Abstract
Research efforts to achieve the production of artificial seedlings of the Japanese eel Anguilla japonica have progressed in recent decades. However, morphological deformities have been frequently observed in reared leptocephali and glass eels. We examined the effect of water current velocities (5.7–8.3 cm/s) on the body size and morphology of reared leptocephali and metamorphosed glass eels. As the current velocity increased, the size of leptocephali became smaller and the occurrence rate of notochord curvature increased. However, even in low velocities, water current had a long-term negative impact on their morphology. Sixty-five percent of metamorphosed glass eels had one of the eight types of vertebral deformities: compression, luxation, fusion, brachyspina, modification, lordosis, kyphosis, or scoliosis. Although their occurrence rate was unrelated to current velocity, there was a tendency for some deformities to be localised in a certain area of the vertebral column. In particular, compression frequently occurred in caudal vertebrae in faster currents. Most vertebral deformities began before the completion of metamorphosis. Therefore, appropriate management of the water current during the leptocephalus stage is important for establishing mass production of morphologically normal glass eels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Japanese eel Anguilla japonica is an important fisheries resource that has been used as traditional food in Japan for a long period [1–3]. Wild-caught glass eels that recruit to Japan and other parts of East Asia in the winter season are raised in aquaculture ponds in several countries [4–6]. The present eel aquaculture system totally depends on the capture of wild glass eels, which results in an unstable supply. It has been suggested that the overexploitation of glass eels is one possible reason for the decline in Japanese eel resources over the past few decades [1]. This species is now on the Red List by the Ministry of the Environment of Japan (http://www.env.go.jp/press/16264.html, assessed 25 April 2016) and the IUCN Red List of Threatened Species [7]. Consequently, there has been an increased focus on research to establish methods for the complete aquaculture of eggs to adults. The mass production of artificial glass eel seedlings has been expected to reduce wild glass eel catches and to stabilize the seedling supply to the eel farming industry. Recently, research on these methods has progressed considerably with the completion of the life cycle in the laboratory and the production of the next generation [8, 9]. However, a commercial-scale production of artificial glass eels has not been considered practical yet.
A major problem in producing glass eels in captivity is the high mortality of eel larvae, called leptocephali, partly because a high proportion of larvae and glass eels have morphological deformities [10, 11], which are uncommon in wild-caught individuals. Both notochord deformities of leptocephali and vertebral deformities of glass eels cause anomalous swimming behavior, feeding failure, and eventually death for some [11]. The morphology of leptocephali drastically transforms during metamorphosis [12], but a more than 80 % larval deformity rate carries over to glass eel deformities [11]. Thus, if glass eels survive and start growing as yellow eels, their morphological deformities would have a negative effect on commercialization efforts.
A few studies have been conducted to understand the causes of morphological deformities in cultured eel larvae. The influence of environmental factors, such as water temperature and salinity, on the occurrence of morphological deformities during the leptocephalus stage was evaluated [13–15] in conjunction with optimal conditions [16–19]. However, the deformities of both late-stage leptocephali and glass eels have not been examined to date. The influence of other environmental factors related to hydrodynamic flow conditions on the occurrence of morphological deformities has been evaluated in other fishes [20, 21], but not in eels. Currently, tank water flow is an important technique for rearing eel larvae [22]. The planktonkreisel, which was originally developed for the maintenance of zooplankton [23], was designed to have a fast revolving current to keep reared eel leptocephali suspended in the water column and prevent fouling of the tank walls [22]. However, wild leptocephali never encounter a fast water current in the ocean, and this may have an influence on the morphology of reared eel leptocephali because they have an undeveloped bone structure, only small amounts of muscle, and a long larval period of almost half a year.
The aim of the present study was to examine the effect of various water current velocities on the body size, morphology, and occurrence of deformities of Japanese eel leptocephali and glass eels reared in captivity. Further, we described eight types of vertebral deformities in glass eels and analyzed the influence of current velocity on their occurrence. These data will be useful for improving artificial seedling production techniques for this species.
Materials and methods
Obtaining and rearing larvae
Induced spawning of adult eels and larval rearing were performed according to previously established methods [22, 24]. Eggs were obtained from artificially matured parent eels on 27 April 2013 and 4 May 2013 and allowed to hatch in a 50-L polycarbonate tank in seawater (35 psu) at 25 °C. The hatched larvae were then kept in a 180-L polycarbonate cylindrical tank with a gentle upwelling flow produced by supplying water from the bottom of the tank (2 L/min) under the same temperature and salinity conditions until 5 days post-hatching (dph). Then, about 1000 leptocephali were transferred to a 19-L planktonkreisel tank [22] and fed an artificial diet made from shark eggs and other materials [25], five times per day in half-diluted seawater (17.5 psu) at 23 °C until 23 dph. The water supply rate to the tank was set at 1.5 L/min, and the mean velocity of the revolving current 1 cm inside from the tank wall was 6.7 cm/s (Fig. 1b).
Planktonkreisel tanks used for rearing Japanese eel Anguilla japonica larvae in four water current velocities. The tanks are shown from left to right as a slow, b medium-slow, c medium-fast, and d fast treatments. The rate of water supply to each tank was equal, and the current velocities of each group were regulated by the number of water inflow tubes
Experimental design
To examine the effect of water current, 23 dph leptocephali (n = 862) were randomly divided into four groups in separate planktonkreisel tanks (Fig. 1) and reared for 200 days. The leptocephali were fed the same diet as before the start of the experimental period by turning off the water flow for 15 min five times per day. The water supply rate to each tank was equally set at 1.5 L/min, the number of inlet tubes with inner diameters of 3 mm ranged from two to five tubes per tank and were used to regulate current velocities (Fig. 1). These inlet tubes produced a stable revolving current in each tank. Because the water supply was the same among the tanks and the inner diameter of the tubes was the same, the water flow was slowest in the tank with five tubes and faster in the tanks with four, three, and two tubes in order. The velocities of the revolving current 1 cm inside from the tank walls were measured using a current meter (KENEK, VE10). The mean (±SD) velocities of the four tanks were 5.7 ± 0.2 cm/s (slow), 6.7 ± 0.1 cm/s (medium-slow), 7.4 ± 0.1 cm/s (medium-fast), and 8.3 ± 0.2 cm/s (fast), respectively.
All leptocephali were sampled at 60 dph (n = 100–134) and 186 dph (n = 61–74), anesthetized in a 0.02 % MS-222 solution, digitally photographed (Nikon, D80, 50 mm macro lens) and returned to their respective tanks. They were not fed after 209 dph to induce metamorphosis by starvation [18]. After completing their metamorphosis, all glass eels (n = 80) were sampled on 223 dph and fixed in a 5 % formalin 1/4 strength seawater solution. The metamorphosis rate was calculated by the formula: metamorphosis rate (%) = number of glass eels/initial number of larvae × 100. The survival rate was calculated by the formula: survival rate (%) = number of survived fish at 223 dph/initial number of larvae × 100. Experiments were conducted according to the principles and procedures approved by the Institutional Animal Care and Use Committee of the University of Tokyo.
Morphometric measurements and observation of deformities
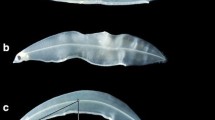
The total length (TL), preanal length (PAL), and body depth (BD) of leptocephali were measured to the nearest 0.01 mm using the digitalized images and the public domain NIH Image program. If the leptocephali had a curved body (kyphosis), a curved line along the notochord connecting the tips of the upper jaw and the caudal fin was considered as the TL. In this study, two types of notochord abnormalities were observed in leptocephali: a “notochord kyphosis”, where there was a dorsal (Λ-shaped) curvature of the notochord column, as reported by Okamura et al. [11], and a “crinkled tail”, where there was a shrinkage or a necrosis of the caudal region (Fig. 2).
Photographs of typical examples of deformed Japanese eel Anguilla japonica leptocephali at 60 days post-hatching observed in the present study. Notochord kyphosis, a dorsal (Λ-shaped) curvature of the notochord (upper), a crinkled tail and shrinkage or necrosis of the caudal region (lower). Scale bar 1 mm
In glass eels, the TL, PAL, and BD were measured under a stereomicroscope (Nikon, SMZ 1500). When a fixed glass eel was deformed, the TL was measured with the body stretched out straight between two glass slides. They were radiographed by a soft X-ray (Softex, PRO-TEST100, 40 kV, 3 mA, 20 s), and the transparent negative films were observed on an illuminated table. Subsequently, glass eel bones were stained with alizarin red S following the standard method. The skin and muscle on the left side of the body of the stained samples were removed with razors to expose the vertebrae and then observed under a stereomicroscope. Based on both the X-radiographs and stained samples, the total number of vertebrae (TV) was determined, and vertebral deformities were observed and recorded. Glass eel deformities were categorised according to Yoshikawa [26] and Witten et al. [27]. To analyze the location of vertebral deformities, the location was recorded as an independent vertebra number counted from the head toward the caudal fin. The heavily continuous brachyspina observed in three fishes of the fastest water velocity group was excluded from the counts of brachyspina locations.
Statistical analyses
The mean TL, PAL, BD, PAL/TL, BD/TL, and TV of fishes among the four current velocity groups were analyzed using a one-way analysis of variance and assessed for increasing and decreasing trends by the Jonckheere–Terpstra trend test. Metamorphosis and survival rates among the four current velocity groups were tested by the Pearson Chi square test. An association between the deformity rate and current velocity was tested by the Pearson Chi square test, and increasing or decreasing trends were assessed by the Cochran–Armitage trend test. The frequency of vertebral deformities of compression, luxation, and brachyspina on the prehaemal (1–45th) and caudal vertebrae (≥46th) (number of prehaemal vertebrae 42–45 [28] and 40–45 [29]) was analyzed by the Pearson Chi square test and residual analysis. Statistical analyses were performed using Excel Stat 2008 with a criterion of significance of p < 0.05.
Results
Leptocephali
At 60 dph, there was no significant trend in TL, PAL, BD, PAL/TL, or BD/TL among the four current velocity treatment groups (Table 1). However, the mean TL at 186 dph significantly decreased with increasing current velocity (Jonckheere–Terpstra test, p = 0.03), whereas there was a significant increase in PAL/TL with increasing current velocity (Jonckheere–Terpstra test, p < 0.01; Table 1).
Notochord abnormalities were often observed in 60 dph larvae (Fig. 2). The occurrence of kyphosis in 60 dph larvae significantly increased with increasing current velocity (Cochran–Armitage trend test, p < 0.01; Fig. 3), and more than 80 % of the leptocephali in the fast current velocity group had that deformity. Meanwhile, in decreasing current velocity, the larvae with crinkled tails significantly increased (Cochran–Armitage trend test, p < 0.01; Fig. 3). At 186 dph, more than 55 % of the grown larvae had notochord kyphosis regardless of the current velocity. While some grown leptocephali with crinkled tails were observed, this was not significant.
The survival rates of eel larvae after 200 dph were almost the same among the four current velocity groups (slow 59 %, medium-slow 61 %, medium-fast 61 %, and fast 59 %; χ 2 test, p = 0.95). Also, there was no significant difference in the metamorphosis rates among the four groups (7.8, 8.9, 8.6, and 12.8 %, respectively; χ 2 test, p = 0.64).
Glass eels
The mean TL and PAL of glass eels decreased significantly with increasing current velocity (Jonckheere–Terpstra test, p < 0.01; Table 2), whereas the BD had no apparent trend. The PAL/TL and BD/TL were not significantly different among the current velocity groups, indicating that it did not affect body proportions. The mean TVs were not significantly different among the four groups and were within the range of 110–117, which were roughly in agreement with the TV range found in nature (112–119) [28, 29]. The exception was one individual from the fastest water current group that had only 108 vertebrae and a compressed body of 35.5 mm TL (Table 2).
In total, 65 % of all glass eels examined had morphological deformities, and they had vertebral abnormalities. Vertebral deformity was categorised into eight types as follows: “compression”, a disappearance of the intervertebral space (Fig. 4a, b); “luxation”, a vertebra that had slipped upward or downward (Fig. 4c, d); “fusion”, an adherence of two vertebrae (Fig. 4e, f); “brachyspina”, a shortened vertebra (Fig. 4g, h); “modification”, a triangular or trapezoid-shaped vertebra (Fig. 4i, j); “lordosis”, a dorsal bend of the spinal column (Fig. 4k, l); “kyphosis”, a ventral bend of the spinal column (Fig. 4m, n); and “scoliosis”, a lateral bend of the spinal column (Fig. 4o, p).
Photographs of stained bone (left panels) and radiographs (right panels) of the vertebral deformities of Japanese eel Anguilla japonica glass eels showing: a, b compression. c, d luxation. e, f fusion. g, h brachyspina. i, j modification. k, l lordosis. m, n kyphosis. o, p scoliosis. Photographs show lateral views except for scoliosis that were dorsoventral views. Scale bars 1 mm
Among these vertebral deformities, the incident rates of compression (31.3 %), luxation (32.5 %), and brachyspina (28.8 %) were significantly higher compared with the other five types of deformities (χ 2 test, p < 0.01; Fig. 5). The deformities of fusion, modification, lordosis, kyphosis, and scoliosis were observed in different current velocity groups, and their incident rates ranged from 1.3 to 12.5 % (Fig. 5). Most glass eels with brachyspina had shortened vertebrae, and three individuals had especially small body sizes (35.5, 42.8, and 43.0 mm TL) with continuous brachyspina from the middle of the body to the caudal end. One of these individuals (35.5 mm TL; TV 108) also had lordosis and kyphosis. Some glass eels had two (18.8 %), three (11.3 %), or four (3.8 %) types of deformities. However, there was no relationship between the incidence rates of these vertebral deformities and current velocity (χ 2 test, p = 0.45, Cochran–Armitage test, p = 0.24; Fig. 5).
The vertebra location of all deformities was apparently influenced by the strength of the current velocity (χ 2 test, p < 0.01; Fig. 6). On the whole, vertebral deformities frequently occurred in caudal vertebrae and were rarely observed in the middle part of the body where there is a boundary between the prehaemal and caudal vertebrae. The locations of deformities for the two slower current groups (slow and medium-slow currents) showed similar patterns (χ 2 test, p = 0.11), and the deformities appeared in both prehaemal and caudal vertebrae (Fig. 6a, b). The locations of deformities in the two faster current groups (medium-fast and fast currents) were different from the slower groups (χ 2 test, p < 0.01) appearing more frequently on caudal vertebrae than prehaemal vertebrae (χ 2 test, p = 0.78; Fig. 6c, d).
Compression occurred at similar rates in all four current velocity groups (χ 2 test, p = 0.75; Fig. 5). However, the location of occurrence was different among the current velocity groups (χ 2 test, p < 0.01) occurring in both prehaemal and caudal vertebrae in the two slower current velocity groups and only in the caudal vertebrae in the two faster current groups (Fig. 7a). Luxation and brachyspina were observed in all locations of the vertebrae with no difference in the location of occurrence (χ 2 test, p = 0.77 and 0.44, respectively; Fig. 7b, d). Vertebrae with fusion, kyphosis, and scoliosis were observed in both prehaemal and caudal vertebrae (Fig. 7c, g, h). Some individuals with kyphosis had deformities in the anterior prehaemal vertebrae just after the head. Modification and lordosis were only observed in more posterior caudal vertebrae (Fig. 7e, f).
Discussion
Water current has been important for rearing eel leptocephali because the current prevents the fragile larvae from contacting the walls of the tank and keeps them suspended in the water column. However, the effects of water current on the occurrence of morphological deformities at the leptocephalus and glass eel stages have been unclear. Okamura et al. [22] suggested that leptocephali reared in planktonkreisels may end up with some degree of dorsal body curvature (notochord kyphosis) as a result of constantly swimming against the strong current in one direction because of their positive rheotaxis [30]. Most of the severely deformed leptocephali eventually died due to feeding failure.
The present study showed that water current had an impact on eel larvae. The occurrence of notochord curvature in leptocephali was significantly increased in the faster current velocities during the early stage (60 dph), but more than 55 % of larvae had a curved notochord regardless of current velocity at the later stage (186 dph). This suggests that the strength of the water current apparently affects the morphology of developing leptocephali, but even a slow current has a long-term negative impact on their morphology. The heavily notochord curvature often observed in the strong currents may have caused the leptocephali to die, because they cannot swim forward due to their round body forms and fail to feed on the bottom of the tank. Furthermore, leptocephali became smaller when in a faster current velocity, and this was consequently reflected in the size of glass eels at metamorphosis (Tables 1, 2). This again suggests that water current has a strong influence on larval growth. These small-sized leptocephali and their death due to poor growth may be the result of greater energy loss by swimming in a strong current over a long period. However, the crinkled tails found in early stage leptocephali reared under slower current conditions suggest that these currents cannot keep the leptocephali suspended in the water column, and their bodies and tail tips may be affected by touching the walls of the tank, resulting in a caudal fin abnormality. It is likely that the most heavily affected leptocephali died prior to 186 dph (Fig. 2).
Compared to wild-caught glass eels, the size of artificial glass eels in the present study (mean 49.3 ± 4.5 mm) was considerably smaller, even in slower currents. The mean TL of wild-caught metamorphosing leptocephali and glass eels of Japanese eel was 56.9 ± 3.6 and 55.8 ± 2.7 mm, respectively [31]. There are some possible reasons for this difference: one may be a nutritional deficiency of the artificial diet used in this study [25] or because glass eels were stimulated to start metamorphosis by 2 weeks of starvation until the end of the experiment. It is likely that such a compulsive starvation would induce metamorphosis at a smaller size. While the TV range of glass eels in this study (110–117) was within the range of wild ones [28, 29] (with one exception, a glass eel with a TV of 108 due to severe morphological deformities of the brachyspina and repeated vertebral curvatures), indicating that eels have less plasticity in vertebral number.
Although there was no significant relationship between the occurrence of each vertebral deformity in glass eels and current velocity, there were some trends where they were located regardless of current velocity. Compression, fusion, kyphosis, and scoliosis were sometimes observed in anterior prehaemal vertebrae of glass eels in slower current conditions. These deformities might occur during the leptocephalus stage. A possible cause may be due to the frequent contact of the head with the bottom of the tank. Because eel leptocephali tend to swim downward due to negative phototaxis [30], the head often touches the bottom of the tank when the speed of revolving current is not enough to keep them suspended in the water column. Moreover, since larvae are fed diets on the bottom of the tank, they sometimes bump against the bottom of the tank. This contact may be the cause of these abnormalities behind the head.
Furthermore, compression, modification, kyphosis, and lordosis were frequently observed in the posterior part of caudal vertebrae of glass eels in all current velocity groups, while these deformities were rare at the boundary between the prehaemal and caudal vertebrae [28, 29]. In other teleost fish species, it has been suggested that current velocity might be related to the occurrence of notochord or vertebral deformities. For example, lordosis in fish such as common carp Cyprinus carpio and European seabass Dicentrarchus labrax may be caused by the intense muscular activity of the posterior part of the vertebrae under strong current conditions, which is induced by rheotaxis [21, 32]. It was also reported that lordosis was induced by vigorous tail beat activities in red seabream Pagrus major juveniles when their caudal fin was removed [33]. The body shape and swimming mode of eels are totally distinct from all other fishes with spindle shaped bodies. Therefore, they usually show a serpentine undulation that is referred to as anguilliform locomotion. However, the amplitude of this undulatory locomotion increases at the posterior part of body in anguilliform fishes [34]. The kinematic behaviour of flat leptocephali is basically similar to cylindrical glass eels and the activity at the tail part should be higher than at other parts of the body, which might be responsible for the high incidence of such vertebral deformities observed in this study.
The causes of almost all vertebral deformities observed in this study possibly began before or during the period of metamorphosis, since deformed glass eels already had abnormal vertebrae just after metamorphosis. Okamura et al. [11] reported that severe deformities, especially notochord kyphosis or lordosis, during the leptocephalus stage were sustained during the glass eel stage as abnormal vertebrae. Therefore, it is likely that some of the vertebral column curvatures are some type of aftereffect of notochord curvatures in leptocephali. However, Yoshikawa [26] reported that cultured Japanese eels in a rearing pond sometimes had similar vertebral deformities such as kyphosis, lordosis, luxation, and brachyspina, and that these deformities apparently occurred during their growth in the culture pond and increased as they grew. However, the four deformity types of compression, fusion, modification, and scoliosis, which were observed in this study, were not reported in cultured eels in the previous study [26]. Therefore, the mechanism of occurrence of those deformities probably differs from those in the present study.
The metamorphosing period may also be important for normal vertebrae development in eels. In general, a strong water current induces abnormal tensions and contractions of muscles to the skeleton in fish such as carp [32]. During the later stage of metamorphosis in leptocephali, the muscle tissue that was limited to a thin external layer drastically increases in thickness [12, 35–38]. Therefore, it is also possible that the water current induces an abnormal musculature formation and its connections to developing vertebrae in metamorphosing leptocephali, and this affects vertebral development. Furthermore, these unfavourable processes are possibly exacerbated by inducing metamorphosis by starvation.
The present study suggests that the notochord curvature in leptocephali and vertebral deformities in glass eels occur due to swimming in a water current during the leptocephalus stage regardless of the current velocity. In addition, a faster current might hinder larval growth due to the energy cost of swimming into the current. However, at present, it is difficult to rear eel larvae without a water current because they are extremely fragile and easily damaged by touching the rearing tank walls. Moreover, in still water, a lower jaw deformity in leptocephali, where the lower jaw becomes fixed in a downward position, often occurs due to the frequent contact of the lower jaw with the bottom of the tank [22]. Therefore, to avoid these larval deformities, other methods to suspend eel larvae in the water column are required for rearing eel larvae safely. For example, using other environmental factors such as light intensity, light sources or wavelength and the shape or size of rearing tanks should be investigated.
In various teleost species, morphological deformities of larvae are induced by genetic and environmental factors such as temperature, salinity, pH, light, density, oxygen level, water quality, nutrient, and hydrodynamic conditions [39–46]. Although it might be difficult to remove all of these causes in captivity, determining the causes of the morphological deformities and especially those correlated with the survival, growth and commercial value of the eels that are produced is necessary to establish the efficient production of morphologically normal glass eels.
References
Tsukamoto K, Aoyama J, Miller MJ (2003) Present status of the Japanese eel: resources and recent research. In: Casselman JM, Cairns DK (eds) Eels at the edge: science, status and conservation concerns. Proceedings of the 2003 International Eel Symposium. American Fisheries Society Symposium Publication, Bethesda pp 21–35
Kuroki M, Oijen MJP, Tsukamoto K (2014) Eels and the Japanese: an inseparable, long-standing relationship. In: Tsukamoto K, Kuroki M (eds) Eels and Humans. Springer, Tokyo, pp 91–108
Kuroki M, Righton D, Walker AM (2014) The importance of Anguillids: a cultural and historical perspective introducing papers from the World Fisheries Congress. Ecol Freshw Fish 23:2–6
Tsukamoto K (1990) Recruitment mechanism of the eel, Anguilla japonica, to the Japanese coast. J Fish Biol 36:659–671
Tsukamoto K, Umezawa A (1990) Early life history and oceanic migration of the eel, Anguilla japonica. La Mer 28:188–198
Lee W-C, Chen Y-H, Lee Y-C, Liao IC (2003) The competitiveness of the eel aquaculture in Taiwan, Japan, and China. Aquaculture 221:115–124
Jacoby DMP, Casselman JM, Crook V, DeLucia M-B, Ahn H, Kaifu K, Kurwie T, Sasal P, Silfvergrip AMC, Smith KG, Uchida K, Walker AM, Gollock MJ (2015) Synergistic patterns of threat and the challenges facing global anguillid eel conservation. Glob Ecol Conserv 4:321–333
Okamura A, Horie N, Mikawa N, Yamada Y, Tsukamoto K (2014) Recent advances in artificial production of glass eels for conservation of anguillid eel populations. Ecol Freshw Fish 23:95–110
Tanaka H (2015) Progression in artificial seedling production of Japanese eel Anguilla japonica. Fish Sci 81:11–19
Okamura A, Yamada Y, Horie N, Utoh T, Mikawa N, Tanaka S, Tsukamoto K (2007) Effects of water temperature on early development of Japanese eel Anguilla japonica. Fish Sci 73:1241–1248
Okamura A, Yamada Y, Mikawa N, Horie N, Tanaka S, Tsukamoto K (2011) Notochord deformities in reared Japanese eel Anguilla japonica larvae. Aquaculture 317:37–41
Kuroki M, Fukuda N, Yamada Y, Okamura A, Tsukamoto K (2010) Morphological changes and otolith growth during metamorphosis of Japanese eel leptocephali in captivity. Coast Mar Sci 34:31–38
Kurokawa T, Okamoto T, Gen K, Uji S, Murashita K, Unuma T, Nomura K, Matsubara H, Kim SK, Ohta H, Tanaka H (2008) Influence of water temperature on morphological deformities in cultured larvae of Japanese eel, Anguilla japonica, at completion of yolk resorption. J World Aquacult Soc 39:726–735
Kurokawa T, Shibahara H, Gen K, Nomura K, Tanaka H (2013) Determination of periods of sensitivity to low-salinity and low-temperature conditions during the early development of cultured Japanese eel Anguilla japonica larvae with respect to the rate of morphological deformity at completion of yolk resorption. Fish Sci 79:673–680
Okamoto T, Kurokawa T, Gen K, Murashita K, Nomura K, Kim S-K, Matsubara H, Ohta H, Tanaka H (2009) Influence of salinity on morphological deformities in cultured larvae of Japanese eel, Anguilla japonica, at completion of yolk resorption. Aquaculture 293:113–118
Horie N, Utoh T, Nimawa N, Yamada Y, Okamura A, Tanaka S, Tsukamoto K (2008) Influence of artificial fertilization methods of the hormone-treated Japanese eel Anguilla japonica upon the quality of eggs and larvae (Comparison between stripping-insemination and spontaneous spawning methods). Nippon Suisan Gakkaishi 74:26–35 (in Japanese with English abstract)
Okamura A, Yamada Y, Mikawa N, Horie N, Utoh T, Kaneko T, Tanaka S, Tsukamoto K (2009) Growth and survival of eel leptocephali (Anguilla japonica) in low salinity water. Aquaculture 296:367–372
Okamura A, Yamada Y, Mikawa N, Horie N, Tsukamoto K (2012) Effect of starvation, body size, and temperature on the onset of metamorphosis in Japanese eel (Anguilla japonica). Can J Zool 90:1378–1385
Kuroki M, Seo MY, Okamura A, Watanabe S, Tsukamoto K, Kaneko T (2016) Morphofunctional features of ionocytes in Japanese eel Anguilla japonica leptocephali acclimated to half-diluted and full-strength seawater. Ichthyol Res. doi:10.1007/s10228-016-0520-0
Chatain B (1994) Abnormal swimbladder development and lordosis in sea bass (Dicentrarchus labrax) and sea bream (Sparus auratus). Aquaculture 119:371–379
Divanach P, Papandroulakis N, Anastasiadis P, Koumoundouros G, Kentouri M (1997) Effect of water currents on the development of skeletal deformities in sea bass (Dicentrarchus labrax L.) with functional swimbladder during postlarval and nursery phase. Aquaculture 156:145–155
Okamura A, Yamada Y, Horita T, Horie N, Mikawa N, Utoh T, Tanaka S, Tsukamoto K (2009) Rearing eel leptocephali (Anguilla japonica Temminck & Schlegel) in a planktonkreisel. Aquacult Res 40:509–512
Greve W (1968) The “planktonkreisel”, a new device for culturing zooplankton. Mar Biol 1:201–203
Ohta H, Kagawa H, Tanaka H, Okuzawa K, Iinuma N, Hirose K (1997) Artificial induction of maturation and fertilization in the Japanese eel, Anguilla japonica. Fish Physiol Biochem 17:163–169
Okamura A, Yamada Y, Horie N, Mikawa N, Tanaka S, Kobayashi H, Tsukamoto K (2013) Hen egg yolk and skinned krill as possible foods for rearing leptocephalus larvae of Anguilla japonica Temminck & Schlegel. Aquacult Res 44:1531–1538
Yoshikawa M (2008) The patterns of the vertebra deformities in cultured Japanese eels Anguilla japonica and consideration about the reason for their existence. B Shizuoka Pref Res Inst Fish 43:19–27 (in Japanese with English abstract)
Witten PE, Gil-Martens L, Huysseune A, Takle H, Hjelde K (2009) Towards a classification and an understanding of developmental relationships of vertebral body malformations in Atlantic salmon (Salmo salar L.). Aquaculture 295:6–14
Ege V (1939) A revision of the genus Anguilla Shaw, a systematic, phylogenetic and geographical study. Dana Rep 16:1–256
Watanabe S, Aoyama J, Tsukamoto K (2004) Reexamination of Ege’s (1939) use of taxonomic characters of the genus Anguilla. B Mar Sci 74:337–351
Yamada Y, Okamura A, Mikawa N, Utoh T, Horie N, Tanaka S, Miller MJ, Tsukamoto K (2009) Ontogenetic changes in phototactic behavior during metamorphosis of artificially reared Japanese eel Anguilla japonica larvae. Mar Ecol Prog Ser 379:241–251
Shinoda A, Aoyama J, Miller MJ, Otake T, Mochioka N, Watanabe S, Minegishi Y, Kuroki M, Yoshinaga T, Yokouchi K, Fukuda N, Sudo R, Hagihara S, Zenimoto K, Suzuki Y, Oya M, Inagaki T, Kimura S, Fukui A, Lee TW, Tsukamoto K (2011) Evaluation of the larval distribution and migration of the Japanese eel in the western North Pacific. Rev Fish Biol Fisher 21:591–611
Backiel T, Kokurewicz B, Ogorzalek A (1984) High incidence of skeletal anomalies in carp, Cyprinus carpio, reared in cages in flowing water. Aquaculture 43:369–380
Kihara M, Ogata S, Kawano N, Kubota I, Yamaguchi R (2002) Lordosis induction in juvenile red sea bream, Pagrus major, by high swimming activity. Aquaculture 212:149–158
D’Aout K, Aerts P (1999) A kinematic comparison of forward and backward swimming in the eel Anguilla anguilla. J Exp Biol 202:1511–1521
Hulet WH (1978) Structure and functional development of the eel leptocephalus Ariosoma balearicum (Delaroche, 1809). Phil Trans R Soc Lond B 282:107–138
Smith DG (1984) Elopiformes, Notacanthiformes and Anguilliformes: relationships. In: Moser HG et al. (eds) Ontogeny and systematics of fishes. American Society of Ichthyologists and Herpetologists, Special Publication 1. Allen Press, Lawrence pp 94–102
Egginton S, Johnston IA (1982) A morphometric analysis of regional differences in myotomal muscle ultrastructure in the juvenile eel (Anguilla anguilla L.). Cell Tissue Res 222:579–596
Tsukamoto Y, Okiyama M (1997) Metamorphosis of the Pacific tarpon, Megalops cyprinoides (Elopiformes, Megalopidae) with remarks on development patterns in the Elopomorpha. B Mar Sci 60:23–36
Cahu C, Zambonino Infante JL, Takeuchi T (2003) Nutritional components affecting skeletal development in fish larvae. Aquaculture 227:245–258
Sawada Y, Hattori M, Sudo N, Kato K, Takagi Y, Ura K, Kurata M, Okada T, Kumai H (2006) Hypoxic conditions induce centrum defects in red seabream Pagrus major (Temminck and Schlegel). Aquac Res 37:805–812
Lall SP, Lewis-McCrea LM (2007) Role of nutrients in skeletal metabolism and pathology in fish—an overview. Aquaculture 267:3–19
Georgakopoulou E, Katharios P, Divanach P, Koumoundouros G (2010) Effect of temperature on the development of skeletal deformities in Gilthead seabream (Sparus aurata Linnaeus, 1758). Aquaculture 308:13–19
Darias MJ, Mazurais D, Koumoundouros G, Cahu CL, Zambonino-Infante JL (2011) Overview of vitamin D and C requirements in fish and their influence on the skeletal system. Aquaculture 315:49–60
Haga Y, Du S-J, Satoh S, Kotani T, Fushimi H, Takeuchi T (2011) Analysis of the mechanism of skeletal deformity in fish larvae using a vitamin A-induced bone deformity model. Aquaculture 315:26–33
Villamizar N, Blanco-Vives B, Migaud H, Davie A, Carboni S, Sánchez-Vázquez FJ (2011) Effects of light during early larval development of some aquacultured teleosts: a review. Aquaculture 315:86–94
Boglione C, Gisbert E, Gavaia P, Witten PE, Moren M, Fontagné S, Koumoundouros G (2013) Skeletal anomalies in reared European fish larvae and juveniles. Part 2: main typologies, occurrences and causative factors. Rev Aquacult 5(Suppl. 1):S121–S167
Acknowledgments
We thank Drs. Yoshiaki Yamada, Noriyuki Horie, and Naomi Mikawa of the IRAGO Institute for their kind support during the experiment. We also thank Drs. Toyoji Kaneko and Takashi Yamakawa for valuable suggestions on the radiographic observations, Dr. Shun Watanabe for facilitating this study and Dr. Michael J. Miller for helping to improve the manuscript. This study was partly supported by a Grant-in-aid (No. 25450270, 26252030) from the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kuroki, M., Okamura, A., Takeuchi, A. et al. Effect of water current on the body size and occurrence of deformities in reared Japanese eel leptocephali and glass eels. Fish Sci 82, 941–951 (2016). https://doi.org/10.1007/s12562-016-1015-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-016-1015-7