Abstract
This study aimed at quantifying leucine requirement of fingerling Catla catla [3.75 (mean body length) ± 0.15 (SE) cm, 0.66 (mean body weight) ± 0.04 (SE) g] by conducting a 12-week feeding trial. Six casein- and gelatin-based (33 % crude protein, 14.0 kJ g−1 calculated digestible energy) semipurified diets containing different concentrations of leucine (0.73, 0.97, 1.24, 1.46, 1.74 and 1.97 % dry diet) were fed to triplicate groups of fish to apparent satiation thrice daily at 08:00, 12:30 and 17:30 hours. Maximum absolute weight gain (AWG, 7.45 g fish−1), protein gain (PG, 1.31 g fish−1), leucine gain (LG, 85.33 mg fish−1), RNA/DNA ratio (4.62) and best feed conversion ratio (FCR, 1.51) were recorded at 1.74 % dietary leucine. Hemoglobin, hematocrit and red blood cell counts count were also found to be optimum in fish fed diet with 1.74 % leucine. Quadratic regression analysis at 95 % maximum response of AWG and minimum response of FCR against dietary leucine concentrations reflected the requirement at 1.58 and 1.57 % dry diet, respectively. Based on above results, inclusion of leucine ranging from 1.57 to 1.58 % of the dry diet is recommended for developing leucine-balanced commercial feeds for the intensive culture of C. catla.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The development of nutritionally adequate, cost-effective feeds for all stages of cultured fish species is of great importance to the commercial success of aquaculture. Formulation of balanced and cost-effective diets requires complete knowledge of nutritional requirements of the cultured species (Wilson 1985; Lin et al. 2013). Dietary intake of essential amino acids is required to achieve optimum growth, best feed conversion and desirable carcass quality. Leucine, a member of aliphatic side chain amino acid family, is essential for normal growth and reproductive potential of the fish (Abidi and Khan 2007). It plays an important role in protein synthesis, promotes insulin release and inhibits protein degradation (Nair et al. 1992). Leucine has also been implicated to play a signaling role in enhancing the availability of specific eukaryotic initiation factors (Anthony et al. 2000) as well as augmenting the activity of proteins involved in mRNA translation (Davis and Fiorotto 2009; Wu et al. 2010). It also supplies gluconeogenic precursors via the formation of alanine in muscle (Brooks 1987). The essential branched-chain amino acid leucine amounts to about 4.6 % of the total amino acids (Takala et al. 1980).
Dietary requirements of leucine have been developed for various cultivable fish species such as chinook salmon, Oncorhynchus tshawytscha (Chance et al. 1964); rainbow trout, O. mykiss (Ogino 1980); Atlantic salmon, Salmo salar (Rollin 1999); Mossambique tilapia, Oreochromis mossambicus (Jauncey et al. 1983); white sturgeon, Acipenser transmontanus (Ng and Hung 1995); rohu, Labeo rohita (Murthy and Varghese 1997; Abidi and Khan 2007); mrigal, Cirrhinus mrigala (Benakappa and Varghese 2003; Ahmed and Khan 2006); red sea bream, Pagrus major (Forster and Ogata 1998); European sea bass, Dicentrarchus labrax, gilthead seabream, Sparus aurata and turbot, Psetta maxima (Kaushik 1998); yellow croaker, Pseudosciaena crocea (Yan et al. 2010); channel catfish, Ictalurus punctatus (Wilson et al. 1980); and stinging catfish, Heteropneustes fossilis (Farhat and Khan 2014).
The Indian major carp C. catla is the most important, fast-growing commercially cultured fish (FAO 2006–2012). Because of its high nutritional value and good taste, it has greater consumer demand (ICLARM 2001). This fish is used as the integral component in carp polyculture system. Catla, along with the other Indian major carps, also form the mainstay of culture practices, contributing approximately 5.4 million tonnes to the total aquaculture production (59.9 million tonnes) in 2010 (FAO 2012). To improve the production process of this species, it is important to understand dietary leucine requirement in order to prepare leucine-balanced feeds. Although data on leucine requirement of fry C. catla are available (Ravi and Devaraj 1991), information on leucine requirement for fingerling C. catla is warranted. Therefore, this study was carried out to determine the dietary leucine requirement of fingerling C. catla using growth, feed conversion ratio, RNA/DNA ratio, protein gain, leucine gain and carcass composition as the sensitive parameters. Relevance of hematological indices in assessing the health status of fish has been reported by several authors (Buentello et al. 2007; Ahmed 2012; Farhat and Khan 2014). Considering the importance of hematological parameters in assessing the health status of fish in response to dietary amino acids, these tools were also utilized to estimate the dietary leucine requirement of this fish.
Materials and methods
Experimental diets
Six isonitrogenous (33 % crude protein) and isocaloric (14.0 kJ g−1 calculated digestible energy) semipurified diets using casein (fat free), gelatin and L-crystalline amino acids with graded levels of leucine (0.75, 1.0, 1.25, 1.5, 1.75 and 2.0 % dry diet) were formulated. The experimental diets were designated as L1, L2, L3, L4, L5 and L6. The amino acids profile of the experimental diets excluding the test amino acid leucine was simulated to that of 33 % whole chicken egg protein. The composition of the experimental diets is given in Table 1. Casein and gelatin served as intact protein sources and provided 0.75 % leucine in the experimental diets. To make the intended concentrations of dietary leucine in the semipurified diets, the amount of leucine was increased at the expense of glycine on protein-to-protein basis (N × 6.25). Since the protein contributed by glycine is highest than that by any other nonessential amino acids, we replaced glycine for leucine in this study. As the levels of leucine increased in the experimental diets, the proportion of dextrin was decreased correspondingly to maintain the energy content of all diets. The analyzed amino acid composition of the experimental diets is presented in Table 2. Amino acid analyses of diets revealed the l-leucine content to be 0.73, 0.97, 1.24, 1.46, 1.74 and 1.97 % of the dry diet. The levels of leucine in the semipurified diets were fixed on the basis of information available on other Indian major carps (Murthy and Varghese 1997; Ahmed and Khan 2006; Abidi and Khan 2007; NRC 2011). Level of protein in the semipurified diets was fixed to be slightly lower than the reported requirement (35 %, Khan and Jafri 1991; Dars et al. 2010). This reduction was made to ensure maximum utilization of the limiting amino acid from the diet (Wilson 2002). Method of preparation of experimental diets was the same as detailed earlier (Zehra and Khan 2013a). Briefly, pre-weighed quantities of crystalline L-amino acids and salt mixture were thoroughly stirred in hot water (80 °C) in a steel bowl attached to a Hobart electric mixer (K5SS, Hobart Corp., Troy, OH, USA). The pH of the resulting mixture was adjusted to neutral with 6N NaOH solution (Nose et al. 1974). Crystalline L-amino acids (CAA) were coated with some amount (5 % of the diet) of cooked carboxymethyl cellulose (CMC). Gelatin was dissolved separately in a volume of water with constant heating and stirring and then transferred to the CMC-bound pre-coated CAA mixtures. These pre-coated CAA mixtures were further coated with cooked casein at 80 °C. The mixer bowl was removed from heating and dextrin added. Vitamin and oil premixes were added to the lukewarm bowl (50 °C) one by one with constant mixing. Lastly, rest 5 % carboxymethyl cellulose was added to the above mixture and the speed of the blender was gradually increased as the diet started to harden. The dough was passed through a pelletizer fitted with a 2-mm die to obtain pellets that were dried in a hot air oven at 40 °C to reduce the moisture content below 10 %. The dry pellets thus obtained were crumbled, sieved (500 µm) and stored at 4 °C until used. The coating of amino acids with carboxymethyl cellulose followed by casein and gelatin provided sufficient water stability. In addition to providing sufficient water stability, coating of crystalline L-amino acids also reduces the absorption rate of the amino acids (Cho et al. 1992) and leaching (Alam et al. 2004) and optimizes their use for protein gain. To determine the leaching loss of the amino acids from the test diets, the dried samples after immersion in water for 30 min were also subjected to amino acid analysis. The amino acid analysis of these dietary samples exhibited no significant change in their amino acid composition.
Estimation of water stability of the diets
Water stability of the diet was estimated by the method of Fagbenro and Jauncey (1995). Briefly, representative samples (5 g) of semipurified diets were placed on a sieve and slowly immersed in a 70-L experimental tanks containing deionized water (water volume 55 l) at 27 °C for 10 min. The sieves were removed, and the crumbles were allowed to drain for 1 min, oven-dried at 105 °C for 2 h, cooled in a desiccators and reweighed. The water stability of the semipurified diets in all experiments was calculated which was found to be about 97 %.
Experimental design and feeding trial
Induced bred fry C. catla were procured from G.B. Pant University of Agriculture and Technology, Pantnagar, transported to the wet laboratory in oxygen-filled polythene bags, given a prophylactic dip in KMnO4 solution (1:3,000) and stocked in indoor circular aqua blue colored fish tanks (1.22 m in diameter; 0.91 m in height; water volume 600 L) for 2 weeks. The tank contained 1,800 fry at a stocking density of 3 fry per liter. Each tank was supplied with well water. The water temperature was maintained constant at 27 ± 1 (SE) °C and dissolved oxygen at 6.8 ± 0.4 (SE) mg L−1. Water flow rate was maintained at 1–1.5 L min−1. A natural photoperiod was 12-h dark/12-h light cycle. The fish were acclimated on a casein- and gelatin (33 % CP)-based H-440 diet (Halver 2002) and reared to fingerling stage.
Fingerling C. catla [3.75 (mean body length) ± 0.15 (SE) cm, 0.66 (mean body weight) ± 0.04 (SE) g] were taken from the above acclimated fish lot and stocked in 70-L circular polyvinyl troughs (water volume 55 L) fitted with a continuous water flow-through (1–1.5 L min−1) system at the rate of 25 fish per trough in triplicate for each dietary treatment level. Fish were fed test diets in the form of dry crumbles (500 µm) to apparent satiation thrice daily at 08:00, 12:30 and 17:30 hours. The fish were carefully observed during feeding to ensure satiety. Pre-weighed amount of feed was supplied to the fish, and the unconsumed feed, if any, was collected soon after active feeding, dried and weighed to measure the actual amount of feed consumed for calculating the feed conversion ratio. Initial and weekly weights of fish were recorded on a top-loading balance (Precisa 120A; 0.1 mg sensitivity, Oerlikon AG, Zurich, Switzerland) after anesthetizing the fish with standardized tricaine methanesulfonate dose (MS-222; 100 µg ml−1). Because of the anesthetic and handling stress, fish did not accept the feed and hence were not fed on the day they were weighed. The feeding trial lasted for 12 weeks. Fecal matter was siphoned before every feeding. The water quality indices including water temperature (27.4–28.2 °C), dissolved oxygen (6.4–7.2 mg L−1), free carbon dioxide (5.9–9.1 mg L−1), pH (7.3–7.5), total ammonia nitrogen (0.29–0.32 mg L−1), nitrites (0.04–0.07 mg L−1) and total alkalinity (74.6–83.5 mg L−1) for this fish were monitored daily during the feeding trial and recorded following standard methods (APHA 1992). The experiment was conducted with a natural photoperiod of 12-h dark/12-h light cycle.
Sample collection and chemical analyses
Fishes were fasted for 24 h to empty their guts before sampling. At the beginning of the feeding trial, 60 fish were randomly sampled, killed and pooled together. Six subsamples of a pooled sample were analyzed for initial carcass composition. At the end of the experiment, 24 h after the last feeding, 20 fish from each replicate of dietary treatments were killed with an overdose (200 µg mL−1) of the MS-222 and pooled separately. These samples were stored at −20 °C for subsequent proximate analysis. Three subsamples of the pooled samples were analyzed for final carcass composition. Proximate composition of experimental diets, and initial and final carcass was estimated using standard methods (AOAC 1995). Dry matter of the samples was determined by oven drying at 105 ± 1 °C for 22 h in a thermostat (Yorko Instruments, New Delhi, India), crude protein by digesting the dried samples in sulfuric acid (12 mL) at high temperature (420 °C) in the presence of potassium sulfate (7.0 g) and copper sulfate (0.8 g) as catalysts (Kjeltec TecatorTM Technology 2300, Hoganas, Sweden), crude lipid by solvent extraction with petroleum ether (B.P. 40–60 °C) for 2–4 h (Socs Plus equipment, SCS 4, T. Nagar, Chennai, India), and ash content was determined by incinerating 2 g of dried samples in a Muffle Furnace (S.M. Scientific Instrument (p) Ltd., Jindal Company, Delhi, India) at 650 °C for 2–3 h. Amino acid analysis of casein, gelatin, experimental diets and initial and final carcass sample was performed according to Khan and Abidi (2011). Briefly, 0.3 mg sample was hydrolyzed in 1 mL of 6N HCl for about 22 h under a nitrogen atmosphere at 110 °C. The samples thus obtained were diluted in 0.02N HCl. The hydrolyzed samples were filtered using microfilter (0.45-micron cellulose acetate membrane, Corning, Tokyo, Japan) and then injected in an automatic Amino Acid Analyzer (Hitachi L- 8800, Tokyo, Japan). Recovery hydrolysis was performed in 4N methanesulfonic acid instead of 6N HCl for the analysis of tryptophan which followed the decomposition at 110 °C temperature for 22 h. After this, 4N NaOH was added to adjust the pH to approximately 2. This was then diluted again in 0.02N HCl. However, the recovery hydrolysis of sulfur amino acids methionine and cystine was performed in 2 mL of performic acid for 4–24 h. After this, 0.3 mL of 48 % HBr was added and the decomposition was performed at 110 °C for 22 h. The samples were then dried solid under reduced pressure. After this, 1 mL of 0.2N NaOH was added and sample was then left standstill for about an hour. Lastly, the pH and volume of the sample were adjusted using 0.05N and 0.1N HCl.
Determination of RNA and DNA
At the termination of feeding trial, three fish from each replicate of the treatment group (n = 3 × 3) were randomly killed with an overdose (200 µg mL−1) of MS-222 and white muscle was removed. Three subsamples of the muscle samples for each replicate of the treatment group were taken for the determination of RNA and DNA. RNA and DNA were determined as per the method adopted by Zehra and Khan (2013a).
Hematological analyses
At the termination of the feeding trial, blood samples were collected in heparinized vials through cardiac puncture of the fish. The blood from five fish from each replicate of the treatment group was pooled to obtain enough samples for hematological analysis. Red blood cell counts (RBCs) and hemoglobin (Hb) were analyzed as per the method adopted by Vani et al. (2012). Hematocrit levels were determined by drawing fresh blood into microhematocrit tubes and centrifuged in a microhematocrit centrifuge at 10,000 g for 5 min (Goldenfarb et al. 1971).
Evaluation of growth performance
Growth performance of the experimental diets was measured by calculating the following parameters:
-
Absolute weight gain (g fish−1) = Final body weight (g) − initial body weight (g)
-
Feed conversion ratio = Dry feed intake (g)/Wet weight gain (g)
-
Feed efficiency = Wet weight gain (g)/Dry feed intake (g)
-
Protein gain (g fish−1) = Final body protein (%) × final body weight (g) − initial body protein (%) × initial body weight (g)
-
Leucine gain (mg fish−1) = Final body leucine content (%) × final body weight (g) − initial body leucine content (%) × initial body weight (g) × 1,000
Statistical analyses
All the data were subjected to one-way analysis of variance (Sokal and Rohlf 1981). Differences among treatment means were determined by Tukey’s honestly significant difference (HSD) test at a P < 0.05 level of significance. Dietary leucine requirement for the fingerling stage of C. catla was estimated by the quadratic regression analysis of absolute weight gain and feed conversion ratio against the varying levels of dietary leucine (Shearer 2000). The quadratic equation employed was Y = aX 2 + bX + c. The leucine requirement for maximum growth performance is defined as the point on the abscissa representing 95 % of the value of the upper asymptote on the ordinate (Dias et al. 2003). Statistical analyses were done using Origin (version 6.1; Origin Software, San Clemente, CA).
Results
Absolute weight gain (AWG, g fish−1), feed conversion ratio (FCR), protein gain (PG, g fish−1), leucine gain (LG, mg fish−1) and RNA/DNA ratio of fingerling C. catla fed diets with different concentrations of leucine are given in Table 3. These parameters improved significantly with the increasing concentrations of leucine up to a level of 1.74 % dry diet (L5) and declined significantly thereafter. The leucine requirement of fingerling C. catla was obtained by quadratic regression analysis of AWG and FCR at 95 % of maximum (Y 95 %max) and minimum responses (Y 95 %min). The Y 95 %max of AWG (Fig. 1) and Y 95 %min of FCR (Fig. 2) yielded the leucine requirement to be 1.58 and 1.57 % of dry diet, respectively. Feed intake did not show significant differences (P > 0.05) among the varying treatment groups (Table 3). A 100 % survival was recorded in all the dietary treatments.
Carcass composition of fingerling C. catla did not show significant (P > 0.05) variations with the increase in dietary leucine levels (Table 4). Moisture content showed a negative trend to that of the dietary leucine. Carcass protein of fish fed varying levels of dietary leucine was not significantly affected. Carcass fat increased (P > 0.05) with the incremental levels of leucine from 0.73 (L1) to 1.97 % (L6) of the dry diet.
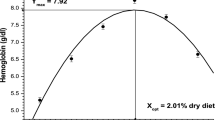
Table 5 reveals the effect of increasing levels of dietary leucine on hematological indices of fish. Hemoglobin (g dl−1), hematocrit (%) and RBCs (106 × mm−3) significantly (P < 0.05) increased with the increasing concentrations of leucine up to 1.74 % of the dry diet (L5) and thereafter (L6), a decline was noted.
Discussion
Leucine is essential for the optimal growth and health of fish because it is abundantly needed for protein synthesis in muscle tissues (Kim and Lee 2013). Hence, it is crucial to establish the leucine requirement to maximize the growth performance of fish. Leucine requirement of fish has been estimated by implementing different statistical models in various studies (Ravi and Devaraj 1991; Rodehutscord et al. 1997; Abidi and Khan 2007; Farhat and Khan 2014). The choice of a statistical model is important in interpreting the nutritional requirement experiments. Generally, nonlinear models are regarded as most appropriate for evaluating results from dose–response experiments because the response to improved dietary concentrations of a limiting nutrient is not linear (Cowey 1992; Rodehutscord et al. 1997). The efficiency of supplemented individual amino acids was found to decrease with the increasing dietary concentrations of amino acids, resulting in plateaus that were described by nonlinear functions (Rodehutscord et al. 1997). Due to the large variability among nonlinear models, it might be hard to know whether the selected nonlinear model is appropriate, particularly if no prior knowledge is available. The choice of a statistical model is based on coefficient of determination (R 2) and sum of squares of regression (SSR). Models with smaller values of SSR and higher determination coefficient (R 2) are selected to fit the data. The dose–growth response data were subjected to sigmoid, exponential, four parameter kinetics and quadratic regression analyses in this study. Since quadratic regression analysis yielded smaller sum of square and highest values of R 2 than that obtained by other models, quadratic model was adopted to estimate the dietary leucine requirement of fingerling C. catla in this study. The response of fish to an essential nutrient is not maximal at a single point for a nonlinear function. Therefore, the consensus is that 95 % of maximum response should be used to estimate requirement with nonlinear function (Rodehutscord et al. 1997; NRC 2011). Thus, 95 % of maximum response (Y 95 %max) of absolute weight gain and feed conversion ratio using quadratic equations was calculated, and the dietary leucine requirement of fingerling C. catla was found to range between 1.57 and 1.58 % dry diet, equivalent to 4.75–4.79 % dietary protein that is higher than the requirement reported for other fish species including chinook salmon, O. tshawytscha 3.9 % (Chance et al. 1964); coho salmon, O. kisutch 3.4 % (Arai and Ogata 1993); rainbow trout, O. mykiss 4.4 % (Kaushik 1998); white sturgeon, A. transmontanus 4.3 % (Ng and Hung 1995); red seabream, 4.2 %; Japanese flounder, C. major 3.9 % (Forster and Ogata 1998); common carp, Cyprinus carpio 3.3 % (Nose 1979); mrigal, C. mrigala 3.9 % (Ahmed and Khan 2006); channel catfish, I. punctatus 4.5 % (NRC 2011); lower than the requirement of Atlantic salmon, S. salar 5.2 % (Rollin 1999); yellow croaker, L. crocea 6.8 % (Yan et al. 2010); and approximately equal to the requirement reported for rohu, L. rohita 4.7 % (NRC 2011) of dietary protein.
The discrepancies in the amino acids requirements may be due to differences in fish size, age, feeding levels, flow rate, stock density and the environmental and culture conditions adopted by different laboratories (Chiu et al. 1988; Forster and Ogata 1998; Luzzana et al. 1998; Abidi and Khan 2009). Different statistical models adopted may also be responsible for the variations in the leucine requirement of varying species. Dietary protein level may be attributed to differences in the leucine requirements among species. Dietary metabolizable energy, availability of dietary amino acids, antagonism and imbalance among amino acids may also be responsible for the wide variations in amino acid requirements (Ishibashi and Ohta 1999).
Leucine requirement of fingerling C. catla determined in this study (4.75–4.79 % dietary protein) is higher than the requirement reported by Ravi and Devaraj (1991) for fry stage of this fish (3.70 % dietary protein). Ravi and Devaraj (1991) reported the leucine requirement by subjecting the weight gain data to broken-line regression analysis which has been reported to underestimate the requirement (Shearer 2000). However, in this study, the leucine requirement is worked out on the basis of quadratic regression analysis which is a good fit as indicated by high R2 values obtained for AWG (0.987) and FCR (0.952). Adoption of these statistical models may influence the estimate of the leucine requirements in both the studies. Moreover, leucine requirement reported by Ravi and Devaraj (1991) is based on weight gain only, whereas in this study, in addition to weight gain, the leucine requirement is also based on the sensitive parameters such as feed conversion ratio, protein gain, leucine gain, RNA/DNA ratio, hematological parameters and carcass composition. In addition to these, the different dietary protein levels adopted in this study (33 % dry diet) and that fixed by Ravi and Devaraj (1991; 40 % dry diet) might also be responsible for the variation in the leucine requirements of C. catla.
A number of empirical methods have been applied to establish the quantitative essential amino acid requirements of fish. Of the various methods used, most quantitative essential amino acid requirements have been established by dose–response studies (Bureau and Encarnacao 2006). The methodological approaches are also based on analyses such as plasma or tissue amino acid concentration and rates of amino acid oxidation (NRC 2011). A high correlation between muscle or whole-body amino acid profile and amino acid patterns for fish has been demonstrated by several investigators (Wang et al. 2005; NRC 2011). A/E ratios have been used as a method of estimating the requirements of all essential amino acids when only one is known by relating the A/E ratio of each EAA to that of the A/E ratio of the known amino acid (Wilson 2002; NRC 2011). Estimating amino acid requirements based on A/E ratios tends to yield higher values than those determined by dose–response studies (Wilson 2002). This method does not consider important factors that influence the efficiency of utilization of some amino acids (Rodehutscord et al. 2000; Encarnacao et al. 2004, 2006). Therefore, the use of amino acid profile from the whole body or muscle is recommended for feed formulation only when dietary requirement data determined by dose–response and growth studies are not available (Bicudo et al. 2009). Since leucine requirement of fingerling C. catla is determined by dose–response study, this data could be used to determine the other essential amino acid requirements of this fish based on A/E ratios.
Growth of fish is the most important issue for aquaculturists and it affected by the various factors in the diets such as protein, amino acids, lipids, fatty acids, carbohydrate, vitamins and minerals (Zehra and Khan 2014a). Dietary leucine concentrations had an impact on growth performance of different fish species (Ng and Hung 1995; Forster and Ogata 1998; Benakappa and Varghese 2003; Ahmed and Khan 2006; Abidi and Khan 2007; Yan et al. 2010). In this study, absolute weight gain showed a quadratic response (Y 95 %max) reaching the highest value at 1.58 % dietary leucine. Further inclusion of dietary leucine resulted in slight reduction in weight gain. This reduction in growth at higher level of dietary leucine is probably a consequence of the amino acid toxicity or dietary amino acid imbalance. Stress due to excess dietary leucine in fish body might have increased the fish’s overall energy demand leading to diversion of energy toward catabolism, therefore, adversely affecting growth. The growth depression in fish fed diets containing excess level of leucine as evident in this study was also noted by earlier workers (Murthy and Varghese 1997; Abidi and Khan 2007; Ng and Hung 1995; Forster and Ogata 1998; Benakappa and Varghese 2003; Ahmed and Khan 2006; Yan et al. 2010). In this study, the weight gain achieved by C. catla fed varying levels of dietary leucine is similar to that obtained by Zehra and Khan (Zehra and Khan 2013a, b, c, 2014a, b) for the same species.
Determination of essential amino acid requirements in studies using the dose–response approach requires addition of large amount of crystalline amino acids to obtain graded levels of test amino acid. The incorporation, however, often leads to depressed growth performance and biased amino acid requirement estimates because of crystalline amino acid leaching, different absorption kinetics of crystalline amino acids and poor diet palatability (Ambardekar et al. 2009). Coating of crystalline amino acids can reduce the solubility and non-synchronous absorption of free amino acid relative to the protein-bound ones (Segovia-Quintero and Reigh 2004; Zhou et al. 2012). In this study, crystalline amino acids were coated with precooked carboxymethylcellulose (CMC) at 50 °C in water followed by casein and gelatin which has reduced the leaching. The pre-coating of crystalline amino acid with CMC also reduced leaching in several studies (Alam et al. 2002, 2004; Wang et al. 2005; Liu et al. 2014). Coating the amino acid with CMC not only reduces the leaching of crystalline amino acid but also delays the passage time and absorption of amino acids.
Antagonism between branched-chain amino acids generally arises in animals from an excess of leucine over isoleucine and valine because the requirement of branched-chain amino acid is affected by each other (De’Mello and Lewis 1971). Choo et al. (1991) have reported that excessive leucine resulted in depressed growth and protein deposition of rainbow trout likely due to antagonism among BCAA. Excesses of leucine are extremely disruptive to utilization of isoleucine and valine, especially when these two amino acids are marginal or limiting (Smith and Austic 1978; Waldroup et al. 2002). In this study, growth reduction at surfeit level of dietary leucine (L6) may not be due to the antagonistic effects of branched-chain amino acids as the levels of isoleucine (1.18 % dry diet) and valine (1.02 % dry diet) in the experimental diets were fixed as per the reported requirements (Zehra and Khan 2013b; unpublished data from our laboratory).
Protein synthesis and deposition are known to be the most efficient when all the required amino acids are present simultaneously at the sites of synthesis (Ng et al. 1996). In the present study, protein gain improved with the increasing levels of leucine up to 1.74 % of the dry diet (L5). This improvement suggests that this level of leucine probably prevented the catabolism of amino acids and led to maximum protein gain at this level of dietary leucine. Carcass protein was not affected significantly with the increasing levels of dietary leucine in this study. Carcass fat showed a positive trend (P > 0.05) with the increasing concentrations of dietary leucine. This increase in carcass fat may be because of the fact that leucine is a ketogenic amino acid, the carbon skeleton of which is converted to acetyl-CoA and acetoacetate in muscle tissue, and these intermediates can be used to synthesize fatty acids (Hyun et al. 2007; Erwan et al. 2009). Ahmed and Khan (2006) have reported improvement in protein deposition up to the requirement level and a linear positive correlation in carcass fat with the increase in dietary leucine. However, Yan et al. (2010) have reported no marked variations in these parameters with the increasing concentrations of leucine.
The RNA/DNA ratio has been used as a sensitive indicator of nutritional condition in several fish species (Mustafa and Zofair 1985; Bulow 1987; Mustafa et al. 1991; Buckley et al. 1999; Abidi and Khan 2009). Cellular RNA is essential for the biosynthesis of protein (Clemmesen 1994). Since RNA/DNA ratio reflects the cellular ability to produce RNA and proteins, this parameter measures the potential for growth. Any factor that prevents or slows down growth is reflected by a reduced RNA/DNA ratio. The reduced values of RNA/DNA ratio in fish fed diets containing lower levels of dietary leucine (L1–L4) in this study were mainly due to the imbalanced leucine content in these diets which probably led to inefficient utilization of leucine for protein synthesis. The highest RNA/DNA ratio at 1.74 % dietary leucine indicates that this level might be optimum to maximize protein synthesis in fingerling of C. catla. In this study, RNA/DNA ratio registered for fingerling C. catla ranged from 2.18 to 4.62, which is almost similar to that reported in C. mrigala (Zehra and Khan 2012), L. rohita (Abidi and Khan 2009) and C. catla (Zehra and Khan 2013a, b, c, 2014a). However, this range is lower than that reported by Mohanta et al. (2008) in Puntius gonionotus.
Erythrocytes’ membranes have a hydrophobic lipid bilayer with a protein skeletal meshwork attached to its inner surface by binding to integral (transmembrane) proteins, so when dietary amino acid deficiency occur, erythropoiesis is affected (Harvey 1997). Low RBCs count coupled with low hemoglobin content at lower levels of dietary leucine in this study may be the result of inadequate amount of leucine available for erythropoiesis. Hematological characteristics of fingerling C. catla attained its peak at 1.74 % (L5) dietary leucine indicate that optimum level of dietary leucine is essential to support the maximum Hb, Hct % and blood cell formation as also reported by Farhat and Khan (2014) in stinging catfish.
In this study, out of the above 1.74 % leucine requirement, 0.73 % is satisfied by casein and gelatin and the rest 1.01 % by crystalline leucine. In order to make leucine-balanced diets, selected feed ingredients should contribute 1.74 % leucine. If the selected feed ingredients do not contain this amount, then the difference in leucine requirement and that contributed by the ingredients should be fulfilled by adding crystalline leucine.
A decrease in feed intake may be regarded as the primary factor responsible for reduced growth in fish fed diets deficient in amino acids in several studies (NRC 2011; Farhat and Khan 2014). However, in this study, feed intake was found to insignificantly different in all the treatment groups indicating that reduced growth in fish fed leucine-deficient diets was not due to the reduced feed intake but because of the deficiency of dietary leucine. It was stated that fish, like other animals, eat primarily to satisfy their energy demands (Cho and Kaushik 1985; NRC 2011). In the present study, almost similar digestible energy in all the diets may explain the similarity in feed intake. Almost constant (P > 0.05) feed intake with the increasing levels of dietary amino acids has also been reported by several workers (Bicudo et al. 2009; Zehra and Khan 2013a, b, c).
Generally, deficiency of most essential amino acids leads to failure of weight gain and loss of appetite rather than pathological signs. The pathological signs have also been recorded in several studies. These pathological signs include spinal deformities, bilateral cataracts and caudal fin erosion (Walton et al. 1982, 1984, 1986; Breck et al. 2003). However, in this study, no such pathological signs except poor growth and feed efficiency were observed during the length of this feeding trial. Absence of leucine deficiency signs in this study may be because of the fact that the lowest level of leucine was adequate to prevent the nutritional pathologies in this fish species.
On the basis of the quadratic regression analysis of the AWG and FCR against varying levels of dietary leucine, the requirement of fingerling C. catla is recommended in the range of 1.57–1.58 % dry diet, equivalent to 4.75–4.79 % dietary protein. The information generated during this study would be helpful in formulating leucine-balanced feeds for the intensive culture of this fish species.
References
Abidi SF, Khan MA (2007) Dietary leucine requirement of fingerling Indian major carp, Labeo rohita (Hamilton). Aquacult Res 38:478–486
Abidi SF, Khan MA (2009) Dietary arginine requirement of fingerling Indian major carp, Labeo rohita (Hamilton) based on growth, nutrient retention efficiencies, RNA/DNA ratio and body composition. J Appl Ichthyol 25:707–714
Ahmed I (2012) Dietary amino acid l-tryptophan requirement of fingerling Indian catfish, Heteropneustes fossilis (Bloch), estimated by growth and haemato-biochemical parameters. Fish Physiol Biochem 38:1195–1209
Ahmed I, Khan MA (2006) Dietary branched-chain amino acid valine, isoleucine and leucine requirements of fingerling Indian major carp, Cirrhinus mrigala (Hamilton). Br J Nutr 96:50–460
Alam MS, Teshima S, Koshio S, Ishikawa M (2002) Influence of different dietary amino acid patterns on growth and body composition of juvenile Japanese flounder, Paralichthys olivaceus. Aquaculture 210:359–369
Alam MS, Teshima S, Koshio S, Ishikawa M (2004) Effects of supplementation of coated crystalline amino acids on growth performance and body composition of juvenile kuruma shrimp Marsupenaeus japonicas. Aquacult Nutr 10:309–316
Ambardekar AA, Reigh RC, Williams MB (2009) Absorption of amino acids from intact dietary proteins and purified amino acid supplements follows different time-courses in channel catfish (Ictalurus punctatus). Aquaculture 291:179–187
American Public Health Association (1992) Standard methods for the examination of water and wastewater, 18th ed. APHA, Washington DC
Anthony JC, Anthony TG, Kimball SR, Jefferson LS (2000) Orally administered leucine stimulates protein synthesis in skeletal muscle of post absorptive rats in association with increased eIF4F formation. J Nutr 130:139–145
Arai S, Ogata H (1993) Quantitative amino acid requirements of fingerling coho salmon. In: By Collie MR, McVey JP (eds) Proceedings of the twentieth U.S. Japan symposium on aquaculture nutrition, UJNR. Department of Commerce, Newport, OR, pp 19–28
Association of Official Analytical Chemists, AOAC (1995) In: Cuniff P (ed) Official methods of analysis, 16th ed. Arlington, VA
Benakappa S, Varghese TJ (2003) Isoleucine, leucine and valine requirement of juvenile Indian major carp, Cirrhinus cirrhosus (Bloch, 1975). Acta Ichthyol Piscat 33:161–172
Bicudo AJA, Sado RY, Cyrino JEP (2009) Dietary lysine requirement of juvenile pacu Piaractus mesopotamicus (Holmberg, 1887). Aquaculture 297:151–156
Breck O, Bjerkas E, Campbell P, Arnesen P, Haldorsen P, Waagbo R (2003) Cataract preventative role of mammalian blood meal, histidine, iron and zinc in diets for Atlantic salmon (Salmo salar L.) of different strains. Aquacult Nutr 9:341–350
Brooks GA (1987) Amino acid and protein metabolism during exercise and recovery. Med Sci Sports Exer 19:S150–S156
Buckley LJ, Caldarone E, Ong TL (1999) RNA-DNA ratio and other nucleic acid-based indicators for growth and condition of marine fishes. Hydrobiologia 401:265–277
Buentello JA, Reyes-Becerril M, Romero-Geraldo MJ, Ascencio-Valle FJ (2007) Effects of dietary arginine on hematological parameters and innate immune function of channel catfish. J Aquat Anim Health 19:195–203
Bulow FJ (1987) RNA-DNA ratio as indicators of growth in fish: a review. In: Summerfelt RC, Hall GE (eds) The age and growth of fish. Iowa State University Press, Ames, pp 45–64
Bureau DP, Encarnacao PM (2006) Adequately defining the amino acid requirements of fish: the case example of lysine. In: Cruz Suarez LE, Ricque Marie D, Tapia Salazar M, Nieto Lopez MG, Villarreal Cavazos, Puello Cruzy AC, Garcia Ortega A (eds) Avances en Nutricion Acuicola VIII. VIII Simposium Internacional de Nutricion Acuicola. 15–17 Noviembre. Universidad Autonoma de Nuevo Leon, Monterrey, Nuevo Leon, Mexico. ISBN 970-694-333-5
Chance RE, Mertz ET, Halver JE (1964) Nutrition of salmonids fishes. XII. Isoleucine, leucine, valine and phenylalanine requirements of chinook salmon and interrelations between isoleucine and leucine for growth. J Nutr 83:177–185
Chiu YN, Austic RE, Rumsey GL (1988) Effect of feeding level and dietary electrolytes on the arginine requirement of rainbow trout (Salmo gairdneri). Aquaculture 69:79–91
Cho CY, Kaushik SJ (1985) Effects of protein intake on metabolizable and net energy values of fish diets. In: Cowey CB, Mackie AM, Bell JG (eds) Nutrition and feeding in fish. Academic Press, London, pp 95–117
Cho CY, Kaushik S, Woodward B (1992) Dietary arginine requirement of young rainbow trout (Oncorhynchus mykiss). Comp Biochem Physiol A 102:211–216
Choo P, Smith TK, Cho CY, Ferguson HW (1991) Dietary excesses of leucine influence growth and body composition of rainbow trout. J Nutr 121:1932–1939
Clemmesen C (1994) The effect of food availability, age or size on the RNA/DNA ratio of individually measured herring larvae: laboratory calibration. Mar Biol 118:377–382
Cowey CB (1992) Nutrition: estimating requirements of rainbow trout. Aquaculture 100:177–189
Dars BA, Narejo NT, Dayo A, Lahsari PK, Laghari MY, Waryani B (2010) Effect of different protein on growth and survival of Catla catla (Hamilton). Reared in glass aquaria. Sindh Univers Res J (Sci Ser) 42:65–68
Davis TA, Fiorotto ML (2009) Regulation of muscle growth in neonates. Curr Opinion Clin Nutr Metabol Care 12:78–85
De’Mello JPF, Lewis D (1971) Amino acid interactions in chick nutrition. 4 Growth, food intake and plasma amino acid patterns. Br J Poult Sci 12:345–358
Dias J, Arzel J, Aguirre P, Corraze G, Kaushik S (2003) Growth and hepatic acetyl coenzyme-A carboxylase activity are affected by dietary protein level in European seabass (Dicentrarchus labrax). Comp Biochem Physiol B 135:183–196
Encarnacao P, de Lange C, Rodehutscord M, Hoehler D, Bureau W, Bureau DP (2004) Diet digestible energy content affects lysine utilization, but not dietary lysine requirements of rainbow trout (Oncorhynchus mykiss) for maximum growth. Aquaculture 235:569–586
Encarnacao P, de Lange C, Bureau DP (2006) Diet energy source affects lysine utilization for protein deposition in rainbow trout (Oncorhynchus mykiss). Aquaculture 261:1371–1381
Erwan E, Alimon AR, Sazili AQ, Yaakub H, Karim R (2009) Effects of varying levels of l-leucine and metabolizable energy in finisher diet on carcass composition and meat sensory characteristics of broiler chickens. Pakist J Nutr 8:792–796
Fagbenro O, Jauncey K (1995) Water stability, nutrient leaching and nutritional properties of moist fermented fish silage diets. Aquacult Eng 14:l43–153
FAO (2006–2012) Cultured aquatic species information programme. Catla catla. Cultured Aquatic Species Information Programme. Text by Jena, J. K. In: FAO Fisheries and Aquaculture Department [online]. Rome
FAO (2012) The state of world fisheries and aquaculture 2012. FAO Fisheries and Aquaculture Department, Rome
Farhat, Khan MA (2014) Response of fingerling stinging catfish, Heteropneustes fossilis (Bloch) to varying levels of dietary l-leucine in relation to growth, feed conversion, protein utilization, leucine retention and blood parameters. Aquacult Nutr 20:291–302
Forster I, Ogata HY (1998) Lysine requirement of juvenile Japanese flounder Paralichthys olivaceus and juvenile red sea bream Pagrus major. Aquaculture 161:131–142
Goldenfarb PB, Bowyer FP, Hall E, Brosious E (1971) Reproducibility in the hematology laboratory: the microhematocrit determination. Am J Clin Pathol 56:35–39
Halver JE (2002) The vitamins. In: Halver JE, Hardy RW (eds) Fish nutrition. Academic Press, San Diego, pp 61–141
Harvey JW (1997) The erythrocyte: physiology, metabolism, and biochemical disorders. In: Kaneko JJ, Harvey JW, Bruss ML (eds) Clinical biochemistry of domestic animals. Academic Press, San Diego, pp 157–203
Hyun Y, Kim JD, Ellis M, Peterson BA, Baker DH, McKeith FK (2007) Effect of dietary leucine and lysine levels on intramuscular fat content in finishing pigs. Can J Anim Sci 87:303–306
ICLARM (2001) The world fish center annual report
Ishibashi T, Ohta Y (1999) Recent advances in amino acid nutrition for efficient poultry production: review. Asian Aus J Anim Sci 12:1298–1309
Jauncey K (1982) The effects of varying dietary protein level on the growth, food conversion, protein utilization and body composition of juvenile tilapias (Sarotherodon mossambicus). Aquaculture 27:43–54
Jauncey K, Tacon AGJ, Jackson AJ (1983) The quantitative essential amino acid requirements of Oreochromis mossambicus. In: Fishelson L, Yaron Z (eds) Proceedings of first international symposium on Tilapia in aquaculture, pp. 328–337, May 8–13
Kaushik SJ (1998) Whole body amino acid composition of European seabass (Dicentrarchus labrax), gilthead seabream (Sparus aurata) and turbot (Psetta maxima) with an estimation of their IAA requirement profiles. Aquat Liv Res 11:355–358
Khan MA, Abidi SF (2011) Dietary arginine requirement of Heteropneustes fossilis fry (Bloch) based on growth, nutrient retention and hematological parameters. Aquacult Nutr 17:418–428
Khan MA, Jafri AK (1991) Dietary protein requirement of two size classes of the Indian major carp, Catla catla Hamilton. J Aquacult Trop 6:79–88
Kim SS, Lee KJ (2013) Comparison of leucine requirement in olive flounder (Paralichthys olivaceus) by free or synthetic dipeptide forms of leucine. Anim Feed Sci Tech 183:195–201
Lin Y, Gong Y, Yuan Y, Gong S, Yu D, Li Q, Luo Z (2013) Dietary l-lysine requirement of juvenile Chinese sucker, Myxocyprinus asiaticus. Aquacult Res 44:1539–1549
Liu FJ, Liu YJ, Tian LX, Chen WD, Yang HJ, Du ZY (2014) Quantitative dietary leucine requirement of juvenile Pacific white shrimp, Litopenaeus vannamei (Boone) reared in low-salinity water. Aquacult Nutr 20:332–340
Luzzana U, Hardy RW, Halver JE (1998) Dietary arginine requirement of fingerling coho salmon (Oncorhynchus kisutch). Aquaculture 163:137–150
Mohanta KN, Mohanty SN, Jena JK, Sahu NP (2008) Optimal dietary lipid level of silver barb, Puntius gonionotus fingerlings in relation to growth, nutrient retention and digestibility, muscle nucleic acid content and digestive enzyme activity. Aquacult Nutr 14:350–359
Murthy HS, Varghese TJ (1997) Dietary requirement of the Indian major carp, Labeo rohita, for the essential amino acid leucine. Mysore J Agricult Sci 31:348–351
Mustafa S, Zofair SM (1985) Seasonal variations in protein, RNA and DNA concentrations in major carps, Catla catla, Labeo rohita and Cirrhinus mrigala. Jpn J Ichthyol 32:258–262
Mustafa S, Lagardere JP, Pastoureaud A (1991) Condition indices and RNA:DNA ratio in overwintering European sea bass, Dicentrarchus labrax, in salt marshes along the Atlantic coast of France. Aquaculture 96:367–374
Nair KS, Schwartz RG, Welle S (1992) Leucine as a regulator of whole body and skeletal muscle protein metabolism in humans. Am J Physiol 263:E928–E934
Ng WK, Hung SSO (1995) Estimating the ideal dietary essential amino acid pattern for growth of white sturgeon, Acipenser transmontanus (Richardson). Aquacult Nutr 1:85–94
Ng WK, Hung SSO, Herold MA (1996) Poor utilization of dietary free amino acids by white sturgeon. Fish Physiol Biochem 15:131–142
Nose T (1979) Summary report on the requirements of essential amino acids for carp. In: Halver JE, Tiews K (eds) Finfish nutrition and fish feed technology. Heenemann, Berlin, pp 145–156
Nose T, Arai S, Lee D, Hashimoto Y (1974) A note on amino acids essential for growth of young carp. Bull Jpn Soc Sci Fish 40:903–908
NRC, National Research Council (2011) Nutrient requirements of fish and shrimps. National Academy Press, Washington
Ogino C (1980) Requirements of carp and rainbow trout for essential amino acids. Bull Jpn Soc Sci Fish 46:171–174
Ravi J, Devaraj KV (1991) Quantitative essential amino acid requirements for growth of catla, Catla catla (Hamilton). Aquaculture 96:281–291
Rodehutscord M, Becker A, Pack M, Pfeffer E (1997) Response of rainbow trout (Oncorhynchus mykiss) to supplements of individual essential amino acids in a semipurified diet, including an estimate of the maintenance requirement for essential amino acids. J Nutr 127:1166–1175
Rodehutscord M, Borchert F, Gregus Z, Pack M, Pfeffer E (2000) Availability and utilization of free lysine in rainbow trout (Oncorhynchus mykiss) 1. Effect of dietary crude protein level. Aquaculture 187:163–176
Rollin X (1999) Critical study of indispensable amino acids requirements of Atlantic salmon (Salmo salar L.) fry. PhD thesis, Universite catholique de Louvain, Louvain
Segovia-Quintero MA, Reigh RC (2004) Coating crystalline methionine with tripalmitin-polyvinyl alcohol slows its absorption in the intestine of Nile tilapia, Oreochromis niloticus. Aquaculture 238:355–367
Shearer KD (2000) Experimental design, statistical analysis and modeling of dietary nutrient requirement studies for fish: a critical review. Aquacult Nutr 6:91–102
Smith TK, Austic RE (1978) The branched-chain amino acid antagonism in chicks. J Nutr 108:1180–1191
Sokal RR, Rohlf FJ (1981) Biometry. W.H. Freeman and Company, New York
Takala T, Hiltunen K, Hassinen E (1980) The mechanism of ammonia production and the effect of mechanical work load on proteolysis and amino acid catabolism in isolated perfused rat heart. Biochem J 192:285–295
Vani T, Saharan N, Roy SD, Ranjan R, Pal AK, Siddaiah GM, Kumar R (2012) Alteration in haematological and biochemical parameters of Catla catla exposed to sub-lethal concentration of cypermethrin. Fish Physiol Biochem 38:1577–1584
Waldroup PW, Kersey JH, Fritts CA (2002) Influence of branched-chain amino acid balance in broiler diets. Int J Poult Sci 1:136–144
Walton MJ, Cowey CB, Adron JW (1982) Methionine metabolism in rainbow trout fed diets of differing methionine and cystine content. J Nutr 112:1525–1535
Walton MJ, Cowey CB, Adron JW (1984) The effect of dietary lysine levels on growth and metabolism of rainbow trout (Salmo gairdneri). Br J Nutr 52:115–122
Walton MJ, Cowey CB, Coloso RM, Adron JW (1986) Dietary requirements of rainbow trout for tryptophan, lysine and arginine determined by growth and biochemical measurements. Fish Physiol Biochem 2:161–169
Wang S, Liu YJ, Tian LX, Xie MQ, Yang HJ, Wang Y, Liang GY (2005) Quantitative dietary lysine requirement of juvenile grass carp Ctenopharyngodon idella. Aquaculture 249:419–429
Wilson RP (1985) Amino acid and protein requirements of fish. In: Cowey CB, Mackie AM, Bell JG (eds) Nutrition and feeding in fish. Academic Press, London, pp 1–15
Wilson RP (2002) Amino acids and protein. In: JE Halver, Hardy RW (eds), Fish nutrition, 3rd edn. Academic Press, San Diego, CA, pp. 143-179
Wilson RP, Poe WE, Robinson EH (1980) Leucine, isoleucine, valine and histidine requirements of fingerling channel catfish. J Nutr 110:627–633
Wu G, Bazer FW, Burghardt RC, Johnson GA, Kim SW, Li XL, Satterfield MC, Spencer TE (2010) Impacts of amino acid nutrition on pregnancy outcome in pigs: mechanisms and implications for swine production. J Anim Sci 88:e195–e204
Yan L, Qinghui A, Kangsen M, Wei X, Zhenyan C, Zhigang HE (2010) Dietary leucine requirement for juvenile large yellow croaker Pseudosciaena crocea (Richardson, 1846). J Ocean Univ China 9:371–375
Zehra S, Khan MA (2012) Dietary protein requirement for fingerling Channa punctatus (Bloch), based on growth, feed conversion, protein retention and biochemical composition. Aquacult Int 20:383–395
Zehra S, Khan MA (2013a) Dietary arginine requirement of fingerling Indian major carp, Catla catla (Hamilton). J World Aquacult Soc 44:363–373
Zehra S, Khan MA (2013b) Dietary isoleucine requirement of fingerling catla, Catla catla (Hamilton), based on growth, protein productive value, isoleucine retention efficiency and carcass composition. Aquacult Int 21:1243–1259
Zehra S, Khan MA (2013c) Dietary lysine requirement of fingerling Catla catla (Hamilton) based on growth, protein deposition, lysine retention efficiency, RNA/DNA ratio and carcass composition. Fish Physiol Biochem 39:503–512
Zehra S, Khan MA (2014a) Dietary threonine requirement of fingerling Indian major carp, Catla catla (Hamilton) estimated by growth, protein retention efficiency, threonine deposition, haematological parameters and carcass composition. Aquacult Res. doi:10.1111/are.12487
Zehra S, Khan MA (2014b) Total sulphur amino acid requirement and maximum cysteine replacement value for methionine for fingerling Catla catla (Hamilton). Aquacult Res. doi:10.1111/are.12493
Zhou H, Chen N, Qiu X, Zhao M, Jin L (2012) Arginine requirement and effect of arginine intake on immunity in largemouth bass, Micropterus salmoides. Aquacult Nutr 18:107–116
Acknowledgments
The authors are grateful to the Chairman, Department of Zoology, Aligarh Muslim University, Aligarh, India, for providing necessary laboratory facilities and also to late Prof. John E. Halver for supporting the Fish Nutrition Research Programme at this laboratory. We also gratefully acknowledge the financial assistance of Maulana Azad National Fellowship-University Grant Commissions for minority students for the year 2010–2011 awarded to one of us (Seemab Zehra) and generous funding received under DST-FIST Programme, New Delhi, in the priority area of Fish Nutrition.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zehra, S., Khan, M.A. Dietary leucine requirement of fingerling Catla catla (Hamilton) based on growth, feed conversion ratio, RNA/DNA ratio, leucine gain, blood indices and carcass composition. Aquacult Int 23, 577–595 (2015). https://doi.org/10.1007/s10499-014-9837-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-014-9837-8