Abstract
The high mortality rate of reared Japanese eel Anguilla japonica larvae is largely due to lower growth rate and the higher rate of deformed larvae. To establish an effective rearing protocol for this species, we examined the effects of water temperature and feeding regimes on their growth and notochord kyphosis. Larvae at 165 days post hatching were reared for 28 days at mean temperatures of 24, 25 and 27 °C, and were fed 4 or 6 times per day. Larval growth rate was significantly higher in larvae reared at 24–25 °C and fed 6 times per day. However, growth rate was significantly reduced at 27 °C, suggesting a shortage of metabolic energy due to an elevated cost of the higher basal metabolic rate at higher temperatures and low nutritional performance of currently used artificial diet. Notochord kyphosis was promoted by elevated water temperature, and two-way ANOVA showed that water temperature and feeding frequency had combined effects on the deformity. These findings suggest the importance of concurrently manipulating both environmental and nutritional factors to produce healthy eel larvae in captivity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite progress in breeding techniques for leptocephalus larvae of the Japanese eel Anguilla japonica, these techniques are not yet practiced commercially (Okamura et al. 2014; Tanaka 2015). This is due in part to the lengthy rearing period for this species, usually over 200 days until metamorphosis into glass eels (Okamura et al. 2009b), entailing a high production cost. In contrast, wild A. japonica leptocephali metamorphose 80–160 days after hatching, as determined by the analysis of their otolith microstructures and microchemistry (Arai et al. 1997). Furthermore, the rate of morphological abnormalities is higher in reared than in wild larvae. Over 50% of reared larvae were reported to have notochord deformities, such as kyphosis or scoliosis, with some severely deformed larvae unable to ingest sufficient food and often dying before metamorphosis (Okamura et al. 2011).
The lengthy leptocephalus phase in captivity is apparently due to nutritional deficiencies of their artificial diet (Okamura et al. 2014). However, suboptimal environmental conditions in captivity may also result in lower growth rates and higher rates of deformities. Wild leptocephali usually experience water temperatures of 27–28 °C (Otake et al. 1998; Tsukamoto et al. 2011), much higher than the water temperatures of 21–23 °C usually used for rearing in captivity (Tanaka et al. 2001; Okamura et al. 2009b). Moreover, wild leptocephali migrate vertically in the ocean on a daily basis (Otake et al. 1998), indicating that they have a type of biorhythm (Yamada et al. 2009), and they usually experience different environmental conditions during the day and at night. For practical reasons, captive larvae are usually fed during the daytime (Okamura et al. 2009b). In contrast, wild larvae may ingest food both at night and during the day, although to date there is no information about natural feeding rhythms in wild leptocephali.
To date, several attempts have been made to identify appropriate environmental conditions for rearing eel larvae in captivity. Light is an important factor, as it can allow the distribution of eel larvae in the water column to be manipulated during feeding (Yamada et al. 2009; Masuda et al. 2012). Salinity directly affects larval survival and growth rates. Half-diluted seawater [about 17.5 practical salinity units (psu)] saves energy for osmoregulation and considerably improves growth performance (Okamura et al. 2009a). Temperatures of 25–27 °C, which are higher than usual (21–22 °C) (Tanaka et al. 2001), have been suggested to promote the growth of eel larvae (Masuda et al. 2013). However, we have recently found that rearing at higher temperatures (25–27 °C) increased the percentage of larvae with notochord kyphosis, a dorsal curvature of the notochord column (Okamura unpublished data). Therefore, it is essential that the effects of water temperature on growth and deformity in eel larvae be tested under laboratory conditions.
This study was designed to determine an effective rearing protocol for A. japonica larvae. We therefore tested the effects of relatively higher water temperature (24–27 °C) on growth and notochord deformity. We also examined the combined effects of water temperature and feeding regime (4 or 6 times per day) on growth and deformity. The findings of this study may contribute to the more efficient production of artificial glass eels under controlled laboratory conditions, not only for A. japonica but for other temperate species of anguillid eels.
Materials and methods
Collection of eel larvae
Leptocephalus larvae of A. japonica were obtained as described (Ohta et al. 1997; Kagawa et al. 2005; Horie et al. 2008). A female of age 2 years and body weight (BW) of about 700 g, feminized by feeding a diet containing 10 mg 17β-estradiol (E2) per kg diet weight for about 6 months, beginning at the glass eel stage (Tachiki et al. 1997), was injected weekly with 40 mg/kg BW pituitary extract of chum salmon Oncorhynchus keta while being maintained at 20 °C and 31 psu until the final maturation phase of oocytes. The fish was subsequently injected with 2 mg/kg BW 17α-hydroxyprogesterone (17α-OHP) (Sigma, St. Louis, MO, USA) and 100 IU/kg BW human chorionic gonadotropin (HCG) (SankyoYell Yakuhin, Tokyo) to induce ovulation. Three males of age 1 year and BW of about 250 g, were each injected weekly with 200 IU HCG while being maintained at 20 °C and 31 psu until spermiation. Immediately following the induction of ovulation, the female and the three males were placed in a 1000-l round black polyethylene tank, maintained at 23 °C and 35 psu and covered with a light-shading sheet. The fish were allowed to spawn and fertilize eggs, both of which occurred within about 15 h.
Fertilized eggs were transferred to a 100-l polycarbonate tank by siphoning. The eggs were incubated in the dark in flowing UV-sterilized seawater, maintained at 25 °C and 35 psu, until hatching about 24 h after fertilization. The hatched larvae were transferred to a 180-l polycarbonate tank and maintained for 6 days under the same conditions.
About 1000 larvae were reared in a 19-l acrylic planktonkreisel tank (Okamura et al. 2009b) supplied with 50% diluted seawater (17.5 psu) at a constant water temperature of 23 °C at a flow rate of 1.5 ml/min, starting at 6 days post hatching (dph). The tanks were set in a darkroom and a fluorescent lamp (about 200 lx at the surface of the tank) set above each tank was turned on during each 15-min feeding session. The larvae were fed an artificial diet 4 times per day, at 0830, 1130, 1430, and 1730, until 165 dph. The diet contained 48 g of eggs of the spiny dogfish Squalus acanthias captured in the north Pacific, 35 ml of krill extract (squeezed from Antarctic krill in our laboratory), 6 g of albumen peptide (RunPep; Pharma Foods International, Kyoto, Japan) and 0.25 g of a vitamin mixture containing vitamins A, B1, B2, B6, D3, E, K, and C, as well as pantothenic acid, niacin, folic acid and inositol (FISH AID-C; Japan Nutrition, Tokyo, Japan). During each feeding session, 20–50 ml of the artificial diet, which varied depending on larval growth, was given to the larvae.
Experimental design
At 165 dph, a total of 242 larvae were randomly divided into 6 groups to assess all combinations of two feeding regimes and three temperature regimes. Each group was divided into two subgroups, each containing 18–22 larvae, resulting in 12 tanks (Table 1). Planktonkreisels supplied with 50% diluted seawater at a flow rate of 1.5 ml/min were also used as experimental tanks and were set up under the dark conditions described above. The two feeding treatments included feedings 4 times per day, at 0830, 1130, and 1430, and 1730, and 6 times per day, at 0530, 0830, 1130, 1430, 1730, and 2330. During each feeding session, 20 ml of the artificial diet was supplied to each tank. The three temperature conditions included average temperature of 24 °C (range 23–25 °C), average temperature of 25 °C (23–27 °C), and maintenance at a constant 27 °C (Table 1). These treatments were continued for 28 days. In this paper, the effects of daily temperature fluctuations between 23 and 25 or 27 °C on eel larvae cannot be evaluated by the present data set, so each temperature condition was managed with an average temperature, respectively.
Data collection
All specimens were anesthetized with 0.02% MS-222 (Sigma, St. Louis, MO, USA) and digitally photographed (Nikon D80, 50-mm macro lens) before (165 dph) and after (193 dph) treatment. The total length (TL) and body depth (BD) of each larva were measured to the nearest 0.001 mm on the digitalized images using the public domain NIH Image software (developed at the US National Institutes of Health and available from http://rsb.info.nih.gov/nih-image/index.html, accessed 23 February 2016). If the larvae had a curved body (kyphosis), a curved line along the notochord connecting the tips of the upper jaw and the caudal fin was considered as the TL. The daily changes in TL (TL growth rate) and BD (BD growth rate) were calculated by subtracting initial values from final values and dividing by the number of days taken for the experiment.
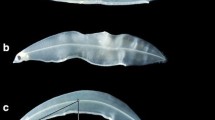
To evaluate the severity of notochord kyphosis (a dorsal [Λ-shaped] curvature of the notochord column, Okamura et al. 2011), the degree of body curvature (DBC), defined as the angle formed by the line connecting the tips of the upper jaw and the caudal fin and the line connecting the tip of the upper jaw and the midpoint of the notochord (Fig. 1), was measured. The daily changes in DBC (DBC change rate) were also calculated by subtracting initial values from final values and dividing by the number of days.
Schematic representation of a reared leptocephalus of Anguilla japonica with notochord kyphosis. The degree of body curvature (DBC), a criterion for the severity of kyphosis, was defined as the angle formed by the line connecting the tips of the upper jaw and the caudal fin and the line connecting the tip of the upper jaw and the midpoint of the notochord
In addition, daily TL and BD growth rates and DBC change rate before starting the experiment, from 6 to 165 dph, was also calculated to use as a reference. These data are shown in Figs. 2a, 3a and 4a.
Total length (TL) growth rates of the larvae reared under different feeding (A) and temperature (B) regimes for 28 days (165–193 dph). The data prior to the experiment (6–165 dph) are also shown (a). Each bar represents the mean ± standard error. Significant differences are indicated by different letters
Body depth (BD) growth rates of the larvae reared under different feeding (A) and temperature (B) regimes for 28 days (165–193 dph). The data prior to the experiment (6–165 dph) are also shown (a). Each bar represents the mean ± standard error. Significant differences are indicated by different letters
Changes in degree of body curvature (DBC) of the larvae reared under different feeding (A) and temperature (B) regimes for 28 days (165–193 dph). The data prior to the experiment (6–165 dph) are also shown (a). Each bar represents the mean ± standard error. Significant differences are indicated by different letters
Data analyses
The consistency of initial mean TL, BD and DBC in the six treatment groups was determined by one-way analysis of variance (ANOVA), followed by the Tukey–Kramer post hoc multiple comparison test. Differences between initial and final mean TL, BD and DBC were analyzed by Student’s t-tests. The combined effects of feeding and temperature regimes on changes in TL, BD and DBC over 28 days was calculated by two-way ANOVA, with any differences assessed by the Tukey–Kramer post hoc multiple comparison test. Survival rates during the experiment were compared by the log-rank test. Because each pair of subgroups showed similar trends, the data of each pair were combined and analyzed. All statistical analyses were performed using Excel Stat (Version 2008, SSRI, Tokyo, Japan). Differences were considered significant at p < 0.05.
Results
The six groups of larvae showed no significant differences in initial mean TL, BD and DBC (Tukey’s test, p > 0.05) (Table 2). Although few larvae died over the 28-day treatment period, there were no significant differences in survival rate among the six groups (log-rank test, p > 0.05), indicating that feeding and temperature regimes did not affect their survival (Table 2).
All groups of larvae showed significant growth over 28 days (t-test, p < 0.01) (Table 2). However, body curvature differed among treatment groups (Table 2). DBCs of larvae fed 4 times per day and maintained at a mean temperature of 25 °C and those fed 6 times and maintained at a mean temperature of 24 °C showed no changes over 28 days, whereas larvae in the four other treatment groups showed significant changes in DBC (t-test, p < 0.01) (Table 2).
Two-way ANOVA showed that feeding and temperature regimes did not have any effects on the TL growth rate of larvae (p > 0.05), whereas the BD growth rate of larvae was significantly affected by feeding (p < 0.01) and temperature (p < 0.05) regimes (Table 3), but that feeding and temperature did not show interactive effects on BD growth (p = 0.68). Changes in DBC were significantly affected by temperature (p < 0.01), but not by feeding (p = 0.32) regimes. However, these two factors had combined effects on DBC changes (p < 0.05) (Table 3).
The mean TL and BD growth rates of larvae fed 6 times per day (TL 0.25 mm/day; BD 0.08 mm/day) were significantly higher than those of larvae fed 4 times (TL 0.20 mm/day; BD 0.06 mm/day) (Tukey’s test, p < 0.05) (Figs. 2A, 3A). The TL of larvae reared at mean water temperatures of 24 (0.24 mm/day) and 25 °C (0.25 mm/day) showed that they grew significantly faster than those reared at 27 °C (0.19 mm/day) (Tukey’s test, p < 0.05) (Fig. 2B). BD showed higher growth at 25 °C (0.08 mm/day) than at 27 °C (0.06 mm/day) (Tukey’s test, p < 0.05) (Fig. 3B).
Daily changes in DBC were greater in larvae reared at 27 °C (Tukey’s test, p < 0.05) (Fig. 4B), with the most severely curved bodies observed in larvae reared at a constant 27 °C and fed 6 times per day (Table 2), indicating combined effects of temperature and feeding regimes as shown by two-way ANOVA (Table 3). Moreover, daily changes in DBC apparently increased as mean water temperature increased, indicating that the severity of curvature was temperature dependent (Fig. 4B).
Discussion
The present study showed that feeding and water temperature regimes influenced both the growth and notochord deformity of eel larvae. Frequent feeding times improved their growth and mean water temperatures of 24–25 °C are more effective for larval growth than 27 °C. However, notochord kyphosis was promoted by elevated temperature, as well as by higher feeding frequency, suggesting that unfavorable environmental conditions and nutritional states in larvae synergistically influenced the deformity. These results suggest that manipulating both environmental and nutritional factors can regulate the healthy growth of eel larvae in captivity.
Growth
Optimum water temperature for the growth of A. japonica leptocephali has been investigated in both field surveys and laboratory experiments. In nature, A. japonica spawn at depths between 150 and 200 m in the seamount areas west of the Mariana Islands, and the eggs and hatched larvae float to the top of the thermocline (25–27 °C) at a depth of 150 m (Tsukamoto et al. 2011; Aoyama et al. 2014), where the larvae possibly start feeding. After growing to a certain size, they migrate vertically on a daily basis while moving west in the North Equatorial Current. Individuals of TL 10–20 mm have been caught at night at depths of 50–100 m and temperatures of 26–29 °C, and during the day at depths of 130–250 m and water temperatures of 17–26 °C (Otake et al. 1998). As the swimming ability of leptocephali increases with their growth, so the temperature range they experience expands.
In captivity, A. japonica leptocephali have been reared at water temperatures of 21–23 °C (Tanaka et al. 2001; Okamura et al. 2009b). Although these lower temperatures were found to prevent bacterial growth in rearing tanks, thereby enhancing larval survival rates, they also lengthened rearing duration until metamorphosis, which often exceeded 250 days (Okamura et al. 2009b). The average TL growth rate of A. japonica larvae reared at 21–23 °C has been reported to be 0.1–0.3 mm/day (Okamura et al. 2014), which is roughly consistent with the growth rate of larvae prior to the present experiment, when larvae were reared at 23 °C (0.22 mm/day) (Fig. 2a). However, these are apparently lower than those of wild larvae (0.3–0.5 mm/day) (Tsukamoto 1990; Ishikawa et al. 2001).
The present study showed that the TL growth rates of eel larvae reared at 24–25 °C were not particularly higher (0.24–0.25 mm/day) than those of wild larvae, suggesting that these temperatures are still suboptimal for eel larvae. Furthermore, a higher water temperature (27 °C) significantly reduced both TL and BD growth (Figs. 2B, 3B). This significant reduction probably indicates a shortage of metabolic energy in larvae reared at higher temperatures. Because the artificial diet presently used, consisting mainly of shark eggs, is still regarded as suboptimal (Okamura et al. 2014), its nutritional performance is probably insufficient to compensate for the energy cost of the higher basal metabolic rate of eel larvae reared at higher water temperatures. Moreover, the higher degree of notochord curvature at higher water temperatures possibly affected their proper growth, especially TL elongation.
On the other hand, BD growth rates at 24–27 °C (0.06–0.08 mm/day) (Fig. 3B) were apparently greater than prior to the experiment (0.04 mm/day) (Fig. 3a), although these larvae differ in age. Unlike TL growth in deformed larvae, the BD growth seems to be not disturbed by the existence of notochord deformity, and thus can be used as an index of growth in deformed eel larvae. If this is so, these data suggest the possibility that warmer temperatures (> 24 °C) than usually used (< 23 °C) are fundamentally preferable for the growth of eel larvae, which is consistent with predictions from field surveys (Tsukamoto et al. 2011; Aoyama et al. 2014).
Deformity
Fish deformities are caused by various environmental stresses, such as suboptimal salinity, water current and water temperature (e.g., Divanach et al. 1997, Cook et al. 2005, Mathes et al. 2010). Exposure of unfavorable water temperatures to larval and adult fishes has been found to induce various abnormalities in the development of the somite, notochord and skeleton. For example, overly high and low water temperatures during the hatching period have been reported to induce assimilation vertebrae in the redfin Tribolodon hakonensis (Komada 1982). Abnormal formation of somites in zebrafish has been reported to be induced by high water temperatures during the early somitogenesis stage (Roy et al. 1999). We previously reported that temperatures under 25 °C affected early eel larvae (0–6 dph), resulting in open lower jaws and pericardial edema (Okamura et al. 2007). The present study also found that water temperature had a marked effect the pathology of notochord kyphosis in eel larvae, strongly suggesting the importance of water temperature.
Notochord kyphosis in eel larvae was not only affected by water temperature but by feeding frequency, suggesting an interactive effect between environmental temperature and nutritional conditions. Overfeeding of nutritionally imbalanced diets, consisting mainly of shark or hen’s eggs, may promote notochord deformities in eel larvae, a condition further enhanced by higher temperatures. Similarly, nutritional imbalance (e.g., vitamin C deficiency) has been reported to induce vertebral malformation in other fish species, including the rainbow trout Oncorhynchus mykiss, with higher water temperatures increasing the incidence of malformation (Sato et al. 1983). Therefore, both the nutritional balance of artificial diets and an appropriate water temperature are simultaneously required to rear fish larvae.
Nutritional imbalances, such as vitamin C or E deficiency, or vitamin D or tyrosine excess, have been found to cause skeletal abnormalities in many fish species (Lall and Lewis-McCrea 2007). Because of its involvement in collagen biosynthesis, a major constituent of the spinal cord (Stemple 2005), vitamin C is especially important in maintaining the structures of the spinal cord and notochord (Barnes and Kodicek 1972; Padh 1991). A deficiency in vitamin C may result in abnormally formed notochord and spinal structures (Sato et al. 1983), as observed in eel larvae. In this study, a commercial vitamin mixture, which included vitamin C (ca. 150 mg/kg), was added to the artificial diet, but it could not be determined whether this concentration met the demands of eel larvae. Although 25 mg vitamin C per kg diet is regarded as sufficient for juvenile and growing fishes (Lall and Lewis-McCrea 2007), larval fishes have a higher requirement due to increased metabolism and growth (Dabrowski 1992). Eel larvae are administered a slurry-type diet (Tanaka et al. 2001), which easily diffuses into the water, resulting in a very low rate of uptake of water soluble nutrients by eel larvae. Therefore, the amounts of vitamin C and other water soluble nutrients may be insufficient for normal development of eel larvae. Further studies are required to assess these possibilities.
References
Aoyama J, Watanabe S, Miller MJ, Mochioka N, Otake T, Yoshinaga T, Tsukamoto K (2014) Spawning sites of the Japanese eel in relation to oceanographic structure and the west Mariana Ridge. PLoS One 9:e88759. https://doi.org/10.1371/journal.pone.0088759
Arai T, Otake T, Tsukamoto K (1997) Drastic changes in otolith microstructure and microchemistry accompanying the onset of metamorphosis in the Japanese eel Anguilla japonica. Mar Ecol Prog Ser 161:17–22. https://doi.org/10.3354/meps161017
Barnes MJ, Kodicek E (1972) Biological hydroxylations and ascorbic acid with special regard to collagen metabolism. Vitam Horm 30:1–43. https://doi.org/10.1016/S0083-6729(08)60793-1
Cook MA, Guthrie KM, Rust MB, Plesha PD (2005) Effects of salinity and temperature during incubation on hatching and development of lingcod Ophiodon elongatus Girard, embryos. Aquac Res 36:1298–1303. https://doi.org/10.1111/j.1365-2109.2005.01346.x
Dabrowski K (1992) Ascorbate concentration in fish ontogeny. J Fish Biol 40:273–279. https://doi.org/10.1111/j.1095-8649.1992.tb02572.x
Divanach P, Papandroulakis N, Anastasiadis P, Koumoundouros G, Kentouri M (1997) Effect of water currents on the development of skeletal deformities in sea bass (Dicentrarchus labtax L.) with functional swim bladder during postlarval and nursery phase. Aquaculture 156:145–155. https://doi.org/10.1016/S0044-8486(97)00072-0
Horie N, Utoh T, Mikawa N, Yamada Y, Okamura A, Tanaka S, Tsukamoto K (2008) Influence of artificial fertilization methods of the hormone-treated Japanese eel Anguilla japonica upon the quality of eggs and larvae (comparison between stripping-insemination and spontaneous spawning methods). Nippon Suisan Gakkaishi 74:26–35 (in Japanese with English abstract)
Ishikawa S, Suzuki K, Inagaki T, Watanabe S, Kimura Y, Okamura A, Otake T, Mochioka N, Suzuki Y, Hasumoto H, Oya M, Miller MJ, Lee TW, Fricke H, Tsukamoto K (2001) Spawning time and place of the Japanese eel Anguilla japonica in the North Equatorial current of the western North Pacific Ocean. Fish Sci. https://doi.org/10.1046/j.1444-2906.2001.00366.x
Kagawa H, Tanaka H, Ohta H, Unuma T, Nomura K (2005) The first success of glass eel production in the world: basic biology on fish reproduction advances new applied technology in aquaculture. Fish Physiol Biochem 31:193–199
Komada N (1982) Vertebral anomalies in the cyprinid fish, Tribolodon hakonensis. Jpn J Ichthyol 29:185–192
Lall SP, Lewis-McCrea LM (2007) Role of nutrients in skeletal metabolism and pathology in fish—an overview. Aquaculture 267:3–19. https://doi.org/10.1016/j.aquaculture.2007.02.053
Masuda Y, Imaizumi H, Usuki H, Oda K, Hashimoto H, Teruya K (2012) Artificial completion of the Japanese eel, Anguilla japonica, life cycle: challenge to mass production. Bull Fish Res Agen 35:111–117
Masuda Y, Jinbo T, Imaizumi H, Hashimoto H, Oda K, Matsuda K, Teruya K, Usuki H (2013) Regulation of water temperature, feeding frequency and larval stocking density leads to shorter duration of larval stage of Japanese eel Anguilla japonica. Nippon Suisan Gakkaishi 79:198–205 (in Japanese with English abstract)
Mathes MT, Hinch SG, Cooke SJ, Crossin GT, Patterson DA, Lotto AG, Farrell AP (2010) Effect of water temperature, timing, physiological condition, and lake thermal refugia on migrating adult Weaver Creek sockeye salmon (Oncorhynchus nerka). Can J Fish Aquat Sci 67:70–84. https://doi.org/10.1139/F09-158
Ohta H, Tanaka H, Kagawa H, Okuzawa K, Iinuma N (1997) Artificial fertilization using testicular spermatozoa in the Japanese eel Anguilla japonica. Fish Sci 63:393–396
Okamura A, Yamada Y, Horie N, Utoh T, Mikawa N, Tanaka S, Tsukamoto K (2007) Effects of water temperature on early development of Japanese eel Anguilla japonica. Fish Sci 73:1241–1248. https://doi.org/10.1111/j.1444-2906.2007.01461.x
Okamura A, Yamada Y, Mikawa N, Horie N, Utoh T, Kaneko T, Tanaka S, Tsukamoto K (2009a) Growth and survival of eel leptocephali (Anguilla japonica) in low-salinity water. Aquaculture 296:367–372. https://doi.org/10.1016/j.aquaculture.2009.08.039
Okamura A, Yamada Y, Horita T, Horie N, Mikawa N, Utoh T, Tanaka S, Tsukamoto K (2009b) Rearing eel leptocephali (Anguilla japonica Temminck & Schlegel) in a plankton kreisel. Aquac Res 40:509–512. https://doi.org/10.1111/j.1365-2109.2008.02127.x
Okamura A, Yamada Y, Mikawa N, Horie N, Tanaka S, Tsukamoto K (2011) Notochord deformities in reared Japanese eel Anguilla japonica larvae. Aquaculture 317:37–41. https://doi.org/10.1016/j.aquaculture.2011.04.024
Okamura A, Horie N, Mikawa N, Yamada Y, Tsukamoto K (2014) Recent advances in artificial production of glass eels for conservation of anguillid eel populations. Ecol Freshw Fish 23:95–110. https://doi.org/10.1111/eff.12086
Otake T, Inagaki T, Hasumoto H, Mochioka N, Tsukamoto K (1998) Diel vertical distribution of Anguilla japonica leptocephali. Ichthyol Res 45:208–211. https://doi.org/10.1007/BF02678565
Padh H (1991) Vitamin C: newer insights into its biochemical functions. Nutr Rev 49:65–70. https://doi.org/10.1111/j.1753-4887.1991.tb07407.x
Roy MN, Prince VE, Ho RK (1999) Heat shock produces periodic somitic disturbances in the zebrafish embryo. Mech Dev 85:27–34. https://doi.org/10.1016/S0925-4773(99)00039-8
Sato M, Kondo T, Yoshinaka R, Ikeda S (1983) Effect of water temperature on the skeletal deformity in ascorbic acid-deficient rainbow trout. Nippon Suisan Gakkaishi 49:443–446
Stemple DL (2005) Structure and function of the notochord: an essential organ for chordate development. Development 132:2503–2512. https://doi.org/10.1242/Dev.01812
Tachiki H, Nakagawa T, Tamura K, Hirose K (1997) Effects of oral administration of estradiol-17β to young on gonadal sex and growth of Japanese eel Anguilla japonica. Suisanzoshoku 45:61–66 (in Japanese with English abstract)
Tanaka H (2015) Progression in artificial seedling production of Japanese eel Anguilla japonica. Fish Sci 81:11–19. https://doi.org/10.1007/s12562-014-0821-z
Tanaka H, Kagawa H, Ohta H (2001) Production of leptocephali of Japanese eel (Anguilla japonica) in captivity. Aquaculture 201:51–60. https://doi.org/10.1016/S0044-8486(01)00553-1
Tsukamoto K (1990) Recruitment mechanism of the eel, Anguilla japonica, to the Japanese coast. J Fish Biol 36:659–671
Tsukamoto K, Chow S, Otake T, Kurogi H, Mochioka N, Miller MJ, Aoyama J, Kimura S, Watanabe S, Yoshinaga T, Shinoda A, Kuroki M, Oya M, Watanabe T, Hata K, Ijiri S, Kazeto Y, Nomura K, Tanaka H (2011) Oceanic spawning ecology of freshwater eels in the western North Pacific. Nat Commun 2:179. https://doi.org/10.1038/ncomms1174
Yamada Y, Okamura A, Mikawa N, Utoh T, Horie N, Tanaka S, Miller M, Tsukamoto K (2009) Ontogenetic changes in phototactic behavior during metamorphosis of artificially reared Japanese eel Anguilla japonica larvae. Mar Ecol Prog Ser 379:241–251. https://doi.org/10.3354/meps07912
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Okamura, A., Horie, N., Mikawa, N. et al. Influence of temperature and feeding regimes on growth and notochord deformity in reared Anguilla japonica leptocephali. Fish Sci 84, 505–512 (2018). https://doi.org/10.1007/s12562-018-1188-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-018-1188-3