Abstract
Shrimp farming at low salinity is a trend that will continue to grow globally. Performance of Litopenaeus vannamei postlarvae in the nursery at different salinities with a biofloc technology (BFT) system needs to be explored further, as the nursery is currently used as a transitional stage between the hatchery and grow-out ponds. Hence, this study evaluated the effect of seven salinity levels (2, 4, 8, 12, 16, 25, and 35 ‰) on the performance of L. vannamei postlarvae reared with a BFT system and zero-water exchange at 2000 org/m3. Additionally, this study evaluated the water quality of all salinity treatments. After 28 days of culture, the findings showed that, under biofloc conditions, salinity affected the performance of some variables of water quality in some cases, but only the combination of a high nitrite-N concentration (>4 mg/l) and low salinity (2 and 4 ‰) caused up to 100 % shrimp mortality in the first 2 weeks. In the rest of the treatments (8, 12, 16, 25, and 35 ‰), shrimp survival was >72 %. Shrimp mortality was affected by salinity, especially when it decreased from 35–25–16 to 12 and 8 ‰. The organisms reared at low salinities presented lower final weights and specific growth rate than those reared at higher salinities. An inverse relationship was shown between the ion concentration and the final weight of shrimp.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The expansion of inland saline water farming combined with the salinity tolerance of Litopenaeus vannamei white shrimp has made the latter an attractive aquaculture species in several countries of the American continent (Saoud et al. 2003; Roy et al. 2010). However, research on L. vannamei at low salinity with biofloc technology (BFT) is still limited. It is imperative to conduct studies on this topic for the development of inland saline water farming with BFT systems.
Diseases remain a limiting factor for the aquaculture industry (FAO 2012), and disease outbreaks result not only from the mere presence of pathogens in the system but also from a compromised health status of the cultured animals in combination with suboptimal environmental conditions (Liu and Chen 2004; De Schryver et al. 2012). Therefore, disease prevention and control should not only focus on implementing biosafety measures at the grow-out stage but must be performed with an integral approach involving, among others factors, adequate nutrition and enhancing the immunity of the cultured animals in the nursery system, which is a transitional stage between the hatchery and grow-out ponds.
Additionally, it is necessary to determine the productive performance of the shrimp postlarvae in a wide range of salinities combined with the handling of BFT. The BFT system has been studied on several occasions and reportedly contributes to the maintenance of good water quality in the system and to the nutrition of the cultured animals (Avnimelech 1999). The basic principle of the BFT system is to recycle waste nutrients, including nitrogen, into the microbial biomass that can be used in situ by the cultured animals or be harvested and processed into feed ingredients (De Schryver et al. 2008; Hari et al. 2004, 2006). Heterotrophic microbial aggregates are stimulated to grow by controlling the C/N ratio in the water through the modification of the carbohydrate content in the feed or by the addition of an external carbon source (Avnimelech 1999) such that the bacteria can assimilate the ammonia waste for new biomass production. The BFT systems have been shown to maintain ammonia below toxic levels and to improve the feed nutrient utilization efficiency of the cultured animals (Avnimelech 1999; Hari et al. 2004; Zhao et al. 2012). The BFT system application can also lead to increased growth and survival of shrimp (Emerenciano et al. 2012), and some studies have investigated the immunological potential of the BFT (Xu and Pan 2013, 2014; Kim et al. 2014; Becerra-Dorame et al. 2014). It is widely known that microorganisms, their cell components, and their metabolites can act as immunostimulants that enhance the shrimp innate immune system and provide improved protection against pathogens (Smith et al. 2003; Vazquez et al. 2009).
Some studies have addressed the impact of salinity on growth, survival, molting frequency, oxygen consumption, and immune performance of L. vannamei (Huang 1983; Pante 1990; Ogle et al. 1992; Villarreal et al. 1994; Ponce-Palafox et al. 1997; Xuying et al. 2014). However, the optimal salinity for white shrimp growth is still controversial, and the combined effect of salinity and BFT in L. vannamei postlarvae has rarely been studied.
Culturing L. vannamei at low salinity is a trend that will continue to grow globally (Roy et al. 2010). Thus, the performance of L. vannamei postlarvae in the nursery at different salinities with the BFT system needs to be explored further, since the nursery is currently used as a transitional stage between the hatchery and grow-out ponds.
The objectives of this study were to determine the effects of different levels of salinity on the water quality and performance of L. vannamei postlarvae with the use of a BFT system and zero-water exchange.
Materials and methods
Experimental design and rearing conditions
The effect of seven salinity levels (2, 4, 8, 12, 16, 25, and 35 ‰) on the performance of L. vannamei postlarvae reared with a BFT system and zero-water exchange was evaluated. Three replicate tanks were randomly assigned to each salinity treatment. The experiments were performed in the indoor tank facilities of the Marine Aquaculture Station (EMA), of the Federal University of Rio Grande, Southern Brazil. The plastic tanks (capacity = 50 l, volume used = 40 l) were supplied with diluted marine water at salinities of 2, 4, 8, 12, 16, 25, and 35 ‰ and constant aeration to maintain high dissolved oxygen (DO) concentrations. Each tank was stocked with 80 organisms [2000 orgs/m3, average body weight = 0.016 ± 0.002 g (≈PL’24)]. During this study, there was no water renewal in any of the tanks. Dechlorinated freshwater was added to the tanks to recover the water lost due to evaporation. Shrimp were fed with a commercially formulated feed (40 % protein, 8 % lipid) twice daily (8:00 and 16:00 h). Initially, the feeding rate was established according to Jory et al. (2001), and the feed was adjusted daily a posteriori according to its consumption. The study lasted 28 days.
Acclimation of shrimp and preparation of biofloc
The shrimp used in the experiment were acquired from the Aquatec Ltda., laboratory (Canguaretama, Rio Grande do Norte, Brazil). After the L. vannamei nauplii arrived at the EMA facilities, they were kept in the shrimp hatchery until they reached the post-larval stage (PL’15). A batch of 1800 postlarvae was separated into seven plastic tanks (270 orgs/tank; capacity/tank = 50 l, volume used/tank = 40 l) with final acclimated salinity levels of 2, 4, 8, 12, 16, 25, and 35 ‰. The salinities were adjusted by approximately 3–6 ‰ daily until the salinity in each tank reached the desired level. It took 1, 2, 3, 4, 5, 6, and 8 days to reach the 35, 25, 16, 12, 8, 4, and 2 ‰ levels, respectively. Shrimp were stocked at each salinity treatment at 24 h after acclimation.
Mature bioflocs were used in the study. Eight days before the experiment began, seven tanks (capacity/tank = 400 l, volume used/tank = 200 l) were filled with diluted sea water at salinities of 2, 4, 8, 12, 16, 25, and 35 ‰. To promote biofloc formation, each tank was supplied with an inoculum of 25 % of its total volume, as this water was obtained from a grow-out tank that showed a mature biofloc. During the 8 days, each tank was maintained with constant aeration and a supply of molasses to maintain a C/N ratio of approximately 12:1 to produce a dominant heterotrophic community. The water of each tank was used to fill the three tanks of each salinity treatment.
Shrimp postlarvae performance
During the study, biometrics was performed weekly, weighing 50 shrimp from each experimental tank individually using a digital balance (precision 0.001 g, Sartorius®). Shrimp were returned to their original tanks after weighing. At the study end, all the shrimp that survived in each experimental tank were weighed and counted to evaluate their growth [final weight, specific growth rate (SGR)], survival, feed conversion ratio (FCR), productivity, and final density per treatment. The final weight was calculated using the following formula: Final weight = Final average weight − Initial weight. The SGR (% weight increase/day) was calculated from SGR = 100 × [(ln W f – ln W i)]/t, where W f = mean weight at the end of the period, W i = mean weight at the beginning of the period, and t = time in days of the period (Ricker 1979). The FCR was calculated by dividing the feed supplied (dry weight) by the live weight gain (wet weight) (Hari et al. 2004). Survival (%) was calculated by counting the living organisms and subtracting the stocked organisms in each tank. The survival data were transformed (arcsine of the square root) before analysis (Zar 1996).
Water quality analyses
The photoperiod for the experimental room was natural, and the water temperature was maintained with one heater immersed in each tank. Throughout the experiment, the DO (mg/l), temperature (°C), pH, and salinity (‰) were monitored twice a day (≈08:00 and 16:00 h) utilizing a multi-parameter analyzer (model 556 MPS, YSI Inc., Yellow Springs, OH, USA). The concentrations of total ammonia-N, nitrite-N (mg/l), nitrate-N (mg/l), and phosphate-P (mg/l) were measured weekly according to the methods recommended by UNESCO (1983). Total suspended solids (TSS, mg/l) and alkalinity (mg CaCO3/l) were determined once per week through gravimetry by filtering aliquots of 20 ml of water through GF 50-A glass fiber filters (Strikland and Parsons 1972) and following the methodology proposed by APHA (1998) respectively. Floc volume (ml/l) was evaluated weekly on site using Imhoff cones (Eaton et al. 1995).
At 0, 14, and 28 days of culture, surface water samples were collected from each tank. These samples were analyzed for major ions using standard protocols. Chloride (Cl−) was measured by the argentometric method. Calcium (Ca2+) and magnesium (Mg2+) were measured by the EDTA complexometric titration method (APHA 1998). Potassium (K+) was measured by the tetraphenylborate method (Fries and Getrost 1977), and sulfate (SO4 2−) was measured by the method of barium salt (AWWA 2002). Sodium (Na2+) was measured in a flame spectrophotometer, Micronal B462 (Tecnal-Equipamentos para Laboratórios Ltda., Piracicaba, SP, Brazil).
Statistical analysis
The homoscedasticity of the variances and the normality of the data for the water quality and shrimp performance were first verified. The treatment effects on the water quality parameters were evaluated by one-way repeated measures ANOVA with treatment as the main factor and the sampling date as the repeated measures factor (Gomez and Gomez 1984). The treatment effects on the production parameters were evaluated using one-way ANOVA. Significant differences were tested with Tukey’s multi-comparison test of means. The results were evaluated with a 5 % significance level. The analyses were conducted using Statistica package v6 (StatSoft, Tulsa, OK, USA).
Results
Water quality parameters
The DO, temperature, and pH monitored throughout the study were not significantly different among the salinity treatments (P > 0.05; Table 1). A mean DO = 6.1 ± 0.2 mg/l, temperature = 30.7 ± 0.2 °C, and pH = 8.0 ± 0.0 were maintained.
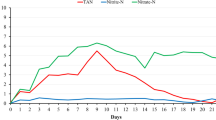
There were significant differences among treatments (P < 0.05; Figs. 1, 2) for the other water quality parameters. The total ammonia-N concentration of the treatments presented significant differences in some sampling periods, but the values of total ammonia-N in any case were higher than 0.5 mg/l, except at the 2 ‰ salinity, at which the values were above 1.0 and 0.5 mg/l at the beginning and at 14 days of culture, respectively (Fig. 1). Additionally, in the treatment of 16 ‰, the total ammonia-N at the beginning was above 0.5 mg/l.
Water quality parameters in the culture of L. vannamei postlarvae under different salinities with the biofloc technology and zero-water exchange. The water quality parameters in the salinity treatments of 2 and 4 ‰ were monitored until the 14th day of culture. Solid arrow to increase the alkalinity, all tanks were supplied with 0.05 g/l/day of sodium bicarbonate (NaHCO3) with a 99.0 % purity (Carbonor®; Furtado et al. 2011, 2014) for three consecutive days after the 2nd and 11th days of culture. Dotted arrow to reduce TSS, all tanks were clarified after the 7th and 20th days of culture (Gaona et al. 2011). Different superscripts within a set of columns for each monitoring period indicate significant differences (P < 0.05)
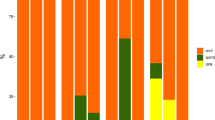
Major ions in the water of the seven salinity treatments. The major ions in the water for the 2 and 4 ‰ treatments were monitored only at the beginning (0 days of culture). Different superscripts within a set of columns for each monitoring period indicate significant differences (0, 14, and 28 days of culture; P < 0.05)
The nitrite-N concentration presented significant differences among the treatments (P < 0.05; Fig. 1). These differences were more evident on the 7th day of culture, where the treatments presented values in the range from 1.5 to 5 mg/l. The highest concentration of nitrite-N remained until the 14th day of culture in the treatments with 2 and 4 ‰, and with a new rise at 21 and 28 days of culture in the treatments of 12 and 8 ‰, respectively. After the 14th day of culture, the rest of the treatments had nitrite-N values lower than 1 mg/l.
The nitrate-N concentration also presented significant differences among the treatments (P < 0.05; Fig. 1). The nitrate-N concentration presented the highest values most of the time with treatments of 25 and 35 ‰. However, all the treatments showed a trend of increasing nitrate-N concentration as the rearing cycle progressed, rising from approximately 0.5–8.0 (day 0) to 38–48 mg/l (day 28). At the beginning of the study, the lowest concentration of nitrate-N (<0.5 mg/l) was presented with the 2 and 4 ‰ treatments.
The phosphate-P concentration fluctuated in the range of <0.0–1.7 mg/l, with significant differences among the treatments (P < 0.05; Fig. 1). At the beginning of the study, the highest phosphate-P concentration was presented at the lower salinities (2, 4, and 8 ‰), but this pattern was not kept after the 21st day of culture.
After the 7th day of culture, alkalinity presented significant differences among treatments (P < 0.05; Fig. 1) in some cases, but without a definite trend. The alkalinity maintained values lower than 50 mg/l at the lower salinities at the beginning of the study.
The TSS concentration presented significant differences among all treatments at the beginning of the study (P < 0.05; Fig. 1). The highest TSS concentration corresponded to the highest salinity, from approximately 370 mg/l at 2 ‰ to 1100 mg/l at 35 ‰.
The floc volume presented significant differences among the treatments (P < 0.05; Fig. 1), with a similar pattern to the TSS concentration, albeit with differences in some cases. Throughout the study, the floc volume in all treatments was higher than 20 ml/l, except on the 14th day of culture. During most of the study, the 25 ‰treatment yielded the highest floc volume.
The concentration of each major ion in the water of all salinity treatments did not present significant differences among the sampling periods (0, 14, and 28 days of culture; P > 0.05; Fig. 2). However, the calcium concentrations in all treatments showed a decreasing trend toward the 14th day of culture and remained in this status until the end of the study. There were no significant differences among some treatments (P > 0.05; Fig. 2) in the concentration of calcium (days 0, 14, and 28), potassium (days 14 and 28), and sulfate (days 0, 14, and 28).
Productive parameters of shrimp postlarvae
The productive parameters of the postlarvae reared in the salinity treatments of 2 and 4 ‰ were not contemplated in the final statistical analysis, as these showed 100 % mortality during the first 2 weeks of culture.
At 28 days of culture, the mean final weight of shrimp postlarvae presented significant differences among the salinity treatments of 8–12 ‰ and 16, 25, and 35 ‰. A similar statistical trend was observed in the survival, SGR, and final density (P < 0.05; Table 2; Fig. 3). The range of the final weight at the end of the study was 0.20 (12 ‰) to 0.75 g (35 ‰). The survival range was 72.9 (8 ‰) to 100 % (16, 25, and 35 ‰). The SGR range at the end of the study was 8.9 (12 ‰) to 13.8 %/day (35 ‰), and the final density was 1457 (8 ‰) to 2000 orgs/m3 (16, 25, and 35 ‰).
Weight performance of L. vannamei postlarvae reared in seven levels of salinity for 28 days with biofloc and zero-water exchange. The average body weight of the 2 and 4 ‰ salinity treatments was monitored until the 11th and 13th days of culture, respectively, and these trials were not included in the final statistical analysis. Different letters for the lines indicate significant differences (P < 0.05)
The FCR showed significant differences between the treatment of 25 ‰ (1.5) and the rest of the treatments (1.7–1.8; P < 0.05; Table 2). The productivity was significantly different among the treatments of 8–12, 16, and 25–35 ‰ (P < 0.05; Table 2), with the lowest productivity in the treatments of 8 (0.11 kg/m3) and 12 ‰ (0.09 kg/m3).
Discussion
The range of mean values of DO, temperature, and pH shown in all treatments applied in this study remained within the levels that were tolerable for growth and survival of shrimp, according to previous reports (van Wyk and Scarpa 1999). However, the rest of the water quality parameters showed significant differences among some salinity treatments; in some treatments, these parameters presented values that can be unsuitable for shrimp culture.
During the first 2 weeks in the 2 and 4 ‰ salinity treatments, the values of total ammonia-N and nitrite-N were the highest (>1 and >4 mg/l, respectively), and the organisms of these treatments depicted a 100 % mortality on the 11th and 13th days of culture, respectively. The highest total ammonia-N concentration recorded in our study was 1.2 mg/l, which was much lower than the concentration at which Mishra et al. (2008) reported a high survival (96.2 %) of shrimp postlarvae when they were exposed to a total ammonia-N concentration of 27 mg/l. Chen and Lin (2003) and Maicá et al. (2012) showed an inverse relationship between salinity and the toxicity of nitrite-N for postlarvae and juveniles of L. vannamei and emphasized that susceptibility to nitrite-N toxicity increased when the animals were exposed to a hypo-osmotic condition. In this study, the nitrite-N concentration in the salinities of 2, 4, 8, and 12 ‰ exceeded the safe range for L. vannamei juveniles cultured at marine salinities (≤1 mg/l) and at ≈2 ‰ (0.45 mg/l) (vanWyk and Scarpa 1999; Gross et al. 2004). Thus, it is probable that the nitrite-N concentration achieved was one of the main factors that contributed to the 100 % mortality in the 2 and 4 ‰ treatments and the 27.1 and 20.0 % mortality in the 8 and 12 ‰ treatments, respectively. At salinities of 2 and 4 ‰, peaks of nitrite-N occurred around the 7th and 14th days, respectively, which agreed with the mortality recorded with those treatments.
In the present study, all the salinity treatments showed a tendency of an increasing nitrate-N concentration according to the progression of the study, suggesting a greater intensity of the nitrification processes. Although the nitrate-N values on the 21st day of culture for the 8 ‰ salinity treatment were similar to those found at the salinities of 25 and 35 ‰; in most of the study, the highest values were found at the higher salinities. The nitrate-N concentration was within the acceptable range (≤60 mg/l; van Wyk and Scarpa 1999), and the increase in nitrate-N concentration with the increase in salinity has been corroborated by other researchers (Decamp et al. 2003; Maicá et al. 2012).
For most of the study, the phosphate-P concentration showed a reducing trend with an increase in salinity, as reported by Maicá et al. (2012). However, this trend was opposite to that reported by Decamp et al. (2003), who noted that the phosphate-P concentration increased as the salinity increased. On the contrary, a trend of an increasing phosphate-P concentration was observed in all treatments as the study progressed, as reported in another work (Maicá et al. 2012).
At the beginning of the study, only in the treatments of higher salinity (25 and 35 ‰) were the values of alkalinity higher than 100 mg/l; in the treatments of lower salinity (2 and 4 ‰), the values were lower than 50 mg/l. For this reason, all the treatment tanks were supplied with sodium bicarbonate for three consecutive days, according to the reports of Furtado et al. (2011, 2014). This procedure was performed after the 2nd and 11th days of culture, as it was determined that it was necessary to maintain an alkalinity concentration above 100 mg CaCO3/l for the biofloc to undergo good development (Ebeling et al. 2006; Furtado et al. 2014).
Since the beginning of the experiment, most treatments had a TSS concentration that exceeded the recommended limit of 500 mg/l for good shrimp performance (Samocha et al. 2007; Gaona et al. 2011). Therefore, to reduce the TSS concentration, all the treatment tanks were clarified, and this procedure was conducted after the 7th and 20th days of culture. After the clarification, only on the 14th day of culture did all treatments have a TSS concentration lower than 400 mg/l. During the entire study, the TSS concentration was higher in the higher salinities, which agreed with that reported by Decamp et al. (2003), Hakanson (2006), and Maicá et al. (2012). These previous studies noted that there was a trend toward increasing suspended particle aggregation and floc size with an increase in salinity, although in our study this was evident only in the floc volume of the salinity treatment of 25 ‰.
Some studies have indicated that shrimp survival in culture systems with seawater, without water exchange and in association with a BFT system, is high (>70 %; Wasielesky et al. 2006; Maicá et al. 2012). However, the survival of marine shrimp reared at low salinity has a wide variation and may be influenced by the species, the age of the organisms, the final salinity at which acclimation occurs, and the ionic composition of the water, among other factors (McGraw et al. 2002; Cheng et al. 2006). In our study, the combination of high nitrite-N concentration and low salinity affected shrimp survival, as previously discussed. Additionally, the survival was affected by salinity, mainly when the salinity decreased from 35–25–16 to 12 and 8 ‰. Similar effects were reported by other authors for both shrimp postlarvae and juveniles, with survival values that range between 2 and 100 % for salinities of 0–36 ‰; i.e., Laramore et al. (2001) with postlarvae at salinities of 4 and 30 ‰ obtained survival rates of 86–100 %, respectively. Jayasankar et al. (2009) reported postlarvae at salinities of 0.0, 0.5, 0.75, and 1.5 ‰ with survival rates of 2, 29, 47, and 85 %, respectively. Decamp et al. (2003) reported that juveniles at salinities of 9, 18, and 36 ‰ reached survival rates of 68, 93, and 94 %, respectively. Finally, Maicá et al. (2012) reported that juveniles at 2, 4, and 25 ‰ reached survival rates of 22, 72, and 97 %, respectively.
Salinity also affected growth of shrimp postlarvae, as the organisms reared at low salinities showed a trend of lower final weight and SGR values than those reared at higher salinities. However, there were no statistically significant differences between the salinity treatments of 8 and 12 ‰, and among the treatments of 16, 25, and 35 ‰. Other authors have reported an inverse relationship between salinity and shrimp growth (Laramore et al. 2001; Cheng et al. 2005; Walker et al. 2009; Maicá et al. 2012).
Salinity refers to the total concentration of all ions in the water, and the major ions contributing to salinity are chloride, sodium, calcium, magnesium, potassium, bicarbonate, and sulfate. Saoud et al. (2003), Davis et al. (2005), and Hou et al. (2012) reported that the ionic composition of water may be a more important limiting factor for shrimp growth and survival than the salinity itself, since the deficiencies in certain ions, such as sodium, calcium, magnesium, and potassium, had a negative impact on the growth and survival of shrimp. In the present work, only the calcium concentration presented a general decreasing trend among sampling periods between day 0 and 14–28 days of culture. However, the rest of the ions were not significantly altered when the postlarvae were reared at 2000 orgs/m3 during 28 days at salinities of 8, 12, 16, 25, and 35 ‰. The impacts of low concentrations of calcium, magnesium, and potassium that prevailed at lower salinities were more evident on the shrimp growth. Although no significant differences were observed among some treatments, there was a trend of an inverse relationship among the ion concentrations and the final weight of shrimp, as reported by other authors (Saoud et al. 2003; Davis et al. 2005; Roy et al. 2007). Additionally, it has been reported that the low growth of shrimp when reared at low salinities is due to the possibility that the organisms use the dietary protein as a source of amino acids not only for weight gain (protein retention), but also for maintenance of the osmotic pressure (Shiau 1998; Rosas et al. 2001). The lack of significant differences among some treatments could be because the shrimp were reared in a system without water exchange in association with bioflocs, an environment of high natural productivity with many nutritional benefits (Wasielesky et al. 2006).
Shrimp maintained in their isosmotic point at a salinity of 25 ‰ (Castille and Lawrence 1981) had the lowest FCR. For the rest of the salinity treatments, the FCR did not present a direct relationship with the salinity, as reported by Decamp et al. (2003).
In summary, salinity affected the performance of some variables of water quality in some cases during the culture of L. vannamei postlarvae in indoor nursery tanks under biofloc conditions, However, only the combination of high nitrite-N concentration (>4 mg/l) and low salinity (2 and 4 ‰) affected significantly shrimp survival. Additionally, survival was affected by salinity, mainly when the salinity decreased from 35–25–16 to 12 and 8 ‰, although high shrimp survival (>72 %) occurred in all salinity treatments. Salinity also affected growth of shrimp postlarvae, as the organisms reared at low salinities showed a trend of lower final weight and SGR than those reared in the higher salinities. Only the calcium concentration presented a general decreasing trend among the sampling periods of 0 day to 14–28 days of culture. The rest of the major ions were not significantly altered when the postlarvae were reared at 2000 orgs/m3 during 28 days at salinities of 8, 12, 16, 25, and 35 ‰, and an inverse relationship existed among the ion concentrations and the final weight of shrimp.
References
APHA (American Public Health Association) (1998) Standard methods for the examination of water and wastewater, 20th edn. American Water Works Association, Washington, DC
Avnimelech Y (1999) Carbon/nitrogen ratio as a control element in aquaculture systems. Aquaculture 176:227–235
AWWA (American Water Works Association) (2002) Simplified procedures for water examination, 5th edn. (M12). American Water Works Association, Denver, CO
Becerra-Dorame MJ, Martinez-Cordova LR, Martínez-Porchas M, Hernández-López J, Lopez-Elías JA, Mendoza-Cano F (2014) Effect of using autotrophic and heterotrophic microbial-based-systems for the pre-grown of Litopenaeus vannamei, on the production performance and selected haemolymph parameters. Aquac Res 45:944–948
Castille FL, Lawrence AL (1981) The effect of salinity in the osmotic, sodium, and chloride concentrations in the haemolymph of euryhaline shrimp of the genus Penaeus. Comp Biochem Physiol 106B:293–296
Chen JC, Lin YC (2003) Acute toxicity of nitrite on Litopenaeus vannamei (Boone) juveniles at different salinity levels. Aquaculture 224:193–201
Cheng KM, Hu CQ, Liu YN, Zheng SX, Qi XJ (2005) Dietary magnesium requirement and physiological responses of marine shrimp Litopenaeus vannamei reared in low salinity water. Aquac Nutr 11:385–393
Cheng KM, Hu CQ, Liu YN, Zheng SX, Qi XJ (2006) Effects of dietary calcium, phosphorus and calcium/phosphorus ratio on the growth and tissue mineralization of Litopenaeus vannamei reared in low-salinity water. Aquaculture 251:472–483
Davis DA, Boyd CE, Rouse DB, Saoud IP (2005) Effects of potassium, magnesium, and age on growth and survival of Litopenaeus vannamei post-larvae reared in inland low salinity well waters in west Alabama. J World Aquac Soc 36:403–406
De Schryver P, Crab R, Defoirdt T, Boon N, Verstraete W (2008) The basics of bio-flocs technology: the added value for aquaculture. Aquaculture 277:125–137
De Schryver P, Defoirdt T, Boon N, Verstraete W, Bossier P (2012) Managing the microbiota in aquaculture systems for disease prevention and control. In: Austin B (ed) Infectious disease in aquaculture: prevention and control. Woodhead Pub Ltd, Cambridge
Decamp O, Cody J, Conquest L, Delanoy G, Tacon AGJ (2003) Effect of salinity on natural community and production of Litopenaeus vannamei (Boone) within experimental zero-water exchange culture systems. Aquac Res 34:345–355
Eaton AD, Clesceri LS, Greenberg AE (eds) (1995) Standard methods for the examination of water and waste water, 10th edn. American Published Health Association, Washington
Ebeling JM, Timmons MB, Bisogni JJ (2006) Engineering analysis of the stoichiometry of photoautotrophic, autotrophic, and heterotrophic control of ammonia-nitrogen in aquaculture production systems. Aquaculture 257:346–358
Emerenciano M, Cuzon G, Goguenheim J, Gaxiola G, AQUACOP (2012) Floc contribution on spawning performance of blue shrimp Litopenaeus stylirostris. Aquac Res 44:75–85
FAO (Food and Agriculture Organization) (2012) The state of world fisheries and aquaculture. Fisheries and Aquaculture Department, FAO, Rome
Fries J, Getrost H (1977) Organic reagents for trace analysis. In: Merck E (ed) Tetraphenylborate method. MERCK, Darmstadt
Furtado PS, Poersch LH, Wasielesky W (2011) Effect of calcium hydroxide, carbonate and sodium bicarbonate on water quality and zootechnical performance of shrimp Litopenaeus vannamei reared in bio-flocs technology (BFT) systems. Aquaculture 32:130–135
Furtado PS, Gaona CAP, Poersch LH, Wasielesky W (2014) Application of different doses of calcium hydroxide in the farming shrimp Litopenaeus vannamei with the biofloc technology (BFT). Aquac Int 22:1009–1023
Gaona CAP, Poersch LH, Krummenauer D, Fóes GK, Wasielesky W (2011) The effect of solids removal on water quality, growth and survival of Litopenaeus vannamei in a biofloc technology culture system. Int J Rec Aquac 12:54–73
Gomez KA, Gomez AA (1984) Statistical procedures for agricultural research, 2nd edn. Wiley, New York
Gross A, Abutbul S, Zilberg D (2004) Acute and chronic effects of nitrite on white shrimp Litopenaeus vannamei cultured in low salinity brackish water. J World Aquac Soc 35:315–321
Hakanson L (2006) The relationship between salinity, suspended particulate matter and water clarity in aquatic systems. Ecol Res 21:75–90
Hari B, Madhusoodana K, Varghese JT, Schrama JW, Verdegem MCJ (2004) Effects of carbohydrate addition on production in extensive shrimp culture systems. Aquaculture 241:179–194
Hari B, Kurup BM, Varghese JT, Schrama JW, Verdegem MCJ (2006) The effect of carbohydrate addition on water quality and the nitrogen budget in extensive shrimp culture systems. Aquaculture 252:248–263
Hou C, Wang F, Dong S, Zhu Y, Yu T (2012) Effects of constant Ca2+ concentration in salinity fluctuations on growth and energy budget of juvenile Litopenaeus vannamei. Aquac Int 20:177–188
Huang HJ (1983) Factors affecting the successful culture of Penaeus stylirostris and Penaeus vannamei at an estuarine power plant site: temperature, salinity, inherent growth variability, damselfly nymph predation, population density and distribution, and polyculture. Ph.D. Thesis, Texas A & M University, College Station, TX, USA
Jayasankar V, Jasmani S, Nomura T, Nohara S, Huong DTT, Wilder MN (2009) Low salinity rearing of the Pacific White Shrimp Litopenaeus vannamei: acclimation, survival and growth of postlarvae and juveniles. Jpn Agric Res Q 43:345–350
Jory DE, Cabrera TR, Dugger DM, Fegan D, Lee PG, Lawrence AL, Jackson CJ, McIntosh RP, Castañeda J (2001) A global review of shrimp feed management: status and perspectives. In: Browdy CL, Jory DE (eds) The new wave: proceedings of the special session on sustainable shrimp culture, aquaculture. The World Aquaculture Society, Baton Rouge
Kim SK, Jang IK, Seo HC, Cho YR, Samocha T, Pang Z (2014) Effect of bioflocs on growth and immune activity of Pacific white shrimp, Litopenaeus vannamei postlarvae. Aquac Res 45:362–371
Laramore S, Laramore CR, Scarpa J (2001) Effect of low salinity on growth and survival of postlarvae and juvenile Litopenaeus vannamei. J World Aquac Soc 32:385–392
Liu CH, Chen JC (2004) Effect of ammonia on the immune response of white shrimp Litopenaeus vannamei and its susceptibility to Vibrio alginolyticus. Fish Shellfish Immunol 16:321–340
Maicá PF, Borba MR, Wasielesky W (2012) Effect of low salinity on microbial floc composition and performance of Litopenaeus vannamei (Boone) juveniles reared in a zero-water exchange super-intensive system. Aquac Res 43:361–370
McGraw WJ, Davis DA, Teichert-Coddington D, Rouse DB (2002) Acclimation of Litopenaeus vannamei postlarvae to low salinity: influence of age, salinity endpoint and rate of salinity reduction. J World Aquac Soc 33:78–84
Mishra JK, Samocha TM, Patnaik S, Speed M, Gandy RL, Ali AM (2008) Performance of an intensive nursery system for the Pacific white shrimp, Litopenaeus vannamei, under limited discharge condition. Aquac Eng 38:2–15
Ogle JT, Beaugez K, Lotz L (1992) Effects of salinity on survival and growth of postlarval Penaeus vannamei. Gulf Res Rep 8:415–421
Pante MJR (1990) Influence of environmental stress on the heritability of molting frequency and growth rate of the penaeid shrimp, Penaeus vannamei. M.Sc. Thesis, University of Houston-Clear lake, Houston, TX, USA
Ponce-Palafox JT, Martinez-Palacios CA, Ross LG (1997) The effects of salinity and temperature on the growth and survival rates of juvenile white shrimp, Penaeus vannamei, Boone, 1931. Aquaculture 157:107–115
Ricker WE (1979) Growth rates and models. In: Hoar WS, Randall DJ, Brett JR (eds) Fish Physiology, Bioenergetics and Growth, vol VIII. Academic Press, New York
Rosas C, Cuzon G, Gaxiola G, Priol YL, Pascual C, Rossignyol J, Contreras F, Sanchez A, Wormhoudt AV (2001) Metabolism and growth of juveniles of Litopenaeus vannamei: effect of salinity and dietary carbohydrate levels. J Exp Mar Biol Ecol 259:1–22
Roy LA, Davis DA, Saoud IP, Henry RP (2007) Effects of varying levels of aqueous potassium and magnesium on survival, growth, and respiration of the Pacific white shrimp, Litopenaeus vannamei, reared in low salinity waters. Aquaculture 262:461–469
Roy LA, Davia DA, Saoud P, Boyd CA, Pine HJ, Boyd CE (2010) Farming culture in inland low salinity waters. Rev Aquac 2:191–208
Samocha TM, Patnaik S, Speed M, Ali AM, Burger JM, Almeida RV, Ayub Z, Harisanto M, Horowitz A, Brook DL (2007) Use of molasses as carbon source in limited discharge nursery and grow-out systems for Litopenaeus vannamei. Aquac Eng 36:184–191
Saoud IP, Davis DA, Rouse DB (2003) Suitability studies of inland well waters for Litopenaeus vannamei culture. Aquaculture 217:373–383
Shiau SY (1998) Nutrient requirements of penaeid shrimp. Aquaculture 164:77–93
Smith VJ, Brown JH, Hauton C (2003) Immunostimulation in crustaceans: Does it really protect against infection? Fish Shellfish Immunol 15:71–90
Strikland JDH, Parsons TH (1972) A practical handbook of seawater analysis. Fish Research Board of Canada Bulletin, Ottawa
UNESCO (United Nations Educational, Scientific and Cultural Organization) (1983) Chemical methods for use in marine environmental monitoring. Manual and guides, Intergovernmental Oceanographic Commission, Paris, France
van Wyk P, Scarpa J (1999) Water quality requirements and management. In: VanWyk P (ed) Farming Marine Shrimp in Recirculating Freshwater Systems. Florida Department of Agriculture and Consumer Services, Tallahassee
Vazquez L, Alpuche J, Maldonado G, Agundis C, Pereyra-morales A, Zenteno E (2009) Immunity mechanisms in crustaceans. Innate Immun 15:179–188
Villarreal H, Hinojosa P, Naranjo J (1994) Effect of temperature and salinity on the oxygen consumption of laboratory produced Penaeus vannamei postlarvae. Comp Biochem Physiol 108A:331–336
Walker SJ, Neill WH, Lawrence AL, Gatlin DM (2009) Effect of salinity and body weight on ecophysiological performance of the Pacific white shrimp (Litopenaeus vannamei). J Exp Mar Biol Ecol 380:119–124
Wasielesky W, Atwood H, Stokes A, Browdy CL (2006) Effect of natural production in a zero exchange suspended microbial floc based super-intensive culture system for white shrimp Litopenaeus vannamei. Aquaculture 258:396–403
Xu WJ, Pan LQ (2013) Enhancement of immune response and antioxidant status of Litopenaeus vannamei juvenile in biofloc-based culture tanks manipulating high C/N ratio of feed input. Aquaculture 412–413:117–124
Xu WJ, Pan LQ (2014) Evaluation of dietary protein level on selected parameters of immune and antioxidant systems, and growth performance of juvenile Litopenaeus vannamei reared in zero-water exchange biofloc-based culture tanks. Aquaculture 426–427:181–188
Xuying J, Sen D, Fang W, Shuanglin D (2014) A comparative study on the nonspecific immunity of juvenile Litopenaeus vannamei ever inhabiting freshwater and seawater. J Ocean Univ China 13:472–478
Zar JH (1996) Biostatistical Analysis, 3rd edn. Prentice Hall, New Jersey
Zhao P, Huang J, Wang XH, Song XL, Yang CH, Zhang XG (2012) The application of bioflocs technology in high-intensive, zero exchange farming systems of Marsupenaeus japonicus. Aquaculture 354–355:97–106
Acknowledgments
The authors are grateful for the financial support provided by the National Council for Scientific and Technological Development (CNPq), Ministry of Fishery and Aquaculture (MPA) and Coordination for the Improvement of Higher Level Personnel (CAPES). W. Wasielesky Jr. is research fellow of CNPq. This work is part of the post-doctoral activities of H. M. Esparza-Leal, Ph.D., at the Universidad Federal do Rio Grande (FURG, Rio Grande do Sul, Brazil), who received post-doc fellowships from CONACYT (Grant 203621) and Instituto Politécnico Nacional (leave of absence COP/COTEBAL/RR-09/14). The authors thank Sandro Fabres for assistance with the water quality analysis during the experimental work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Esparza-Leal, H.M., Amaral Xavier, J.A. & Wasielesky, W. Performance of Litopenaeus vannamei postlarvae reared in indoor nursery tanks under biofloc conditions at different salinities and zero-water exchange. Aquacult Int 24, 1435–1447 (2016). https://doi.org/10.1007/s10499-016-0001-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-016-0001-5