Abstract
A 12-week experiment was conducted to quantify dietary lysine requirement of fingerling Catla catla (3.65 ± 0.05 cm; 0.58 ± 0.02 g) by feeding casein–gelatine-based diets (33.0 % crude protein; 14.3 kJ/g digestible energy) with six levels of l-lysine (1.25, 1.50, 1.75, 2.00, 2.25 and 2.50 % dry diet). The experiment was conducted in eighteen 70-L indoor polyvinyl circular troughs provided with a water flow-through system (1–1.5 L/min). Live weight gain (LWG), feed conversion ratio (FCR), protein deposition (PD), lysine retention efficiency (LRE%) and RNA/DNA ratio were used as the response criteria. Second-degree polynomial regression analysis at 95 % maximum and minimum response of LWG and FCR data exhibited the lysine requirement between 1.8 and 1.9 % dry diet, corresponding to 5.5–5.7 % dietary protein. Regression analysis of PD, LRE and RNA/DNA ratio yielded the requirement between 1.7 and 1.8 % dry diet, corresponding to 5.2–5.5 % dietary protein. Since live weight gain and protein deposition are the key parameters for estimating nutrient requirement, these tools were used to recommend the lysine requirement of fingerling C. catla which ranges between 1.7 and 1.8 % dry diet. Data generated during this study will be useful to formulate lysine-balanced feed for intensive culture of this fish.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fish have quantitative requirements for each essential amino acid. Since amino acids play important and versatile roles in protein metabolism (Wright and Fyhn 2001), dietary inclusion of amino acids should be optimum to achieve maximum growth and health benefits. Of the essential amino acids, lysine is one of the most limiting essential amino acids in ingredients used for the production of commercial fish feeds (Forster and Ogata 1998; Ovi and Eze 2012). Lysine is an essential amino acid present in high proportion in fish muscle tissue, involved in growth and maintenance of positive nitrogen balance and also used in ‘cross-linking’ protein, especially collagen (UNM 2006). Information on dietary lysine requirements of cyprinids such as mrigal Cirrhinus mrigala (Ahmed and Khan 2004); grass carp Ctenopharyngodon idella (Wang et al. 2005); rohu Labeo rohita (Abidi and Khan 2010); common carp, Cyprinus carpio (Zhou et al. 2008); and other cultivable fish species (NRC 2011) are available.
Indian major carp, Catla catla, is a potentially important freshwater fish species cultured in Asia, particularly in the Indian subcontinent as a component of polyculture system. It is a fast growing and an excellent food fish with high market price and adaptability to intensive culture systems. Although information on lysine requirement of the fry stage of C. catla is available (Ravi and Devaraj 1991), no information is available on dietary lysine requirement of fingerling C. catla. Therefore, this study was undertaken to work out the dietary lysine requirement of the fingerling stage of this important cultivated fish species.
Materials and methods
Experimental diet
Six isonitrogenous (33.0 % crude protein) and isocaloric (14.3 kJ/g digestible energy) amino acid test diets using casein (fat-free), gelatine and l-crystalline amino acids with graded levels of l-lysine (1.25, 1.50, 1.75, 2.00, 2.25 and 2.50 % dry diet) were prepared (Table 1). l-crystalline amino acids excluding the test amino acid lysine were used to simulate the amino acid profile of the experimental diets to that of 33.0 % whole chicken egg protein. Diets were made isonitrogenous and isocaloric by adjusting glycine and the dextrin. The levels of lysine in the amino acid test diets were fixed on the basis of information available on other two Indian major carps (Ahmed and Khan 2004; Abidi and Khan 2010). Analysed amino acid composition (% dry diet) of the experimental diets is presented in Table 2. Diets were designated as L1.25, L1.5, L1.75, L2, L2.25 and L2.5. To ensure maximum utilization of the limiting amino acid, the dietary protein level was fixed at 33.0 % which is slightly lower than the optimum protein requirement (35.0 %) of fingerling C. catla reported by Khan and Jafri (1991) and Dars et al. (2010). Method of preparation of experimental diets was the same as detailed earlier (Abidi and Khan 2007). Since l-crystalline amino acids were well coated with casein and gelatine and then bound with 10 % carboxymethyl cellulose, this provided sufficient water stability that was checked according to the method of Fagbenro and Jauncey (1995) and found to be about 98 %.
Experimental design and feeding trial
Induced bred fry C. catla were procured from G. B. Pant University of Agriculture and Technology, Pantnagar. These were then transported to the wet laboratory in oxygen-filled polythene bags, given a prophylactic dip in KMnO4 solution (1:3,000) and stocked in indoor circular aqua-blue coloured, plastic lined (Plastic Crafts Corp, Mumbai, India) fish tanks (1.22 m × 0.91 m × 0.91 m; water volume 600 L) for 3 weeks. During this period, the fish were acclimated to a casein–gelatine-based (33.0 % CP) H-440 diet (Halver 2002) and reared to fingerling stage.
Fingerling C. catla (3.65 ± 0.05 cm; 0.58 ± 0.02 g) were taken from the above acclimated fish lot and stocked in triplicate groups in 70-L circular polyvinyl troughs (water volume 55 L) fitted with a continuous water flow-through (1–1.5 L/min) system at the rate of 25 fish per trough for each dietary treatment level. Fish were fed test diets in the form of dry crumbles (0.20–0.25 mm) to apparent satiation thrice a day at 08.00, 12.30 and 17.30 h. Initial and weekly weights were recorded on a top-loading balance (Precisa 120A; 0.1 mg sensitivity, Oerlikon AG, Zurich, Switzerland) after anaesthetizing the fish with tricaine methanesulphonate (MS-222; 100 μg/mL). Fish were deprived of feed on the day they were weighed. The feeding trial lasted for 12 weeks. Faecal matter was siphoned before every feeding.
Water quality parameters
Water quality parameters were recorded daily during the feeding trial (APHA 1992). The average water temperature, dissolved oxygen, free carbon dioxide, pH, total ammonia nitrogen, nitrites and total alkalinity based on daily measurements were 26.8 ± 1.6 °C, 6.8 ± 0.9 mg/L, 7.9 ± 2.1 mg/L, 7.1 ± 0.5, 0.29 ± 0.03 mg/L, 0.07 ± 0.004 mg/L and 68.1 ± 2.41 mg/L, respectively.
Sample collection
After the end of the 12-week feeding trial, four fish from each replicate of the treatment (n = 3 × 4) were anesthetized with MS-222 (tricaine methanesulphonate; 100 μg/mL). Liver and viscera of each specimen were carefully removed and weights of fish, viscera and liver were recorded to calculate viscerosomatic index (VSI), HSI and condition factor (CF).
Chemical analyses
Proximate composition of experimental diets, and initial and final whole-body carcass was estimated using standard methods (AOAC 1995) for dry matter (oven drying at 105 ± 1 °C for 22 h), crude protein (Kjeltec TecatorTM Technology 2300, Sweden), crude fat (solvent extraction with petroleum ether B.P 40–60 °C for 2–4 h using Socs Plus, SCS 4, Pelican equipments, Chennai, India) and ash (oven incineration at 650 °C for 2–4 h using muffle furnace, S.M. Scientific Instrument (p) ltd. Jindal Company, India 2–4 h). Amino acid analysis of casein, gelatine, experimental diets, initial and final whole-body fish carcass was performed by hydrolysing 0.3 mg sample in 1 mL of 6 N HCl for about 22 h. The sample thus obtained was diluted to 0.02 N HCl and injected in an automatic amino acid analyzer (Hitachi L-8800, Tokyo, Japan). Recovery hydrolysis was performed in 4 N methanesulphonic acid for the analysis of tryptophan and in performic acid for the recovery of sulphur amino acids. At the beginning of the feeding trial, 60 fish were randomly sampled, killed and pooled together. Six subsamples of a pooled sample were analysed for initial whole-body carcass composition. At the end of the experiment, 20 fishes from each replicate of dietary treatments were randomly killed with an overdose of MS-222 and pooled separately. Three subsamples of the pooled samples were analysed for final whole-body carcass composition.
Muscle RNA/DNA ratio determination
Muscle RNA and DNA were determined by the method of Schneider (1957). After the termination of feeding trial, three fish from each replicate of the treatment group (n = 3 × 3) were randomly killed with an overdose of MS-222 and white muscle tissue was removed. Three subsamples of the tissue samples for each replicate of the treatment group were taken for the determination of RNA/DNA ratio. Muscle samples (100 mg) were homogenized for 5 min in 5 % trichloroacetic acid (TCA) at 90 °C and then centrifuged at 5,000 rpm for 20 min. For the determination of RNA, 2.0 mL of distilled water and 3.0 mL of orcinol reagent were added in 1.0 mL of supernatant. The reaction mixture was kept in boiling water bath for 20 min. The greenish-blue colour thus developed was read at 660 nm. For DNA determination, in 1 mL of supernatant, 1.0 mL of distilled water and 4.0 mL of freshly prepared diphenylamine reagent were added. The reaction mixture was kept on a boiling water bath for 10 min. The blue colour developed was measured at 600 nm. Standard curves for RNA and DNA were drawn using different concentrations of yeast RNA and calf thymus DNA, respectively. The values were expressed as μg/100 mg fish muscle tissue on dry basis.
Data analyses
Growth performance of the experimental diets was measured as a function of the weight gain by calculating following parameters:
Statistical analyses
Dietary lysine requirements for fingerling C. catla were estimated by the second-degree polynomial regression analysis (Zeitoun et al. 1976). The equation employed was Y = aX 2 + bX + c. The lysine requirement for maximum growth and protein deposition is defined as the point on the abscissa representing 95 % of the value of the upper asymptote on the ordinate (Dias et al. 2003). Determination of the significance of the trend of these responses was assessed using quadratic contrasts of dietary lysine at a significance level of P < 0.05. All the statistical analyses were performed using Origin (version 6.1; Origin Software, San Clemente, CA, USA).
Results
Growth performance
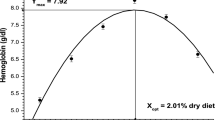
Data related to live weight gain (LWG), feed conversion ratio (FCR), protein deposition (PD) and lysine retention efficiency (LRE%) are illustrated in Table 3. The second-degree polynomial regression analysis of the above parameters at 95 % maximum response exhibited their best values at 1.8 (Fig. 1), 1.9, 1.7 (Fig. 2) and 1.7 % lysine of the dry diet. The equations employed to establish the second-degree polynomial relationship of each variable are as under:
The amino acid test diets were well accepted by the fishes of all the treatment groups and feed intake was not significantly affected by the dietary lysine concentrations (Table 3).
Whole-body composition
Whole-body composition of fish fed varying levels of dietary lysine is presented in Table 4. These parameters were affected by the varying concentrations of dietary lysine. Carcass fat content decreased linearly with the increase in lysine up to 2.0 % of the dry diet (L2), beyond this remained almost unchanged. Moisture content exhibited the reverse trend in contrast to carcass fat. Carcass protein attained the highest value (15.6 %) for the group fed dietary lysine at 1.75 % (L1.75). Dietary treatments did not influence carcass ash content. No mortality was recorded during the entire length of the feeding trial.
Nucleic acid indices
Nucleic acid indices were affected by the varying levels of dietary lysine (Table 4). Muscle DNA concentration was found to decrease with the increase in dietary lysine levels up to 2.0 % (L2) and a slight increase was noted for the groups fed higher levels of lysine at 2.25 and 2.5 % dry diet (L2.25–L2.5). The muscle RNA concentration was found to increase with the increased inclusion of lysine up to 1.75 % of the dry diet (L1.75) and decreased in groups fed higher dietary lysine concentrations at 2.0, 2.25 and 2.5 % (L2, L2.25 and L2.5). The data for RNA/DNA ratio were also subjected to second-degree polynomial regression analysis which at 95 % of maximum response exhibited the lysine requirement at 1.7 % of the dry diet. The second-degree polynomial regression equation employed to calculate the requirement was Y = −5.31X 2 + 20.74X − 15.87 (R 2 = 0.870).
Somatic indices
Somatic indices including HSI, VSI and CF are depicted in Table 4. Fish fed lowest level of dietary lysine (L1.25) had highest HSI value (0.98). Viscerosomatic index was found to decrease with the increase of dietary lysine up to 2.0 % (L2) and then increased in fish fed at higher levels (2.25 and 2.5 %) of dietary lysine (L2.25–L2.5). Graded levels of dietary lysine had an impact on condition factor. Lowest value of CF (0.93) was recorded for the group fed 1.25 % dietary lysine (L1.25). However, it improved (0.93–1.58) with the increase in dietary lysine concentrations up to 2.0 % (L2) and declined for the groups receiving higher dietary lysine concentrations (L2.25–L2.5).
Amino acid composition of fish carcass
The amino acid composition of fish carcass fed diets with increasing levels of lysine is given in Table 5. Lysine content of fish carcass was significantly affected by dietary lysine levels. Fish fed diet containing 1.75 % lysine (L1.75) showed highest lysine content. Whereas lowest lysine content was recorded in group receiving 1.25 % dietary lysine (L1.25). However, no significant (P > 0.05) change in concentrations of other amino acids was noted.
Second-degree polynomial regression analysis at 95 % maximum and minimum response of LWG, FCR, PD, LRE and RNA/DNA ratio against dietary lysine concentrations yielded the optimal values between 1.7 and 1.9 % of the dry diet, corresponding to 5.2–5.8 % of dietary protein.
Discussion
Since live weight gain and protein deposition are the key parameters for estimating requirement (Encarnacao et al. 2004), these parameters were used to estimate the lysine requirement of fingerling C. catla. The second-degree polynomial regression analysis of LWG and PD exhibited the lysine requirement of fingerling C. catla in the range of 1.7–1.8 % dry diet. The requirement obtained in this study is lower than that reported for common carp C. carpio, 2.2 %; rohu L. rohita, 2.3 %; tilapia O. spp., 2.4 %; rainbow trout O. mykiss, 2.4 %; Atlantic Salmon S. salar, 2.4 %; pacific salmon O. spp., 2.2 % (NRC 2011) and comparable to the requirement of O. spp. 1.6 %; channel catfish I. punctatus, 1.6 % of the dry diet (NRC 2011). The above discrepancies in lysine requirements among fishes may be attributed to differences in metabolic requirements of the species and daily protein consumption by fish, dietary formulations and feeding regimes used in the classical dose–response experiments (Cowey 1993; Fagbenro et al. 1998). In addition, use of different mathematical methods for fitting the response and estimating requirement results in different estimates of amino acid requirements (Rodehutscord and Pack 1999).
The lysine requirement of fingerling C. catla worked out during this study (1.7–1.8 % dry diet) is lower than the requirement reported by Ravi and Devaraj (1991) for fry stage of this fish (2.5 % dry diet). Higher amino acid requirement estimates are associated with the smaller size fish, whereas it becomes comparatively lower with the advancing stages probably as a result of the higher rates of protein synthesis and growth displayed by smaller individuals (Houlihan et al. 1986; Moltschaniwskyj and Carter 2010). This may be the reason for the differences in the lysine requirement as fish under study were in fingerling stage requiring lower dietary nutrient requirements for the metabolical and physiological activities than the fry stage of the fish in the study conducted by Ravi and Devaraj (1991). Differences in the lysine requirement of similar species may be due to differences in experimental design, available dietary energy and the composition of the specific dietary protein (Wilson 1984). Ravi and Devaraj (1991) have used the l-crystalline amino acids in unbound form hampering the amino acid utilization by lowering its gut retentivity, hence yielding to a higher requirement estimates. However, in present study, the l-crystalline amino acids were coated with casein and gelatine that have promoted the retention time of the amino acid in the gut leading to more efficient utilization of the ingested amino acids. These methodological biases may probably be the reasons for the differences obtained in the requirement estimates of the two studies.
In the present study, live weight gain of fingerling C. catla was found to improve quadratically up to 1.8 % dietary lysine (Y 95 %max) and then declined with further increase in dietary lysine levels. This trend of weight gain is in agreement with the results reported by earlier workers (Ahmed and Khan 2004; Wang et al. 2005; Mai et al. 2006; Dairiki et al. 2007; Bicudo et al. 2009; Abidi and Khan 2010; Xie et al. 2012; Furuya et al. 2012). The reduction in the growth of fish fed higher than required levels of dietary lysine may either be due to the stress caused by excess amount of amino acid in the body of the fish leading to extra energy expenditure towards deamination and excretion (Abidi and Khan 2010).
The RNA/DNA ratio has been considered to be a promising indicator of growth as it is closely related to the rate of protein synthesis (Tanaka et al. 2007). In the present study, muscle RNA/DNA ratio was found to improve quadratically up to 1.7 % lysine of the dry diet. The dietary imbalance of amino acids affects the efficiency of protein utilization. Lysine is primarily used for protein deposition (Firman 2004) which in this study was found to improve quadratically with the increased inclusion of dietary lysine up to 1.7 % (Y 95 %max), and a reduction was noted for the groups fed dietary lysine above this level. Carcass protein also followed the same trend. Carcass lipid responded negatively with the increasing concentrations of dietary lysine up to 2.0 % (L2). This indicates that a better dietary amino acid balance probably prevents the selective catabolism of amino acids and consequently increases protein synthesis, while decreasing the accumulation of lipid reserves (Tantikitti and Chimsung 2001; Conceicao et al. 2003). The declining trend of carcass fat content as observed in this study may also be due to the fact that lysine is the precursor of l-carnitine (Rebouche 1992) which acts as a lipolytic factor thus contributing to a reduction in body fat deposition (Dias et al. 2001). It also facilitates the removal of short-chain organic acids from mitochondria, thereby freeing intramitochondrial coenzyme A to participate in the β-oxidation and tricarboxylic acid cycle pathways which could avoid accumulation of lipid in fish body (Ozorio et al. 2003). The low carcass lipid content recorded for fish fed incremental levels of dietary lysine as evident in this study is in agreement with Ruchimat et al. (1997), Keshavanth and Renuka (1998), Mai et al. (2006) and Ma et al. (2007). Common adverse effects of lysine deficiency in fish are slow growth rate and poor protein utilization. These deficiency symptoms were also recorded in this study. Hence, optimizing dietary lysine is prerequisite for growth and protein deposition in fingering C. catla.
Second-degree polynomial regression analysis of LWG and PD data exhibited the lysine requirement between 1.7 and 1.8 % dry diet which is recommended to prepare lysine-balanced, cost-effective practical feeds for intensive aquaculture of this valuable Indian major carp species.
Abbreviations
- LWG:
-
Live weight gain
- FCR:
-
Feed conversion ratio
- PD:
-
Protein deposition
- LRE:
-
Lysine retention efficiency
- MS-222:
-
Tricaine methanesulphonate
- VSI:
-
Viscerosomatic index
- HSI:
-
Hepatosomatic index
- CF:
-
Condition factor
- CP:
-
Crude protein
- TCA:
-
Trichloroacetic acid
References
Abidi SF, Khan MA (2007) Dietary leucine requirement of fingerling Indian major carp, Labeo rohita (Hamilton). Aquacult Res 38:478–486
Abidi SF, Khan MA (2010) Growth, protein retention, and body composition of fingerling Indian major carp, rohu, Labeo rohita (Hamilton), fed diets with various levels of lysine. J World Aquac Soc 41:791–799
Ahmed I, Khan MA (2004) Dietary lysine requirement of fingerling Indian major carp, Cirrhinus mrigala (Hamilton). Aquaculture 235:499–511
American Public Health Association (APHA) (1992) Standard methods for the examination of water and wastewater, 18th edn. APHA, Washington, p 1268
Association of Official Analytical Chemists (AOAC) (1995) Official methods of analysis of official analytical chemists international, 16th edn. Association of Official Analytical Chemists, Arlington
Bicudo AJA, Sado RY, Cyrino JEP (2009) Dietary lysine requirement of juvenile pacu Piaractus mesopotamicus (Holmberg, 1887). Aquaculture 297:151–156
Conceicao LEC, Grasdalen H, Ronnestad I (2003) Amino acid requirements of fish larvae and post-larvae: new tools and recent findings. Aquaculture 227:221–232
Cowey CB (1993) Recommendations of technical sessions. In: Gropp JM, Tacon AGJ (eds) Report of the EIFAC workshop methodology for determination of nutrient requirements in fish EIFAC/OP 29. Food and Agricultural Organization of the United Nations, Rome
Dairiki JK, Dias CTS, Cyrino JEP (2007) Lysine requirement of largemouth bass, Micropterus salmoides: a comparison of methods of analysis of dose–response trials data. J Appl Aquacult 19:1–27
Dars BA, Narejo NT, Dayo A, Lashari PK, Laghari MY, Waryani B (2010) Effect of different protein on growth and survival of Catla catla (Hamilton). Reared in glass aquaria. Sindh Univ Res Jour (Sci Ser) 42:65–68
Dias J, Arzel J, Corraze G, Kaushik J (2001) Effects of dietary l-carnitine supplementation on growth and lipid metabolism in European seabass (Dicentrarchus labrax). Aquacult Res 32:206–215
Dias J, Arzel J, Aguirre P, Corraze G, Kaushik S (2003) Growth and hepatic acetyl coenzyme-A carboxylase activity are affected by dietary protein level in European seabass (Dicentrarchus labrax). Comp Biochem Phys B 135:183–196
Encarnacao P, Lange DC, Rodehutscord M, Hoehler D, Bureau W, Bureau DP (2004) Diet digestible energy content affects lysine utilization, but not dietary lysine requirements of rainbow trout (Oncorhynchus mykiss) for maximum growth. Aquaculture 235:569–586
Fagbenro O, Jauncey K (1995) Water stability, nutrient leaching and nutritional properties of moist fermented fish silage diets. Aquacult Eng 14:143–153
Fagbenro OA, Balogun AM, Bello-Olusoji OA, Fasakin EA (1998) Dietary lysine requirement of the African Catfish, Clarias gariepinus. J Appl Aquacult 8:71–77
Firman J (2004) Digestible lysine requirements of male Turkeys in their 1st six weeks. Int J Poult Sci 3:373–377
Forster I, Ogata HY (1998) Lysine requirement of juvenile Japanese flounder (Paralichthys olivaceus) and juvenile red sea bream (Pagrus major). Aquaculture 161:131–142
Furuya WM, Graciano TS, Vidal LVO, Xavier TO, Gongora LD, Righetti JS, Furuya VRB (2012) Digestible lysine requirement of Nile tilapia fingerlings fed arginine-tolysine-Balanced diets. R Bras Zootec 41:485–490
Halver JE (2002) The vitamins. In: Halver JE, Hardy RW (eds) Fish nutrition, 3rd edn. Academic Press, San Diego, pp 61–141
Houlihan DF, McMillan DN, Laurent P (1986) Growth rates, protein synthesis and protein degradation rates in rainbow trout: effect of body size. Physiol Zool 59:482–493
Jauncey K (1982) The effects of varying dietary protein level on the growth, food conversion, protein utilization and body composition of juvenile tilapias (Sarotherodon mossambicus). Aquaculture 27:43–54
Keshavanth P, Renuka P (1998) Effect of dietary l-carnitine supplements on growth and body composition of fingerling rohu, Labeo rohita (Hamilton). Aquacult Nutr 4:83–87
Khan MA, Jafri AK (1991) Dietary protein requirement of two size classes of the Indian major carp, Catla catla Hamilton. J Aquacult Trop 6:79–88
Ma JJ, Xu ZR, Shao QJ, Xu JZ, Hung SSO, Hu WL, Zhuo LY (2007) Effect of dietary supplemental l-carnitine on growth performance, body composition and antioxidant status in juvenile black sea bream, Sparus macrocephalus. Aquacult Nutr 14:464–471
Mai K, Zhang L, Ai Q, Duan Q, Zhang C, Li H, Wan J, Liufu Z (2006) Dietary lysine requirement of juvenile seabass (Lateolabrax japonicas). Aquaculture 258:535–542
Moltschaniwskyj NA, Carter CG (2010) Protein synthesis, degradation, and retention: mechanisms of indeterminate growth in cephalopods. Physiol Bio Zool 83:997–1008
National Research Council (NRC) (2011) Nutrient requirements of fish and shrimp. National Academy Press, Washington
Ovi SO, Eze SS (2012) Lysine requirement and its effect on the body composition of Oreochromis niloticus fingerlings. J Fish Aquat Sci. doi:10.3923/jfas.2012
Ozorio ROA, Verreth JAJ, Aragao CR, Vermeulen CJ, Schrama JW, Verstegen MWA, Huisman EA (2003) Dietary carnitine supplements increased lipid metabolism and decreased protein oxidation in African catfish (Clarias gariepinus) juveniles fed high fat levels. J Aquacult Trop 18:225–238
Ravi J, Devaraj KV (1991) Quantitative essential amino acid requirements for growth of catla, Catla catla (Hamilton). Aquaculture 96:281–291
Rebouche CJ (1992) Carnitine function and requirements during the life cycle. FASEB J 6:3379–3386
Rodehutscord M, Pack M (1999) Estimates of essential amino acid requirements from dose–response studies with rainbow trout and broiler chicken: effect of mathematical model. Arch Anim Nutr 52:223–244
Ruchimat T, Masumoto T, Hosokawa H, Itoh Y, Shimeno S (1997) Quantitative lysine requirement of yellowtail (Seriola quinquiradiata). Aquaculture 158:331–339
Schneider WC (1957) Determination of nucleic acids in tissue by pantose analysis. In: Calowick SP, Kaplon NO (eds) Methods of enzymology. Academic press, New York, p 680
Tanaka Y, Gwak WS, Tanaka M, Sawada Y, Okada T, Miyashita S, Kumai H (2007) Ontogenetic changes in RNA, DNA and protein contents of laboratory-reared Pacific bluefin tuna Thunnus orientalis. Fish Sci 73:378–384
Tantikitti C, Chimsung N (2001) Dietary lysine requirement of freshwater catfish (Mystus nemurus Cuv. and Val.). Aquacult Res 32:135–141
University of Maryland—UNM (2006) Lysine. Edu. Maryland. Available at: http://www.unm. Accessed on 25 July 2006
Wang S, Liu YJ, Tian LX, Xie MQ, Yang HJ, Wang Y, Liang GY (2005) Quantitative dietary lysine requirement of juvenile grass carp Ctenopharyngodon idella. Aquaculture 249:419–429
Wilson RP (1984) Proteins and amino acids. In: Robinson EH, Lovell RT (eds) Nutrition and feeding of channel catfish. Southern Cooperative Services Bulletin No. 296. Texas A & M University, College Station, pp 5–11
Wright PA, Fyhn HJ (2001) Ontogeny of nitrogen metabolism and excretion. In: Wright PA, Anderson PM, Wright PA, Fyhn HJ (eds) Nitrogen excretion, Fish Physiology 20. Academic Press, New York, pp 149–200
Xie F, Ai Q, Mai K, Xu W, Wang X (2012) Dietary lysine requirement of large yellow croaker (Pseudosciaena crocea, Richardson 1846) larvae. Aquacult Res 43:917–928
Zhou XQ, Zhao CR, Jiang J, Feng L, Liu Y (2008) Dietary lysine requirement of juvenile Jian carp (cyprinus carpio var. Jian). Aquacult Nutr 14:381–386
Zeitoun IH, Ullrey DE, Magee WT, Gill JL, Bergen WG (1976) Quantifying nutrient requirements of fish. J Fish Res Board Can 33:167–172
Acknowledgments
The authors are grateful to the Chairman, Department of Zoology, Aligarh Muslim University, Aligarh, India for providing necessary laboratory facilities and also to Prof. John E. Halver for supporting the Fish Nutrition Research Programme at this laboratory. We also gratefully acknowledge the financial assistance from Maulana Azad National Fellowship-University Grant Commissions for minority students for the year 2010–2011 awarded to one of us (Seemab Zehra) and generous funding received under DST-FIST Programme, New Delhi in the priority area of Fish Nutrition.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zehra, S., Khan, M.A. Dietary lysine requirement of fingerling Catla catla (Hamilton) based on growth, protein deposition, lysine retention efficiency, RNA/DNA ratio and carcass composition. Fish Physiol Biochem 39, 503–512 (2013). https://doi.org/10.1007/s10695-012-9715-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-012-9715-0