48:1070–1079. https://doi.org/10.1111/are.12949
Grasshoff N, Ehrhardt M, Kremling K (1983) Methods of sea water analysis, Second edn. Weinheim, Verlag Chemie
Hargreaves JA (2006) Photosynthetic suspended-growth systems in aquaculture. Aquacult Eng 34:344–363. https://doi.org/10.1016/j.aquaeng.2005.08.009
INFOSTAT (2008) InfoStat, software Estadística, v. 2008. Córdoba, Grupo InfoStat, FCA, Universidad Nacional de Córdoba. 334p
Jatobá A, Silva BC, Silva JS, Vieira FN, Mouriño JLP, Seiffert WQ, Toledo TM (2014) Protein levels for Litopenaeus vannamei in semi-intensive and biofloc systems. Aquaculture 432:365–371. https://doi.org/10.1016/j.aquaculture.2014.05.005
Kim E, Yoo S, Ro H-Y, Han H-J, Baek Y-W, Eom I-C, Kim H-M, Kim P, Choi K (2013) Aquatic toxicity assessment of phosphate compounds. Environ Health Toxicol. 28:e2013002. https://doi.org/10.5620/eht.2013.28.e2013002
Krummenauer D, Peixoto S, Cavalli RO, Poersch LH, Wasielesky W (2011) Superintensive culture of white shrimp, Litopenaeus vannamei, in a biofloc technology system in Southern Brazil at different stocking densities. J World Aquac Soc 42:726–733. https://doi.org/10.1111/j.1749-7345.2011.00507
Kumar VS, Pandey PK, Anand T, Bhuvaneswari GR, Dhinakaran A, Kumar S (2018) Biofloc improves water, effluent quality and growth parameters of Penaeus vannamei in an intensive culture system. J Environ Manage 215:206e215–206e215. https://doi.org/10.1016/j.jenvman.2018.03.015
Lara G, Honda M, Poersch LH, Wasielesky WJ (2017) The use of biofilm and different feeding rates in biofloc culture system: the effects in shrimp growth parameters. Aquacult Int 25:1959–1970. https://doi.org/10.1007/s10499-017-0151-0
Li E, Chen L, Zeng C, Chen X, Yu N, Lai Q, Qin JG (2007) Growth, body composition, respiration and ambient ammonia nitrogen tolerance of the juvenile white shrimp, Litopenaeus vannamei, at different salinities. Aquaculture 265:385–390. https://doi.org/10.1016/j.aquaculture.2007.02.018
Liu G, Ye Z, Liu D, Zhu S (2018) Inorganic nitrogen control, growth, and immunophysiological response of Litopenaeus vannamei (Boone, 1931) in a biofloc system and in clear water with or without commercial probiotic. Aquacult Int 26:981–999. https://doi.org/10.1007/s10499-018-0263-1
Maicá PF, de Borba MR, Wasielesky W (2012) Effect of low salinity on microbial floc composition and performance of Litopenaeus vannamei (Boone) juveniles reared in a zero-water-exchange super-intensive system. Aquacult Res 43:361–370. https://doi.org/10.1111/j.1365-2109.2011.02838.x
McGraw W, Teichert-Coddington DR, Rouse DB, Boyd CE (2001) Higher minimum dissolved oxygen concentrations increase penaeid shrimp yields in earthen ponds. Aquaculture 199:311–321. https://doi.org/10.1016/S0044-8486(01)00530-0
Medeiros VA, Fontoura GAT, Dezotti M, Sant'Anna GL (2005) Evaluation of the effect of salinity and the addition of a nutritional supplement in the biological treatment of a complex industrial effluent. In: Congresso Brasileiro de Engenharia Sanitária e Ambiental, 2005, Campo Grande. p.1-15. (in portuguese)
Muhlert ACS, Lima JSG, Machado L, Evangelista RA (2013) Numerical indicators as a tool for assessing the ecological sustainability of marine shrimp farming in Sergipe, Brazil. Interciência 38:615–620 (in portuguese)
Ray AJ, Lotz M (2017) Comparing salinities of 10, 20, and 30‰ in intensive, commercial-scale biofloc shrimp (Litopenaeus vannamei) production systems. Aquaculture 476:29–36. https://doi.org/10.1016/j.aquaculture.2017.03.047
Ray AJ, Lewis BL, Browdy CL, Leffler JW (2010) Suspended solids removal to improve shrimp (Litopenaeus vannamei) production and an evaluation of a plant-based feed in minimal-exchange, superintensive culture systems. Aquaculture 299:89–98. https://doi.org/10.1016/j.aquaculture.2009.11.021
Ray AJ, Dillon KS, Lotz JM (2011) Water quality dynamics and shrimp (Litopenaeus vannamei) production in intensive, mesohaline culture systems with two levels of biofloc management. Aquacult Eng 45:127–136. https://doi.org/10.1016/j.aquaeng.2011.09.001
Rosas C, Cuzon G, Gaxiola G, Le Priol Y, Pascual C, Rossignyol J, Contreras F, Sanchez A, Wormhoudt AV (2001) Metabolism and growth of juveniles of Litopenaeus vannamei: Effect of salinity and dietary carbohydrate levels. J Exp Mar Biol Ecol 259:1–22. https://doi.org/10.1016/S0022-0981(01)00222-2
Santos NBV, Furtado PS, César DE, Wasielesky WJ (2019) Assessment of the nitrification process in a culture of Pacific white shrimp, using artificial substrate and bacterial inoculum in a biofloc technology system (BFT). Cienc Rural 49:e20180306. https://doi.org/10.1590/0103-8478cr20180306
Schveitzer R, Arantes R, Baloi MF, Costódio PFS, Arana LV, Seiffert WQ, Andreatta ER (2013a) Use of artificial substrates in the culture of Litopenaeus vannamei (Biofloc System) at different stocking densities: Effects on microbial activity, water quality and production rates. Aquacult Eng 54:93–103. https://doi.org/10.1016/j.aquaeng.2012.12.003
Schveitzer R, Arantes R, Costódio PFS, do Espírito Santo CM, Arana LV, Seiffert WQ, Andreatta ER (2013b) Effect of different biofloc levels on microbial activity, water quality and performance of Litopenaeus vannamei in a tank system operated with no water exchange. Aquacult Eng 56:59–70. https://doi.org/10.1016/j.aquaeng.2013.04.006
Souza J, Cardozo A, Wasielesky WJ, Abreu PC (2019) Does the biofloc size matter to the nitrification process in Biofloc Technology (BFT) systems? Aquaculture 500:443–450. https://doi.org/10.1016/j.aquaculture.2018.10.051
UNESCO - United Nations Educational, Scientific and Cultural Organization (1983) Chemical methods for use in marine environmental monitoring. Intergovernmental Oceanographic Comission, Paris
von Sperling M (1996) Basic principles of sewage treatment, 2nd edn. Belo Horizonte: Editora UFMG, Minas Gerais (in portuguese)
Wasielesky WJ, Atwood H, Stokes A, Browdy CL (2006) Effect of natural production in a zero exchange suspended microbial floc based super-intensive culture system for white shrimp Litopenaeus vannamei. Aquaculture 258:396–403. https://doi.org/10.1016/j.aquaculture.2006.04.030
Wasielesky WJ, Froes C, Fóes G, Krummenauer D, Lara G, Poersch L (2013) Nursery of Litopenaeus vannamei Reared in a Biofloc System: The Effect of Stocking Densities and Compensatory Growth. J Shellfish Res 32:799–806. https://doi.org/10.2983/035.032.0323
Zhao Z, Xu Q, Luo L, Wang C, Li J, Wang L (2014) Effect of feed C/N ratio promoted bioflocs on water quality and production performance of bottom and filter feeder carp in minimum-water exchanged pond polyculture system. Aquaculture 434:442–448. https://doi.org/10.1016/j.aquaculture.2014.09.006
Zhao Q, Pan L, Ren Q, Hu D (2015) Digital gene expression analysis in hemocytes of the white shrimp Litopenaeus vannamei in response to low salinity stress. Fish Shellfish Immunol 42:400–407. https://doi.org/10.1016/j.fsi.2014.11.020
Acknowledgements
We acknowledge FAPEMIG, CNPq, CAPES, GUABI, and AQUATEC for supporting this research. Kleber Campos Miranda-Filho is a research fellow of CNPq (302286/2018-7).
Author information
Authors and Affiliations
Contributions
All authors contributed equally from the conception and writing of the manuscript. All authors critically revised the manuscript and approved of the final version.
Corresponding author
Ethics declarations
Ethics approval
The study with invertebrate species does not require an ethical approval.
Conflict of interest
The authors declare no competing interests.
Additional information
Handling Editor: Gavin Burnell
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Spelta, A.C.F., Lorenzini, J.P.S., Neves, L.d.C. et al. Dynamics of the water quality parameters in the super-intensive culture of Litopenaeus vannamei in BFT system on artificial brackish water. Aquacult Int 29, 2049–2063 (2021). https://doi.org/10.1007/s10499-021-00734-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-021-00734-1