Abstract
In order to determine the dietary isoleucine requirement of fingerling catla, Catla catla (4.25 ± 0.15 cm, 0.61 ± 0.04 g), six isonitrogenous (33.0 % crude protein) and isocaloric (13.7 kJ/g digestible energy) amino acid test diets containing casein, gelatin and l-crystalline amino acids with graded levels of isoleucine (0.5, 0.75, 1.0, 1.25, 1.5 and 1.75 % of the dry diet) were prepared. Triplicate groups of fish were randomly stocked in eighteen 70-l indoor polyvinyl circular troughs at a density of 25 fingerling per trough provided with a water flow-through system (1–1.5 l min−1). The experimental diets were fed to fish to apparent satiation at 08:00, 12:30 and 17:30 h for 12 weeks. Growth of the fish was found to increase with the incremental levels of dietary isoleucine up to 1.25 % of the dry diet. Quadratic regression analysis at 95 % maximum response of absolute weight gain (6.18 g fish−1), protein productive value (0.32), isoleucine retention efficiency (71.91 g fish−1), RNA/DNA ratio (4.81) and carcass protein (15.7 %) yielded the optimum isoleucine requirement in the range of 1.13–1.18 % of the dry diet, corresponding to 3.42–3.58 % of dietary protein. Data generated in this experiment would be useful to formulate isoleucine-balanced, cost-effective quality feeds for fingerling catla.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Successful and sustainable aquaculture depends upon the provision of nutritionally balanced, environmental friendly and economically viable practical feeds (Singh et al. 2006) that are the crucial elements in the culture of aquatic animals. Therefore, knowledge on nutrition and practical feeding of fish is essential for successful aquaculture. In aquaculture, feed is often the single largest operating cost item and can represent over 50 % of the operating costs in intensive aquaculture (El-Sayed 2004). Protein is a basic and most expensive component of fish feeds, both in terms of quantity and quality. Fish require not only a minimum level of protein but also that the essential amino acids are balanced to meet the requirements of each single one (Dacosta-Calheiros et al. 2003). The adjustment of essential amino acid as per the dietary needs of fish not only improves nutritional efficiency and feed conversion efficiency but also influences the utilization of other nutrients. If the essential amino acid requirements of fish species are known, it would be possible to meet these needs by using combinations of different cost-effective protein ingredients. The complete 10 essential amino acid requirements have been established for only a limited number of cultured fish species such as mrigal, Cirrhinus mrigala; Atlantic Salmon, Salmo salar; Common carp, Cyprinus carpio; rohu, Labeo rohita; tilapia, Oreochromis spp.; channel catfish, Ictalurus punctatus; rainbow trout, Oncorhynchus mykiss; and pacific salmon, Oncorhynchus spp. (NRC 2011).
Of the ten essential amino acids, evaluation of isoleucine requirement is of particular importance because isoleucine along with the other two branched-chain amino acids acts as nutrient regulator of protein synthesis and protein degradation. It is also involved in the insulin biosynthesis and secretion (Kimball and Jefferson 2006). In addition to this, it helps in energy production in the body and has been found to reduce twitching and tremors in animals (Braverman et al. 2003). It is the first limiting of the branched-chain amino acids in meat and bone meal as well as the first limiting of those amino acids not available in commercial feed-grade form (Wang et al. 1997).
The dietary requirements of isoleucine have been estimated for different fish species including chinook salmon, O. tshawytscha (Chance et al. 1964); channel catfish, I. punctatus (Wilson et al. 1980); rainbow trout, O. mykiss (Ogino 1980); Mossambique tilapia, O. mossambicus (Jauncey et al. 1983); rohu, L. rohita (Murthy and Varghese 1996; Khan and Abidi 2007); white sturgeon, Acipenser transmontanus (Ng and Hung 1995); red sea bream, Pagrus major (Forster and Ogata 1998); European sea bass, Dicentrarchus labrax; gilthead seabream, Sparus aurata; turbot, Psetta maxima (Kaushik 1998); Atlantic salmon, S. salar (Rollin 1999); mrigal, C. mrigala (Benakappa and Varghese 2003; Ahmed and Khan 2006); and grass carp, Ctenopharyngodon idella (Di et al. 2009).
Among the three Indian major carps, Catla catla is one of the most important, fast-growing commercially cultured fish (FAO 2006–2012). Because of its high nutritional value and good taste, it has greater consumer demand (ICLARM 2001). This fish is used as the integral component in carp polyculture system. Although information on isoleucine requirement of fry C. catla exists (Ravi and Devaraj 1991), no information on isoleucine requirement of fingerling stage of C. catla is available. Hence, this study was undertaken to determine the isoleucine requirement of fingerling C. catla using the dose–response method.
In the present study, absolute weight gain, feed conversion ratio, protein productive value, isoleucine retention efficiency and carcass protein were used as growth indicators to estimate the isoleucine requirement of this fish. Relevance of RNA and DNA data in growth and condition assessment of fish has been documented by a number of authors (Mustafa 1977; Bulow 1987; Abidi and Khan 2009). Considering the significance of RNA/DNA ratio as an index of protein synthetic machinery in cells and therefore as a sensitive indicator of fish growth in response to nutritional status (Clemmesen 1994), this parameter was also employed, in addition to growth parameters, to quantify the dietary isoleucine requirement.
Materials and methods
Experimental diets
Six isonitrogenous (33.0 % crude protein) and isocaloric (13.7 kJ g−1 digestible energy) amino acid test diets using casein (fat-free), gelatin and l-crystalline amino acids with graded levels of isoleucine (0.5, 0.75, 1.0, 1.25, 1.5 and 1.75 % of dry diet) were prepared (Tables 1, 2). The levels of isoleucine in the amino acid test diets were fixed on the basis of information available on other two Indian major carps (Murthy and Varghese 1996; Khan and Abidi 2007; Benakappa and Varghese 2003; Ahmed and Khan 2006). The dietary protein level was fixed at 33 %, which is lower than the optimum protein requirement (35 %) of fingerling C. catla (Khan and Jafri 1991; Dars et al. 2010). This reduction was made to ensure maximum utilization of the limiting amino acid from the diet (Wilson 2002). The amino acid composition of the experimental diets simulated that of 33 % whole chicken egg protein excluding the test amino acid isoleucine. Analysed amino acid composition of 33 % whole chicken egg protein reflected the arginine, histidine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, valine, cystine, tyrosine, alanine, aspartic acid, glutamic acid, proline and serine contents to be 2.11, 0.69, 3.04, 2.38, 1.36, 2.08, 1.43, 0.5, 2.41, 0.79, 1.49, 1.9, 1.14, 2.34, 2.77 and 0.57 % of the dry matter, respectively. The isoleucine content contributed by the casein and gelatin in experimental diets was 0.45 and 0.03 % of dry diet, respectively. The amount of isoleucine was increased at the expense of glycine, on nitrogen to nitrogen to attain the intended concentrations of dietary isoleucine in the amino acid test diets. Diets were made isonitrogenous and isocaloric by adjusting the levels of glycine and dextrin. The diets were designated as I0.5, I0.75, I1, I1.25, I1.5 and I1.75. Method of preparation of experimental diets was the same as detailed earlier (Abidi and Khan 2007). The l-crystalline amino acids were coated with casein and gelatin, and then 10 % of carboxymethyl cellulose was added to provide sufficient water stability which was checked and found to be 96 %. Water stability of the diet was estimated by the method adopted by Fagbenro and Jauncey (1995). In addition to provide sufficient water stability, coating of l-crystalline amino acids also reduces the absorption rate of the amino acids (Cho et al. 1992) and leaching (Alam et al. 2004). We adopted this approach as a safety measure to prevent the leaching of crystalline amino acid from the test diets.
Experimental design and feeding trial
Fries of C. catla were procured from G.B. Pant University of Agriculture and Technology, Pantnagar, transported to the wet laboratory in oxygen-filled polythene bags, given a prophylactic dip in KMnO4 solution (1:3,000) before stocking in indoor circular aqua-blue-coloured, plastic lined (Plastic Crafts Corp, Mumbai, India) fish tanks (1.22 m in diameter and 0.91 m in height; water volume 600 l) for 3 weeks. During this period, the fish were acclimated on a casein–gelatin (33 % CP)-based H-440 diet (Halver 2002) and reared to fingerling stage.
Fingerling C. catla (4.25 ± 0.15 cm, 0.61 ± 0.04 g) were taken from the above acclimated fish lot and stocked in triplicate groups at the rate of 25 fish per trough for each dietary treatment level in 70-l circular polyvinyl troughs (water volume 55 l) fitted with a continuous water flow-through (1–1.5 l min−1) system. Fish were fed the test diets in the form of dry crumbles (0.20–0.25 mm) to apparent satiation thrice daily at 08:00, 12:30 and 17:30 h. Initial and weekly weights were recorded on a top-loading balance (Precisa 120A; 0.1 mg sensitivity, Oerlikon AG, Zurich, Switzerland). Fish were deprived of feed on the day they were weighed. The feeding trial lasted for 12 weeks. Faecal matter was syphoned before every feeding. Water-quality indices were recorded following standard methods (APHA 1992). The range of water temperature, dissolved oxygen, free carbon dioxide, pH, total ammonia nitrogen, nitrites and total alkalinity, based on daily measurements, were 27.1–28.4 °C, 6.4–7.1, 5.8–10.1, 7.2–7.6, 0.27–0.34, 0.03–0.07 and 68.4–81.6 mg l−1, respectively.
Sample collection
At the beginning of the feeding trial, 60 fish were randomly sampled, killed and pooled. Six subsamples of the pooled sample were analysed for initial carcass composition. At the end of the experiment, 15 fishes from each replicate of all the dietary isoleucine treatments were randomly killed and pooled. Three subsamples of the pooled samples were analysed for final carcass composition. Another five fish from each replicate (n = 3 × 5) were anesthetized with MS-222 (tricaine methane sulphonate; 100 μg ml−1), and liver and viscera of each specimen were carefully removed. Weight of fish, viscera and liver were recorded to calculate viscerosomatic index (VSI), hepatosomatic index (HSI) and condition factor (CF). After taking the weight of fish, viscera and liver, muscle tissue was removed from these fish. Muscle samples (100 mg) were homogenized for 5 min in 5 % of trichloroacetic acid (TCA) at 90 °C and then centrifuged at 5,000 rpm for 20 min and the supernatant removed. Three subsamples of each tissue sample were taken for the determination of RNA/DNA ratio.
Evaluation of growth parameters
Growth performance of the fish in response to the experimental diets was measured as a function of the weight gain by calculating the following parameters:
Chemical analyses
Proximate composition of experimental diets, and initial and final carcass were analysed using standard methods (AOAC 1995) for dry matter (oven drying at 105 ± 1 °C for 22 h), crude protein (Kjeldhal nitrogen × 6.25 using Kjeltec TecatorTM Technology 2300, Sweden), crude fat (solvent extraction with petroleum ether B.P 40–60 °C for 2–4 h using Socs Plus, SCS 4, Pelican equipments, Chennai, India) and ash (oven incineration at 650 °C for 2–4 h using muffle furnace, S.M. Scientific Instrument (p) ltd. Jindal Company, India). Amino acid analysis of casein, gelatin, experimental diets, initial and final fish carcass were performed by hydrolysing 0.3 mg sample in 1 ml of 6 N HCl for about 22 h. The sample thus obtained was diluted in 0.02 N HCl and injected in an Automatic Amino Acid Analyzer (Hitachi L-8800, Tokyo, Japan). Recovery hydrolysis was performed in 4 N methanesulfonic acid for the analysis of tryptophan and in performic acid for the recovery of sulphur amino acids.
Determination of muscle RNA and DNA
Muscle RNA and DNA were determined according to Schneider (1957). For the determination of RNA, 2.0 ml of distilled water and 3.0 ml of orcinol reagent were added to 1.0 ml of supernatant. The reaction mixture was kept in boiling water bath for 20 min. The greenish-blue colour thus developed was read at 660 nm in a spectrophotometer (Genesis 10-UV, Thermo Spectronic, Madison, USA). For DNA determination, 1.0 ml of distilled water and 4.0 ml of freshly prepared diphenylamine reagent were added to 1.0 ml of the supernatant. The reaction mixture was kept on a boiling water bath for 10 min. The blue colour developed was measured at 600 nm. Standard curves for RNA and DNA were drawn using different concentrations of yeast RNA and calf thymus DNA, respectively.
Statistical analyses
All growth data were subjected to one-way analysis of variance (Sokal and Rohlf 1981). Differences among treatment means were determined by Tukey’s honestly significant difference (HSD) test at a P < 0.05 level of significance. Dietary isoleucine requirement of fingerling C. catla was estimated by fitting a polynomial quadratic regression to the dose–growth responses relationship (Shearer 2000). The equation employed was Y = aX 2 + bX + c. The requirement was estimated to be that level eliciting 95 % of maximum response (Rodehutscord et al. 1997; Dias et al. 2003; Gaylord et al. 2005). Data analyses were performed using the statistical software Origin (version 6.1; Origin Software, San Clemente, CA, USA).
Results
Absolute weight gain (AWG), feed conversion ratio (FCR), protein productive value (PPV), isoleucine retention efficiency (IRE) and RNA/DNA ratio of fingerling C. catla fed diets with graded levels of isoleucine over the 12-week feeding trial were found to increase significantly with the increase in dietary isoleucine concentrations up to 1.25 % (Table 3). The best values of AWG (6.52 g fish−1), FCR (1.45), PPV (0.35), IRE (74.13 %) and RNA/DNA ratio (5.20) were observed in fish fed diet I1.25. No significant differences (P > 0.05) in growth parameters were recorded in fish fed 1.5 % of isoleucine diet. However, further increase in dietary isoleucine concentration (1.75 %) led to significant fall (P < 0.05) in growth parameters. Feed intake did not differ significantly among treatment groups. No mortality was recorded in all the treatment groups.
Dietary isoleucine concentrations had an impact on carcass composition of fingerling C. catla (Table 4). Moisture content showed a reverse trend to that of the dietary isoleucine concentrations up to 1.25 % (I1.25). Carcass protein content was found to improve quadratically (P < 0.05) with the increase in dietary isoleucine concentrations up to 1.25 % with the highest value (16.54 %) at this level (I1.25). A further inclusion of dietary isoleucine (I1.5–I1.75) resulted in a slight reduction (P > 0.05) in carcass protein. Carcass fat showed a decreasing trend up to 1.25 % of dietary isoleucine (I1.25) beyond that no significant change was recorded. Carcass ash content remained almost the same except those fed diet I0.5 and I0.75.
Somatic indices including HSI, VSI and CF are illustrated in Table 4. The HSI did not show marked variations with the dietary isoleucine levels except for the fish receiving diet I0.5. Fish fed the lowest level of dietary isoleucine (I0.5) had the highest value of HSI. A negative correlation in VSI with the increasing concentrations of dietary isoleucine was noted up to 1.25 % (I1.25). It was found to increase with the further increase in dietary isoleucine (I1.5–I1.75). The CF improved with the increase in dietary isoleucine and was found to be highest at 1.25 %. Fish fed higher levels of dietary isoleucine at 1.5 (I1.5) and 1.75 % (I1.75) showed significant decline in CF.
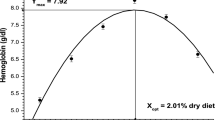
In order to find precise isoleucine requirement, AWG, PPV, IRE, RNA/DNA ratio and carcass protein data were subjected to quadratic regression analysis which at 95 % maximum response exhibited the optimum dietary isoleucine requirement to range between 1.13 and 1.18 % of dry diets which is equivalent to 3.42–3.58 % of dietary protein. The quadratic equations employed for AWG (Fig. 1), PPV (Fig. 2), IRE (Fig. 3), RNA/DNA ratio (Fig. 4) and carcass protein (Fig. 5) are depicted in the respective figures.
Discussion
Dietary isoleucine requirement of fingerling C. catla (3.42–3.58 % dietary protein) worked out in this experiment is found to be higher than that reported for chinook salmon, O. tshawytscha 2.2 % (Chance et al. 1964); common carp, C. carpio 2.5 % (Nose 1979); channel catfish, I. punctatus 2.6 % (Wilson et al. 1980); rainbow trout, O. mykiss 2.4 % (Ogino 1980); Nile tilapia, O. niloticus 3.1 % (Santiago and Lovell 1988); chum salmon, O. keta 2.4 % (Akiyama and Arai 1993); coho salmon, O. kisutch 1.2 % (Arai and Ogata 1993); white sturgeon, A. transmontanus 3.0 % (Ng and Hung 1995); Japanese flounder, Paralichthys olivaceus 2.0 %; red sea bream, P. major 2.2 % (Forster and Ogata 1998); turbot, P. maxima 2.60 % (Kaushik 1998); Atlantic salmon, S. salar 3.2 % (Rollin 1999); mrigal, C. mrigala 3.12–3.15 % (Benakappa and Varghese 2003, Ahmed and Khan 2006) but lower than the requirement reported for milkfish, Chanos chanos 4.0 % (Borlongan and Coloso 1993); grass carp, Ctenopharyngodon idella 4.0–4.23 % (Di et al. 2009) and comparable to the requirement of rohu, L. rohita 3.75 % (Khan and Abidi 2007) and tilapia 3.45 % of dietary protein (NRC 2011). The above discrepancies in amino acid requirements of fish may be affected by fish size and age, adequate levels of other nutrients, flow rate, stock density, and the environmental and culture conditions adopted by different laboratories (Cowey and Luquet 1983; Kim et al. 1992; Forster and Ogata 1998; Luzzana et al. 1998; Abidi and Khan 2009). Nutrient and energy digestibility, amino acid profile and energy content may also alter the amino acid requirements (Simmons et al. 1999; De Silva et al. 2000).
Isoleucine requirement of fingering C. catla in the present study (3.42–3.58 % dietary protein) is higher than the requirement reported by Ravi and Devaraj (1991) for fry stage of this fish (2.35 % dietary protein). Ravi and Devaraj (1991) reported the isoleucine requirement by subjecting the weight gain data to broken-line regression analysis that has been reported to underestimate the requirement (Shearer 2000). However, in this study, the isoleucine requirement is worked out on the basis of quadratic regression analysis indicating high R 2 values (Figs. 1, 2, 3, 4, 5). Adoption of these statistical models may influence the estimate of the isoleucine requirements in both the studies. Moreover, the above low isoleucine requirement reported by Ravi and Devaraj (1991) is based on subjecting the weight gain data to regression analysis. Whereas in this study, the isoleucine requirement is based on subjecting the sensitive growth parameters such as protein productive value, isoleucine retention efficiency, RNA/DNA ratio and carcass protein to quadratic regression analysis in addition to growth. In addition to these, the different dietary protein levels adopted in this study (33 % dry diet) and that reported by Ravi and Devaraj (1991; 40 % dry diet) might also be responsible for the variation in the isoleucine requirements of C. catla. A huge difference in isoleucine requirement estimates in both the studies is evident when requirements are expressed as % protein, and on the other hand, the same difference become minor indeed when requirements are expressed as % dry diet (1.13–1.18 vs. 0.94). Hence, a lower dietary protein level in this study relative to that used by Ravi and Devaraj may also and simply explain higher isoleucine requirement estimates.
Determination of essential amino acid requirements in studies using the dose–response approach requires addition of large amount of crystalline amino acids to obtain graded-level of test amino acid. The incorporation, however, often leads to depressed growth performance and biased amino acid requirement estimates because of crystalline amino acid leaching, different absorption kinetics of crystalline amino acids and poor diet palatability (Ambardekar et al. 2009). Coating of crystalline amino acids can reduce the solubility and non-synchronous absorption of free amino acid relative to the protein-bound ones (Segovia-Quintero and Reigh 2004; Zhou et al. 2012). Ravi and Devaraj (1991) have used the l-crystalline amino acids in unbound form, which might have hampered the amino acid utilization because of reduced gut retention. However, in this study, the l-crystalline amino acids in the experimental diets were coated by casein and gelatin which increases the retention time of the amino acid in the gut leading to more efficient utilization of the ingested amino acids.
In this study, absolute weight gain was found to improve with the increase in concentration of dietary isoleucine up to 1.25 % (I1.25). Further inclusion of isoleucine (I1.5–I1.75) in diets led to reduction in growth of the fish. Similarly, protein productive value was found to improve with the increased inclusion of dietary isoleucine from 0.5 to 1.25 % (I0.5–I1.25). However, a reduction in protein productive value at higher levels of dietary isoleucine (I1.5–I1.75) was recorded. This reduction in growth performance at higher levels of dietary isoleucine may be attributed to amino acid toxicity. It has also been reported that excessive levels of amino acids may become toxic and have an adverse effect on growth because the disproportionate amount of one amino acid affects the absorption and utilization of other amino acids (Murthy and Varghese 1996). The major proportion of the limiting amino acids is used for protein synthesis, while amino acid in excess will be more available for oxidation (Gahl et al. 1996), which may be the cause of growth depression at higher levels of dietary isoleucine.
Nutritional and metabolic interactions among the branched-chain amino acids isoleucine, leucine and valine have been reported for various warm blooded animals, including man (Hambraeus et al. 1976); poultry (De’Mello and Lewis 1971; Smith and Austic 1978); rat (Harper et al. 1970); and the pig (Oestemer et al. 1973). Data on interactions among branched-chain amino acids in fish are not clear-cut and are inconsistent among species (Yamamoto et al. 2004). Chance et al. (1964) reported that isoleucine requirement in chinook salmon was influenced by dietary leucine and that excess dietary isoleucine reduced growth rates when leucine was deficient. In this study, interactions of isoleucine with the other two branched-chain amino acids were not studied.
The RNA/DNA ratio was found to increase with the increased inclusion of dietary isoleucine up to 1.25 % (I1.25). Further inclusion of isoleucine (I1.5–I1.75) resulted in declining RNA/DNA ratio in muscle tissue. Somatic indices including HSI, VSI and CF were also affected by varying concentrations of isoleucine. The HSI value was higher for the group fed the lowest level of dietary isoleucine (I0.5), which may probably be due to excess accumulation of fat in liver. Farhat and Khan (2011) have also reported that the higher value of HSI in fish fed at higher levels of dietary lysine might be due to deposition of fat in liver. A negative correlation in VSI with the increasing concentrations of dietary isoleucine was noted up to 1.25 % (I1.25). Further inclusion of dietary isoleucine at 1.5 (I1.5) and 1.75 % (I1.75) resulted in a slight increment of VSI values. The CF was also found to improve significantly with the increase in the levels of dietary isoleucine and found to be the highest at 1.25 % isoleucine (I1.25) in dry diet. Further increase in dietary isoleucine (I1.5–I1.75) showed a significant drop in CF. Di et al. (2009) also reported the same pattern of RNA/DNA ratio, VSI and CF in grass carp fed graded levels of isoleucine in the diet.
Deficiency of isoleucine causes loss of weight and poor feed conversion in milk fish (Borlongan and Coloso 1993); rohu (Murthy and Varghese 1996; Khan and Abidi 2007); and mrigal (Ahmed and Khan 2006). However, no study has addressed the other pathological signs such as spinal deformities, bilateral cataracts and caudal fin erosion due to deficiency of isoleucine in fish. Except poor growth and feed utilization efficiency, no other diet-related pathological signs were recorded in this study. All fish were found to be in good health condition.
It has been reported that the farmed C. catla deposited significantly higher lipid contents in liver (Hassan et al. 2010). Since the level of fat deposition affects carcass quality, the mobilization of these lipid reserves is essential to improve the carcass quality. As the carcass fat content of this fish was found in the range of 3.26–5.48 % as reported in this study and by earlier workers (Seenappa and Devaraj 1995; Zehra and Khan 2012), this fish is considered to be medium fatty fish as per the classification of Lambertsen (1978). Fatty fish are prone to oxidation. Oxidation of lipids not only produces rancid odours and flavours, but can also decrease nutritional quality and safety by the formation of secondary products (Frankel 1998; Hsieh and Kinsella 1989). Dietary isoleucine contributes to the improvement of the carcass quality as it increases the activity of uncoupling proteins in muscle cells which increased fat burning. Supplementing isoleucine might be a simple way to speed metabolic rate and lose body fat (Di et al. 2009; Nishimura et al. 2010). In this study, carcass fat was found to decrease linearly with the increasing concentrations of dietary isoleucine up to 1.25 % (I1.25). However, carcass protein showed a positive trend with the increasing levels of dietary isoleucine up to 1.25 % (I1.25). A similar trend of carcass fat and carcass protein has also been reported by Di et al. (2009) in grass carp fed graded levels of dietary isoleucine.
In conclusion, the quadratic regression analysis of growth parameters at 95 % maximum response exhibited the optimum dietary isoleucine requirement between 1.13 and 1.18 % of dry diets, corresponding to 3.42–3.58 % of dietary protein, and hence is recommended for fingerling C. catla. Data generated by the present study would be useful in formulating isoleucine-balanced feeds for the intensive culture of fingerling C. catla.
Abbreviations
- AWG:
-
Absolute weight gain
- FCR:
-
Feed conversion ratio
- PPV:
-
Protein productive value
- IRE:
-
Isoleucine retention efficiency
- MS-222:
-
Tricaine methane sulphonate
- VSI:
-
Viscerosomatic index
- HSI:
-
Hepatosomatic index
- CF:
-
Condition factor
- CP:
-
Crude protein
- TCA:
-
Trichloroacetic acid
References
Abidi SF, Khan MA (2007) Dietary leucine requirements of fingerling Indian major carp, Labeo rohita (Hamilton). Aquac Res 38:478–486
Abidi SF, Khan MA (2009) Dietary arginine requirement of fingerling Indian major carp, Labeo rohita (Hamilton) based on growth, nutrient retention efficiencies, RNA/DNA ratio and body composition. J Appl Ichthyol 25:707–714
Ahmed I, Khan MA (2006) Dietary branched-chain amino acid valine, isoleucine and leucine requirements of fingerling Indian major carp, Cirrhinus mrigala (Hamilton). Br J Nutr 96:50–460
Akiyama T, Arai S (1993) Amino acid requirements of chum salmon fry and supplementation of amino acids to diet. In: Collie MR, McVey JP (eds) Proceedings of the Twentieth U.S.—Japan Symposium on Aquaculture Nutrition, UJNR Department of Commerce, Newport, OR, pp 35–48
Alam MS, Teshima S, Koshio S, Ishikawa M (2004) Effects of supplementation of coated crystalline amino acids on growth performance and body composition of juvenile kuruma shrimp Marsupenaeus japonicas. Aquac Nutr 10:309–316
Ambardekar AA, Reigh RC, Williams MB (2009) Absorption of amino acids from intact dietary proteins and purified amino acid supplements follows different time-courses in channel catfish (Ictalurus punctatus). Aquaculture 291:179–187
American Public Health Association (APHA) (1992) Standard methods for the examination of water and wastewater, 18th edn. APHA, Washington, p 1268
Arai S, Ogata H (1993) Quantitative amino acid requirements of fingerling coho salmon. In: Collie MR, McVey JP (eds) Proceedings of the Twentieth U.S. Japan Symposium on Aquaculture Nutrition, UJNR. Department of Commerce, Newport, OR, pp 19–28
Association of Official Analytical Chemists (AOAC) (1995) Official methods of analysis of official analytical chemists international, 16th edn. Association of Official Analytical Chemists, Arlington
Benakappa S, Varghese TJ (2003) Isoleucine, leucine and valine requirement of juvenile Indian major carp, Cirrhinus cirrhosus (Bloch, 1975). Acta Ichthyol Piscat 33:161–172
Borlongan IG, Coloso RM (1993) Requirements of juvenile milkfish (Chanos chanos Forsskal) for essential amino acids. J Nutr 123:125–132
Braverman ER, Pfeiffer CC, Blum K (2003) The healing nutrients within: facts, findings, and new research on amino acids. In: Hirsch C (ed) Branched chain amino acids, 3rd edn. Basic Health Publications, Inc. Laguna Beach, CA, p 434
Bulow FJ (1987) RNA-DNA ratio as indicators of growth in fish: a review. In: Summerfelt RC, Hall GE (eds) The age and growth of fish. Iowa State University Press, Ames, pp 45–64
Chance RE, Mertz ET, Halver JE (1964) Nutrition of salmonids fishes. XII. Isoleucine, leucine, valine and phenylalanine requirements of chinook salmon and interrelations between isoleucine and leucine for growth. J Nutr 83:177–185
Cho CY, Kaushik S, Woodward B (1992) Dietary arginine requirement of young rainbow trout (Oncorhynchus mykiss). Comp Biochem Physiol A 102:211–216
Clemmesen C (1994) The effect of food availability, age and size on the RNA/DNA ratio of individually measured herring larvae: laboratory calibration. Mar Biol 118:337–382
Cowey CB, Luquet P (1983) Physiological basis of protein requirements of fishes. Critical analysis of allowances. In: Prion R, Arnal M, Bonin D (eds) Proceedings of the fourth international symposium on protein metabolism and nutrition. Clermont-Ferrand, France, 5–9 September. INRA Publications, Les Colloques de 1’ INRA, France, pp 365–384
Dacosta-Calheiros MA, Arnason J, Bjornsdottir R (2003) Alternative sources of protein in feed for cultured fish: a case study on Atlantic cod fry (Gasud morhua). The United Nations University, Fisheries Training Programme, Final project, pp 1–33
Dars BA, Narejo NT, Dayo A, Lahsari PK, Laghari MY, Waryani B (2010) Effect of different protein on growth and survival of Catla catla (Hamilton). Reared in glass aquaria. Sindh Univ Res Jour (Sci Ser) 42:65–68
De Silva SS, Gunasekera RM, Gooley G (2000) Digestibility and amino acid availability of three protein rich ingredient incorporated diets by Murray cod Maccullochella peelii peelii (Mitchell) and Australian shortfin eel Anguilla australis Richardson. Aquac Res 31:195–205
De’Mello JPF, Lewis D (1971) Amino acid interactions in chick nutrition. 4 Growth, food intake and plasma amino acid patterns. Br Poult Sci 12:345–358
Di SX, Li L, Hua W, Wen G, Shui WQ, Hui X (2009) Study on isoleucine requirement for juvenile grass carp, Ctenopharyngodon idellus. J Fish China 33:813–822
Dias J, Arzel J, Aguirre P, Corraze G, Kaushik S (2003) Growth and hepatic acetyl coenzyme-A carboxylase activity are affected by dietary protein level in European seabass (Dicentrarchus labrax). Comp Biochem Physiol B 135:183–196
El-Sayed AFM (2004) Protein nutrition of farmed tilapia: searching for unconventional sources. In: Bolivar RB, Mair GC, Fitzsimmons K (eds) Proceedings of the 6th international symposium on tilapia in aquaculture. Central Luzon State University, Manila, Philippines, pp 364–378
Fagbenro O, Jauncey K (1995) Water stability, nutrient leaching and nutritional properties of moist fermented fish silage diets. Aquac Eng 14:l43–l153
FAO (2006-2012) Cultured aquatic species information programme. Catla catla. Cultured Aquatic Species Information Programme. Text by Jena, J.K. In: FAO Fisheries and Aquaculture Department [online]. Rome
Farhat A, Khan MA (2011) Dietary l-lysine requirement of fingerling stinging catfish, Heteropneustes fossilis (Bloch) for optimizing growth, feed conversion, protein and lysine deposition. Aquac Res. doi:10.1111/j.1365-2109.2011.03054.x
Forster I, Ogata HY (1998) Lysine requirement of juvenile Japanese flounder Paralichthys olivaceus and juvenile red sea bream Pagrus major. Aquaculture 161:131–142
Frankel EN (1998) Hydroperoxides. In: Dundee UK (ed) Lipid oxidation, 1st edn. The Oily Press, Dundee, pp 23–41
Gahl MJ, Finke MD, Crenshaw TD, Benevenga NJ (1996) Efficiency of lysine or threonine retention in growing rats fed diets limiting in either lysine or threonine. J Nutr 126:3090–3096
Gaylord TG, Rawles SD, Davis KB (2005) Dietary tryptophan requirement of hybrid striped bass (Morone chrysops × M. saxatilis). Aquac Nutr 11:367–374
Halver JE (2002) The vitamins. In: Halver JE, Hardy RW (eds) Fish nutrition, 3rd edn. Academic Press, San Diego, pp 61–141
Hambraeus L, Bilmazes C, Dippel C, Scrimshaw N, Young VR (1976) Regulatory role of dietary leucine on plasma branched chain amino acid levels in young men. J Nutr 106:320–340
Harper AE, Benevenga NJ, Wohlueter RM (1970) Effects of ingestion of disproportionate amounts of amino acids. Physiol Rev 50:428–558
Hassan M, Chatha SAS, Tahira I, Hussain B (2010) Total lipids and fatty acid profile in the liver of wild and farmed catla catla fish. Grasas Y Aceites, 61: Enero-Marzo, pp 52–57
Hsieh RJ, Kinsella JE (1989) Oxidation of polyunsaturated fatty acids: mechanisms, products and inhibition with emphasis on fish. Adv Food Nutr Res 33:233–241
ICLARM (2001) The World Fish Center annual report
Jauncey K (1982) The effects of varying dietary protein level on the growth, food conversion, protein utilization and body composition of juvenile tilapias (Sarotherodon mossambicus). Aquaculture 27:43–54
Jauncey K, Tacon AGJ, Jackson AJ (1983) The quantitative essential amino acid requirements of Oreochromis mossambicus. In: Fishelson L, Yaron Z (eds) Proceedings of first international symposium on tilapia in aquaculture, May 8–13, pp 328–337
Kaushik SJ (1998) Whole body amino acid composition of European seabass (Dicentrarchus labrax), gilthead seabream (Sparus aurata) and turbot (Psetta maxima) with an estimation of their IAA requirement profiles. Aquat Living Resour 11:355–358
Khan MA, Abidi SF (2007) Dietary isoleucine requirement of fingerling Indian major carp, Labeo rohita (Hamilton). Aquac Nutr 13:424–430
Khan MA, Jafri AK (1991) Dietary protein requirement of two size classes of the Indian major carp, Catla catla Hamilton. J Aquac Trop 6:79–88
Kim KI, Kayes TB, Amundson CH (1992) Requirements for lysine and arginine by rainbow trout (Oncorhynchus mykiss). Aquaculture 106:333–344
Kimball SR, Jefferson LS (2006) New functions for amino acids: effects on gene transcription and translation. Am J Clin Nutr 83:500S–507S
Lambertsen G (1978) Fatty acid composition of fish fats. Comparison based on eight fatty acids. Fisk Dir Skr Ernaering 1:105
Luzzana U, Hardy RW, Halver JE (1998) Dietary arginine requirement of fingerling coho salmon (Oncorhynchus kisutch). Aquaculture 163:137–150
Murthy HS, Varghese TJ (1996) Quantitative dietary isoleucine requirement for growth and survival of Indian major carp, Labeo rohita, (Hamilton) fry. Indian J Exp Biol 34:1141–1143
Mustafa S (1977) Influence of maturation on the concentrations of RNA and DNA in the flesh of the Catfish Clarias batrachus. Trans Am Fish Soc 106:449–451
National Research Council (NRC) (2011) Nutrient requirements of fish and shrimp. National Academy Press, Washington
Ng WK, Hung SSO (1995) Estimating the ideal dietary essential amino acid pattern for growth of white sturgeon, Acipenser transmontanus (Richardson). Aquac Nutr 1:85–94
Nishimura J, Masaki T, Arakawa M, Seike M, Yoshimatsu H (2010) Isoleucine prevents the accumulation of tissue triglycerides and upregulates the expression of PPARα and uncoupling protein in diet-induced obese mice. J Nutr 140:496–500
Nose T (1979) Summary report on the requirements of essential amino acids for carp. In: Halver JE, Tiews K (eds) Finfish nutrition and fish feed technology. Heenemann, Berlin, pp 145–156
Oestemer GA, Hanson LE, Meade RJ (1973) Leucine–isoleucine interrelationship in the young pig. J Anim Sci 36:674–678
Ogino C (1980) Requirements of carp and rainbow trout for essential amino acids. Bull Jpn Soc Sci Fish 46:171–174
Ravi J, Devaraj KV (1991) Quantitative essential amino acid requirements for growth of catla, Catla catla (Hamilton). Aquaculture 96:281–291
Rodehutscord M, Becker A, Pack M, Pfeffer E (1997) Response of rainbow trout (Oncorhynchus mykiss) to supplements of individual essential amino acids in a semipurified diet, including an estimate of the maintenance requirement for essential amino acids. J Nutr 126:1166–1175
Rollin X (1999) Critical study of indispensable amino acids requirements of Atlantic salmon (Salmo salar L.) fry. PhD thesis, Universite catholique de Louvain, Louvain, Belgium
Santiago CB, Lovell RT (1988) Amino acid requirements for growth of Nile tilapia. J Nutr 118:1540–1546
Schneider WC (1957) Determination of nucleic acids in tissue by pantose analysis. In: Calowick SP, Kaplon NO (eds) Methods of enzymology. Academic press, New York, p 680
Seenappa D, Devaraj KV (1995) Effect of different levels of protein, fat and carbohydrate on growth, feed utilization and body carcass composition of fingerlings in Catla catla (Ham.). Aquaculture 129:243–249
Segovia-Quintero MA, Reigh RC (2004) Coating crystalline methionine with tripalmitin-polyvinyl alcohol slows its absorption in the intestine of Nile tilapia, Oreochromis niloticus. Aquaculture 238:355–367
Shearer KD (2000) Experimental design, statistical analysis and modeling of dietary nutrient requirement studies for fish: a critical review. Aquac Nutr 6:91–102
Simmons L, Moccia RD, Bureau DP, Sivak JG, Herbert K (1999) Dietary methionine requirement of juvenile Arctic charr, Salvelinus alpinus (L). Aquac Nutr 5:93–100
Singh RK, Balange AK, Ghughuskar MM (2006) Protein sparing effect of carbohydrates in the diet of Cirrhinus mrigala (Hamilton, 1822) fry. Aquaculture 258:680–684
Smith TK, Austic RE (1978) The branched-chain amino acid antagonism in chicks. J Nutr 108:1180–1191
Sokal RR, Rohlf FJ (1981) Biometry. W.H. Freeman and Company, New York, p 859
Wang X, Castanon F, Parsons CM (1997) Order of amino acid limitation in meat and bone meal. Poult Sci 76:54–58
Wilson RP (2002) Amino acids and protein. In: Halver JE, Hardy RW (eds) Fish nutrition, 3rd edn. Academic Press, San Diego, pp 143–179
Wilson RP, Poe WE, Robinson EH (1980) Leucine, isoleucine, valine and histidine requirements of fingerling channel catfish. J Nutr 110:627–633
Yamamoto T, Shima T, Furutia H (2004) Antagonistic effects of branched chain amino acids induced by excess protein bound leucine in diets for rainbow trout (Oncorhynchus mykiss). Aquaculture 232:539–550
Zehra S, Khan MA (2012) Dietary lysine requirement of fingerling Catla catla (Hamilton) based on growth, protein deposition, lysine retention efficiency, RNA/DNA ratio and carcass composition. Fish Physiol Biochem. doi:10.1007/s10695-012-9715-0
Zhou H, Chen N, Qiu X, Zhao M, Jin L (2012) Arginine requirement and effect of arginine intake on immunity in largemouth bass, Micropterus salmoides. Aquac Nutr 18:107–116
Acknowledgments
The authors are grateful to the Chairman, Department of Zoology, Aligarh Muslim University, Aligarh, India, for providing necessary laboratory facilities and also to Prof. John E. Halver for supporting the Fish Nutrition Research Programme at this laboratory. We also gratefully acknowledge the financial assistance of Maulana Azad National Fellowship-University Grant Commissions for minority students for the year 2010–2011 awarded to one of us (Seemab Zehra) and generous funding received under DST-FIST Programme, New Delhi, in the priority area of Fish Nutrition.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zehra, S., Khan, M.A. Dietary isoleucine requirement of fingerling catla, Catla catla (Hamilton), based on growth, protein productive value, isoleucine retention efficiency and carcass composition. Aquacult Int 21, 1243–1259 (2013). https://doi.org/10.1007/s10499-013-9627-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-013-9627-8