Abstract
One of the difficulties to rear Anguilla japonica larvae is the frequent occurrence of notochord deformities. We tested the effect of salinity on the occurrence of the deformities, because we have been using 50 % diluted seawater (50 % SW) for glass eel production, on the basis of the fact that intermediate salinity saves energy due to lower cost for osmoregulation and contributes higher survival and growth rates. We reared 6-day-old larvae in 50 and 100 % SW for 85 days and observed their morphology. The occurrence rate of deformed larvae, including kyphosis and scoliosis, was significantly higher in 50 % SW (35.8 %) than in 100 % SW (25.4 %), while survival rate was significantly higher in 50 % SW (69.8 %) than in 100 % SW (32.3 %) and growth in 50 % SW (mean body depth: 7.9 ± 5.3 mm) was better than in 100 % SW (6.8 ± 4.6 mm). We speculate that the most of severely deformed larvae could not survive in the tougher condition of 100 % SW, showing the lower occurrence of deformed larvae. Eventually, the yield of normal larvae after 85 days rearing was 1.9-fold higher in 50 % SW than in 100 % SW, implying that the advantage of 50 % SW for rearing eel larvae outweighs the risk of deformities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The need for the artificial production of glass eels of Japanese eel Anguilla japonica as seedlings for aquaculture has been increasing in recent years, because the stock of Japanese eel has been declining over the past decades (Tsukamoto et al. 2009). Although the technology for the glass eel production at a laboratory level has established in 2002 (Tanaka et al. 2003), commercial-scale production of glass eels has not been practical to date, because of the high mortality rate of eel larvae (leptocephali) (Okamura et al. 2013a).
One of the major problems in rearing eel larvae is the high proportion of fish with notochord abnormalities, such as kyphosis, lordosis and scoliosis (Okamura et al. 2011). In some instances, over 50 % of reared larvae had any of these deformities. Severely deformed larvae could not swim normally, and after metamorphosis, these deformed glass eels die due to their inability to ingest food (Okamura et al. 2011). To date, the causative factors of these deformities remain unknown. Probably, these deformities are due to artificial conditions such as water temperature, water flow, salinity, fish density, restricted space, artificial foods or unknown parameters, which may be different to some extent from the natural condition. Especially, we have been practically using an intermediate salinity (50 % SW) of 17–18 practical salinity unit (psu) to rear eel larvae, because such water condition saves energy due to lower cost for osmoregulation and appears to contribute the higher growth and survival of eel larvae (Okamura et al. 2009b; Lee et al. 2013). Even in 50 % SW, eel larvae can also metamorphose into glass eels (Okamura et al. 2009 b). However, the effect of low-salinity water on the occurrence of such deformities is not well validated.
In this study, we investigated the effect of salinity of rearing water on the occurrence of notochord abnormalities in eel larvae in captivity. We compared the growth, survival and occurrence rates of the deformities in larvae reared in 50 % SW and full-strength seawater (100 % SW). On the basis of these data, we evaluate the benefits and disadvantages of using 50 % SW in rearing eel larvae.
Materials and methods
Fish
A. japonica larvae were obtained as described (Kagawa et al. 2005; Ohta et al. 1997; Tanaka et al. 2001). Briefly, fertilized eggs were obtained from parents matured artificially, females by repeated injections of pituitary extracts of chum salmon Oncorhynchus keta and males by repeated injections of human chorionic gonadotropin. Hatched larvae were maintained in a 180-L polycarbonate tank supplied with 100 % SW (34.5 psu) at 25 °C for 6 days until the completion of the mouth opening.
Experimental conditions
To examine the effect of salinity on the occurrence of deformities, we reared larvae in a 10-L planktonkreisel tank (Okamura et al. 2009a) with different salinity waters for 85 days. We transferred larvae of 6 days post-hatch (dph) to tanks containing 50 % (17.5 psu) and 100 % SW (35 psu) at 23 °C. A solution of 50 % SW was prepared by mixing the ground SW with well water. The experiment was conducted in duplicate with each tank containing 400 larvae until 90 dph. Rearing conditions, such as the rate of water supply and water circulation speed, were set according to Okamura et al. (2009a). Feeding operation was carried out five times a day at 09:00, 11:00, 13:00, 15:00 and 17:00. The diet made from shark Squalus acanthias egg, Antarctic krill extract, soybean peptide and some vitamins was used as described in Okamura et al. (2013b). Dead larvae were counted daily and removed from the tanks.
Sampling and measurements
About 30 larvae from each tank were randomly sampled each at 30 and 90 dph and fixed in 5 % formalin—25 % SW solution. One day after fixation, all specimens were digitally photographed (Nikon, D80, 50 mm macro lens). Body depth (BD, mm) was measured to the nearest 0.01 mm on the digitalized images using the public domain NIH Image program (developed at the US National Institutes of Health and available from http://rsb.info.nih.gov/nih-image/index.html, accessed February 1, 2015). The BD is the greatest dimension from lateral view. We did not use total length (TL) for the present analyses, because we assumed that the TL of deformed larvae was often underestimated due to their curved bodies.
Definition of notochord deformities
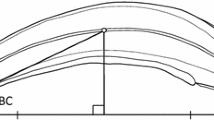
Two types of deformities were observed in the present study (Fig. 1), and the type of deformity was according to Okamura et al. (2011) as follows. Scoliosis is an abnormal lateral zig-zag curvature of the notochord column (Fig. 2b). The posterior part of most scoliotic larvae showed heavy shrinkage axially, whereas the anterior part showed relatively normal morphology. Kyphosis is a dorsal (Λ-shaped) curvature of the notochord column. Typical kyphotic larvae had crescent-shaped bodies, with the curvature often easy to stretch from the head to the tail along the notochord (Fig. 1c). As a criterion to predict the strength of kyphosis, we measured the degree of body curvature (DBC) (Fig. 1c) using the NIH Image. The DBC is the angle formed by the line connecting tips of the upper jaw and the caudal fin and the line connecting the tip of the upper jaw and the midpoint of the notochord. Here, we defined the larvae with a DBC ≥10 were the kyphotic larvae, whereas those with a DBC <10 were the normal larvae, because all larvae with DBC of ≥10 could not swim normally (Okamura et al. 2011).
Typical morphology of Anguilla japonica larvae. a Normal larvae; b deformed larvae with scoliosis; c deformed larvae with kyphosis and the index of degree of body curvature (DBC). The DBC is the angle formed by the line connecting tips of the upper jaw and the caudal fin and the line connecting the tip of the upper jaw and the midpoint of the notochord. Bar = 10 mm
Statistical analyses
Survival rate between the groups was analyzed using the χ 2 test. Mean BW and DBC between the groups were analyzed by the Student t test. Differences in the proportion of occurrence rate of deformities and the yield of respective larvae were tested by the χ 2 test. All analyses were performed using Excel Stat 2008 (SSRI, Tokyo, Japan); p < 0.05 was considered significant.
Results
Survival and growth
The survival and growth rates of larvae were apparently higher in 50 % SW than in 100 % SW (Figs. 2, 3). The survival rates of two tanks in the 50 % SW group were gradually decreased during the experiment and eventually both reached around 70 % at the end of the experiment (Fig. 2). Meanwhile, those in the 100 % SW group rapidly decreased soon after the beginning of feeding, but after 7 weeks they were stably maintained at a 24–39 % level until 90 dph (Fig. 2), and these values were significantly lower than those in the 50 % SW (χ 2 test, p < 0.05). The mean BD of the larvae reared in 50 % SW (3.62 ± 1.64 mm, n = 120) was significantly larger than in 100 % SW (3.12 ± 1.01 mm, n = 114) at 90 dph (Fig. 3) (t test, p < 0.05), which agreed well with our previous results (Okamura et al. 2009b).
Morphology
Two types of notochord deformities occurred in the both groups (Fig. 1b, c). Kyphotic larvae more often occurred than scoliotic larvae, but these deformities were often combined (Table 1). Scoliosis including a combination of kyphosis and scoliosis was rare with a ratio of <2.8 % of survived larvae in 100 % SW, whereas these larvae significantly more often occurred in 50 % SW (χ 2 test, p < 0.05) (Table 1). Proportion of normal larvae in each group was significantly greater in 100 % SW (78.7 %) than in 50 % SW (64.7 %) (χ 2 test, p < 0.05) (Table 1). Mean value of DBC (the strength of body curvature) also showed a significant difference between the two groups (t test, p < 0.05), the value in 50 % SW (30 dph: 1.36 ± 3.38, n = 76; 90 dph: 7.91 ± 5.29, n = 120) being always greater than in 100 % SW (30 dph: 0.10 ± 3.62, n = 66; 90 dph: 6.75 ± 4.6, n = 114) at 30 and 90 dph (Fig. 4). However, the strength of body curvature increased considerably with age in the both groups (Fig. 4), indicating that kyphosis was exacerbated by growth regardless of salinity. In spite of the high rate of deformities, the yield of normal larvae in 50 % SW was 1.9-fold larger than 100 % SW (Table 1).
Discussion
The present study showed that the proportion of deformed A. japonica larvae apparently increases when they were reared in 50 % SW. However, the survival and growth of larvae are improved by rearing in 50 % SW, and consequently, the yield of normal larvae increased as compared with 100 % SW.
There are two possible reasons why deformed larvae increased in 50 % SW. One possibility is that low salinity directly influences the development of A. japonica larvae. In some instance, unfavorable salinity has been reported to affect the occurrence of spinal deformities in other fish species (Cook et al. 2005; Ottesen and Bolla 1998). Bonefish leptocephali reared during metamorphosis in artificial seawater, with a calcium ion concentration under 2 mM, frequently showed arched-body deformation, similar to kyphosis (Pfeiler 1997), suggesting that the lower Ca2+ in 50 % SW, about 5 mM, may be at least partly responsible for notochord deformities in eel leptocephali. During the pre-larval stage (0–6 dph) of A. japonica, low salinity was found to result in frequent lower jaw deformity and pericardial edema, but did not affect the occurrence of notochord deformity (Okamoto et al. 2009).
Another possibility is that salinity affects the survival of deformed larvae. The low occurrence rate of deformed larvae in 100 % SW in this study is possibly due to the fragility of deformed larvae. The growth and survival of eel larvae reared in the intermediate salinity are apparently advantageous. Lee et al. (2013) reported that the tissue osmolality of reared A. japonica larvae ranged from 360 to 540 mOsm/kg·H2O, which was considerably lower than full-strength seawater osmolality (ca. 1050 mOsm/kg·H2O), and this was equivalent to that of 50 % or more diluted sea water. Therefore, such advantage in the survival and growth of leptocephali is derived from the low cost of osmoregulation under near-isoosmotic conditions. In this sense, full-strength seawater may be somewhat strict condition for reared eel larvae. Furthermore, severely deformed larvae cannot swim normally, and they also cannot eat diet efficiently (Okamura et al. 2011). Probably, most of such deformed larvae in 100 % SW would die due to malnutrition. In contrast, it is possible that larvae in 50 % SW even with deformities could survive thanks to lower cost for osmoregulation. If this is so, salinity may not be a major cause of notochord deformity in eel larvae. In fact, the strength of body curvature increased considerably with age regardless of salinity.
In conclusion, the present study showed that using the intermediate-salinity water for rearing eel leptocephali has advantages over the full-strength seawater. Although a number of notochord deformities occurred in the larvae reared in 50 % SW, the survival and growth rates of larvae in 50 % SW were considerably higher than in 100 % SW, resulting in a higher yield of normal larvae in 50 % SW. It is possible that salinity is not a major cause of notochord deformity in eel larvae. Thus, other possible factors influencing the deformity such as temperature, water flow or nutrition should be addressed.
References
Cook MA, Guthrie KM, Rust MB, Plesha PD (2005) Effects of salinity and temperature during incubation on hatching and development of lingcod, Ophiodon elongatus Girard, embryos. Aquacult Res 36:1298–1303
Kagawa H, Tanaka H, Ohta H, Unuma T, Nomura K (2005) The first success of glass eel production in the world: basic biology on fish reproduction advances new applied technology in aquaculture. Fish Physiol Biochem 31:193–199
Lee KM, Yamada Y, Okamura A, Tsukamoto K, Kaneko T (2013) Hyposmoregulatory ability and ion- and water-regulatory mechanisms during the leptocephalus stages of Japanese eel Anguilla japonica. Fish Sci 79:77–86
Ohta H, Kagawa H, Tanaka H, Okuzawa K, Iinuma N, Hirose K (1997) Artificial induction of maturation and fertilization in the Japanese eel, Anguilla japonica. Fish Physiol Biochem 17:163–169
Okamoto T, Kurokawa T, Gen K, Murashita K, Nomura K, Kim SK, Matsubara H, Ohta H, Tanaka H (2009) Influence of salinity on morphological deformities in cultured larvae of Japanese eel, Anguilla japonica, at completion of yolk resorption. Aquaculture 293:113–118
Okamura A, Yamada Y, Horita T, Horie N, Mikawa N, Utoh T, Tanaka S, Tsukamoto K (2009a) Rearing eel leptocephali (Anguilla japonica Temminck & Schlegel) in a planktonkreisel. Aquacult Res 40:509–512
Okamura A, Yamada Y, Mikawa N, Horie N, Utoh T, Kaneko T, Tanaka S, Tsukamoto K (2009b) Growth and survival of eel leptocephali (Anguilla japonica) in low- salinity water. Aquaculture 296:367–372
Okamura A, Yamada Y, Mikawa N, Horie N, Tanaka S, Tsukamoto K (2011) Notochord deformities in reared Japanese eel Anguilla japonica larvae. Aquaculture 317:37–41
Okamura A, Horie N, Mikawa N, Yamada Y, Tsukamoto K (2013a) Recent advances in artificial production of glass eels for conservation of anguillid eel populations. Ecol Freshw Fish 23:95–110
Okamura A, Yamada Y, Horie N, Mikawa N, Tanaka S, Kobayashi H, Tsukamoto K (2013b) Hen egg yolk and skinned krill as possible foods for rearing leptocephalus larvae of Anguilla japonica Temminck & Schlegel. Aquacult Res 44:1531–1538
Ottesen OH, Bolla S (1998) Combined effects of temperature and salinity on development and survival of Atlantic halibut larvae. Aquacult Int 6:103–120
Pfeiler E (1997) Effect of Ca2+ on survival and development of metamorphosing bonefish (Albula sp.) leptocephali. Mar Biol 127:571–578
Tanaka H, Kagawa H, Ohta H (2001) Production of leptocephali of Japanese eel (Anguilla japonica) in captivity. Aquaculture 201:51–60
Tanaka H, Kagawa H, Ohta H, Unuma T, Nomura K (2003) The first production of glass eel in captivity: fish reproductive physiology facilitates great progress in aquaculture. Fish Physiol Biochem 28:493–497
Tsukamoto K, Aoyama J, Miller MJ (2009) Present status of the Japanese eel: resources and recent research. Am Fish Soc Symp 58:21–35
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Okamura, A., Yamada, Y., Mikawa, N. et al. Effect of salinity on occurrence of notochord deformities in Japanese eel Anguilla japonica larvae. Aquacult Int 24, 549–555 (2016). https://doi.org/10.1007/s10499-015-9944-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-015-9944-1