Abstract
Ischemic stroke is the leading cause of human disability and mortality in the world. The main problem in stroke therapy is the search of efficient neuroprotector capable to rescue neurons in the potentially salvageable transition zone (penumbra), which is expanding after brain damage. The data on molecular mechanisms of penumbra formation and expression of diverse signaling proteins in the penumbra during first 24 h after ischemic stroke are discussed. Two basic features of cell death regulation in the ischemic penumbra were observed: (1) both apoptotic and anti-apoptotic proteins are simultaneously over-expressed in the penumbra, so that the fate of individual cells is determined by the balance between these opposite tendencies. (2) Similtaneous and concerted up-regulation in the ischemic penumbra of proteins that execute apoptosis (caspases 3, 6, 7; Bcl-10, SMAC/DIABLO, AIF, PSR), signaling proteins that regulate different apoptosis pathways (p38, JNK, DYRK1A, neurotrophin receptor p75); transcription factors that control expression of various apoptosis regulation proteins (E2F1, p53, c-Myc, GADD153); and proteins, which are normally involved in diverse cellular functions, but stimulate apoptosis in specific situations (NMDAR2a, Par4, GAD65/67, caspase 11). Hence, diverse apoptosis initiation and regulation pathways are induced simultaneously in penumbra from very different initial positions. Similarly, various anti-apoptotic proteins (Bcl-x, p21/WAF-1, MDM2, p63, PKBα, ERK1, RAF1, ERK5, MAKAPK2, protein phosphatases 1α and MKP-1, estrogen and EGF receptors, calmodulin, CaMKII, CaMKIV) are upregulated. These data provide an integral view of neurodegeneration and neuroprotection in penumbra. Some discussed proteins may serve as potential targets for anti-stroke therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stroke is one of the major causes of human death and severe disability, which requires regular attention and care from family members. It is a great medical and social problem. More than 17 million stroke incidences occur in the world each year. 30–35% of strokes induce human death. About 10–15% of survived patients die during a year. Other patients have different neurological problems [1, 2]. In ischemic stroke (70–80% of all strokes) occlusion of cerebral blood vessels very quickly, for few minutes causes oxygen and glucose depletion, ATP deficit, spreading depolarization, excitotoxicity, and tissue infarct. It is impossible to rescue neurons in the infarction core for such short time. However, the injury propagates to neighboring cells and forms the transition zone (ischemic penumbra) between the ischemic core and normal tissue. Tissue damage in the penumbra develops slower, for several hours, and this “therapeutical window” provides time to rescue cells and restrict the infarction volume [1,2,3,4].
Despite extensive testing of hundreds of drugs such as glutamate antagonists, blockers of Ca2+ channels, antioxidants and other pro-survival chemicals, no one effective anti-stroke neuroprotector medication has been found and approved so far. Even drugs, which protect ischemic neurons in in vitro and in vivo experiments, are either ineffective for humans, or cause adverse negative effects [3,4,5,6,7,8,9,10]. Therefore, deep and comprehensive studies of molecular mechanisms that control neurodegeneration and neuroprotection in the ischemic penumbra are needed.
Unlike ischemic core, where cells die mostly through necrosis, apoptosis prevails in the penumbra [4, 10,11,12]. Although necrosis, necroptosis, or autophagy as well as mixed or hybrid cell death modes also occur in the penumbra [13,14,15], we focus on the apoptosis regulation in the penumbra in first hours after ischemic stroke when this tissue may be salvaged. We consider the molecular processes that relate the primary excitotoxic factors to the secondary signaling processes, which control apoptosis in the ischemic penumbra, namely the expression of various pro- and antiapoptotic proteins. Some important problems of stroke biochemistry such as recovery processes that occur after the first post-stroke day and searches of pharmacological anti-stroke agents remain beyond the scope of this review. We also consider only the results of in vivo experiments, but not the data obtained on cultured cells.
Experimental stroke models
The ischemic brain damages vary from mild circulatory disorders (transient ischemic attack, microinsult, or occlusion of small cerebral vessels) to focal or global stroke. Different animal models of cerebral ischemia that display some but not all stroke features are currently used in studies of molecular mechanisms of the ischemic brain damage and in testing of novel anti-stroke neuroprotector agents [16,17,18,19,20].
Global cerebral ischemia induced by ligation of carotid arteries (two-vessel or four-vessel occlusion models) is relevant to cardiac arrest and asphyxia in humans. Its disadvantages include the complicated surgery, occurrence of seizures, variable outcome and poor reproducibility.
Focal ischemic stroke is induced by permanent or transient occlusion of the middle cerebral artery (MCAO). In the permanent model, arterial blockade is created by vessel electrocoagulation, or by intra-arterial insertion of a nylon thread for one or more days. In the transient model, the bloodstream is blocked by a nylon filament for 0.5–2 h. This procedure does not require craniotomy. The duration of occlusion determines the extent of ischemic lesion. The infarct volume is rather large and well reproducible. The ischemic penumbra is similar to that in human stroke. However, MCAO requires anesthesia and significant surgical skill. It is characterized with a risk of vessel rupture, hemorrhage and relatively high mortality. The transient ischemia is followed by tissue reperfusion that causes secondary oxidative injury. The thromboembolic model is similar, but arteries are occluded by an exogenous thrombin clot, or by synthetic microspheres injected into internal carotid artery. This method is more relevant to human stroke, but location, size and duration of ischemia are poorly predictable and reproducible [16,17,18].
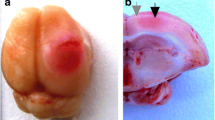
In photothrombotic stroke (PTS) focal cerebral infarct is induced by localized photodynamic effect, in which the hydrophilic dye Rose Bengal that poorly penetrates cells and remains in the cerebral vasculature, is used. Localized light exposure induces photoexcitation of Rose Bengal molecules, oxidative lesion of endothelial membranes, platelet aggregation, and occlusion of small vessels. This stroke model is little invasive and simple. The injury degree, location, and size are well controlled and reproducible. Animal mortality is low. PTS mimics the permanent ischemic occlusion of small vessels [20,21,22,23].
Primary pathological events in the ischemic core
Pathological processes in the ischemic core include several interlinked components (Fig. 1). Vessel occlusion rapidly prevents glucose and oxygen delivery. This inhibits oxidative phosphorylation and suppresses ATP production. Following activation of anaerobic glycolysis causes accumulation of lactic acid and tissue acidosis. ATP depletion leads to failure of Na+/K+- and Ca2+-ATPases, fall of ionic gradients, Na+ and Ca2+ influx, and K+ efflux. Extracellular K+ mediates depolarization of neighboring cells. Water penetration causes swelling of the cell and intracellular organelles, membrane disruption, and edema [1,2,3,4, 10, 17,18,19]. Massive glutamate release activates NMDA and AMPA receptors in neighboring cells and induces their excitotoxic death. NMDA receptors (NMDARs) were suggested to play the major role in excitotoxicity and stroke pathology [24, 25].
Cytosolic Ca2+ overload and oxidative stress are the key factors in ischemic cell damage. Dramatic increase in the cytosolic Ca2+ level occurs due to penetration through NMDARs and voltage-operated Ca2+ channels, which are opened under depolarization. Ca2+ also releases from mitochondria and endoplasmic reticulum. The failure of Ca2+-ATPases in ER and in the plasma membrane, as well as the inability of Na+/Ca2+ exchanger to extrude Ca2+ in the situation of the Na+ gradient fall, also lead to Ca2+ accumulation in the cytosol [26,27,28,29]. The perturbation of mitochondrial electron transport causes leakage of electrons onto oxygen and production of superoxide-anion (O2−) and other reactive species (ROS). Oxidative stress includes lipid peroxidation, protein dysfunction and DNA damage. Severe oxidative stress in the ischemic core causes necrosis, whereas the weaker cell damage in the penumbra elicits predominately apoptosis [30,31,32]. Ca2+-activated neuronal NO synthase, which is often linked spatially with NMDARs, produces nitric oxide that reacts with O2− to produce peroxynitrite (ONOO−), a powerful oxidant [33].
Diffusible factors that spread the injury and induce penumbra formation
Glutamate, K+, ROS, NO, acidosis, and edema are the main pathogenic factors that can spread the injury from the ischemic core and induce formation of the ischemic penumbra (Table 1). Glutamate released from the damaged neurons induces excitotoxicity in neighboring cells [24, 25]. Extracellular K+ depolarizes neighboring cells that leads to opening of voltage-operated Ca2+ channels and Ca2+ channels associated with NMDAR receptors. Both processes result in Ca2+ influx [25,26,27,28,29]. Acidosis disrupts Na+/Ca2+ exchanger, which removes Ca2+ from the cell, and thus, contributes to Ca2+ overload. Cytosolic Ca2+ activates diverse proteinases, lipases, and nucleases that destruct cellular components. Ca2+-activated calpain and cathepsins play the essential role in neurodegeneration. Intracellular Ca2+ induces mitochondrial dysfunction, ROS generation, and NO production [26,27,28].
The relatively long-living ROS such as H2O2 and O2− diffuse between organelles and between cells. They can activate both pro-survival and pro-apoptotic signaling cascades [34]. Oxidative injury of ER and mitochondrial membranes stimulates the release of stored Ca2+ and mitochondrial pro-apoptotic proteins such as cytochrome c, Smac/DIABLO, AIF, endonuclease G into the cytosol [27, 28, 35]. ROS target proteins containing thiol groups in the active center. For example, ROS-sensitive apoptosis signal-regulating kinase 1 (ASK1) activates MAP kinase JNK that stimulates apoptosis. In contrast, ERK, another MAP kinase, maintains cell survival [36, 37]. Ca2+ and ROS-mediated signaling pathways stimulate various transcription factors such as NF-κB, AP-1, CREB, STAT, etc. that regulate expression of proteins involved in cell survival or death [32].
Nitric oxide, another diffusible messenger, exerts dual effects on surrounding tissue. At the initial ischemia stage, NO produced by endothelial NO synthase promotes vasodilatation that maintains blood supply and plays the protective role. However, Ca2+ overload stimulates neuronal NO synthase to produce neurotoxic amount of NO. Later, transcription factor NF-κB stimulates the expression of inducible NOS, which produces NO more efficiently. ROS and peroxynitrite induce oxidative damage to membrane lipids and proteins and cause mitochondrial dysfunction [33, 38].
Cell death types in the ischemic penumbra
Main neuronal death types: necrosis, apoptosis, and autophagic death have been subdivided recently by different subtypes such as intrinsic and extrinsic apoptosis, oncosis, necroptosis, parthanatos, ferroptosis, autophagy, autolysis, pyroptosis, etc. [15]. Necroptosis, a regulated necrosis, in which disruption of the cell membrane is mediated by the RIP1/RIP3/MLKL cascade was found to occur in ischemic stroke [39, 40]. Nevertheless, apoptosis is the dominating cell death modality in the ischemic penumbra [1,2,3,4, 10,11,12].
The ischemic penumbra is spatially inhomogeneous. Electron-microscopy showed the lesion gradient across the penumbra in the rat cerebral cortex: strong tissue destruction near the ischemic core, and weak alteration at the penumbra periphery [20]. According to [3], the necrotic core is surrounded by the narrow transient zone with low ATP level, in which both necrosis and apoptosis occur, and by the wider penumbra with higher ATP level, where apoptosis prevails. Necrosis occurs during first post-stroke minutes, whereas apoptosis develops later, for hours or days. Apoptosis requires the integrity of the plasma membrane, overload of cytosolic Ca2+ that activates various proteases and nucleases, and stimulation of caspase cascade that triggers cell destruction [41]. Apoptosis results in DNA fragmentation, degradation and cross-linking of cytoskeletal and nuclear proteins, formation of apoptotic bodies, expression of ligands for phagocytic cell receptors and finally phagocytosis by microglia and neghboring astrocytes [15, 42]. Apoptotic DNA fragmentation was observed in the penumbra early after stroke. Fragmented nuclei appeared as soon as 0.5–4 h after focal cerebral ischemia and reached the maximum at 24–48 h [12, 15, 41]. Unlike necrosis, which is irreversible and cannot be prevented, apoptosis is highly regulated and can be, in principal, suppressed by different pharmacological agents. Therefore, cerebral tissue in the penumbra is potentially salvageable. Suppression of apoptosis is one of the neuroprotective strategies in stroke therapy [10, 15, 42,43,44].
The dichotomy between necrosis in the infarct core and apoptosis in the penumbra is not strict. Ultrastructural and biochemical data sometimes revealed signs of apoptotic cell death in the infarct core, which is usually considered as a necrotic zone [11]. On the other hand, necrotic, apoptotic, and hybrid cells were found in the ischemic penumbra. The intermediate, or hybrid death of some cells has features of concurrent apoptotic and necrotic alterations such as proapoptotic activation of caspase-3, DNA fragmentation, and nuclear condensation along with pronecrotic membrane deterioration, damage of organelles, and cell swelling [11, 12, 45]. Cells died from necroptosis or autophagy were also observed in the ischemic penumbra [15].
Cerebral ischemia can induce two general pathways of apoptosis. The intrinsic pathway is initiated by injured mitochondria. It includes release of mitochondrial cytochrome c into the cytosol, the following stimulation of caspase-3 and the downstream caspase/proteinase/nuclease cascades that destroy the cell. The extrinsic pathway is triggered by the death receptors at the cell surface. It is mediated by the caspase-8/caspase-3/downstream hydrolytic cascades. The early hypotheses suggested that stroke elicits mainly the caspase-dependent apoptosis in neurons. However, the data accumulating in the last time, showed that apoptosis is widespread in non-neuronal cells, and that caspase-independent mechanisms play a significant role in their death [10, 45].
The key apoptotic proteins have been already identified. However, the complex mechanisms that initiate and regulate apoptosis in various cells under different conditions remain incompletely understood yet. That is why the attempts to treat stroke patients by manipulating apoptotic are not successful so far [10].
Over-expression of apoptotic proteins in the ischemic penumbra
The cell response to acute injury is initially carried out by the proteins present in the cell. However, if the injury is strong and present proteins are unable to cope with the primary lesion, additional proteins are synthesized. In the rodent brain, various apoptotic proteins were shown to be up-regulated in the penumbra during first post-stroke hours. The over-expression of caspases 1, 3, 6, 8, and 9 in the infarct core was observed as soon as 0.5–1 h after MCAO in the rat cerebral cortex. This effect increased during following 12–24 h [45, 46]. The upregulation of Smac/DIABLO and caspases 3, 6, and 7 occurred in the ischemic penumbra at 1–4 h after photothrombotic stroke in the rat cerebral cortex [47]. In the MCAO-induced penumbra the over-expression of caspase 3 and its active truncated form was recorded in neurons at 4 h after stroke. In astrocytes caspases 3 and 6 were over-expressed later, at 12–24 h [45, 48].
Cytochrome c was appeared in the neuronal cytoplasm in the ischemic core and in the penumbra 3 h after MCAO in the rat brain [49, 50]. Other mitochondrial pro-apoptotic proteins AIF, Smac/DIABLO and HtrA2/Omi were over-expressed in the penumbra at 4–12 h after MCAO [50,51,52]. The over-expression of AIF and XAF1 (antagonist of apoptosis inhibitor XIAP) in the rat cerebral cortex was observed later, at 6–24 h after MCAO [50, 51]. These effects were associated with the over-expression of Bid and Bax that form the megapore in the outer mitochondrial membrane, through which these pro-apoptotic proteins pass into the cytosol [50, 53].
Apoptotic proteins and proteins that mediate their release from mitochondria may be potential targets for neuroprotective agents [34]. However, the examination of various inhibitors or activators of such proteins did not confirm their neuroprotection efficiency. Despite the assumption that caspase inhibitors may be neuroprotective, their inhibitory effects were transient, and cell death progressed afterwards. Possibly, modulation of the higher level regulators such as signaling proteins that control apoptosis implementation may be more fruitful. The activities and levels of multiple signaling proteins should be simultaneously studied. Currently, some multiplex analytical methods based on gene and protein expression profiling are developed. These methods provide the information on the simultaneous expression of many proteins in the penumbra tissue.
Expression of apoptosis-regulating proteins in the ischemic penumbra
Using the oligonucleotide-based microarrays, Jin et al. detected transcriptional induction of several pro-apoptotic genes including CASP3, ALG2, GADD 34, GADD153, and E2F1 in the rat hyppocampus at 24 h after transient global ischemia [54]. Lu et al. showed the up-regulation of RNA transcripts encoding calpain, cathepsin C, and ICE-like proteinase involved in apoptosis execution. Simultaneously, immediate early genes c-fos, c-jun, fra-1, c-myc, and HIF-1 were over-expressed in the penumbra 4 or 24 h after permanent MCAO in the rat cerebral cortex [55].
However, the mRNA profiles do not well correlate with the protein profiles [56]. Proteins are the ultimate effectors that perform almost all cellular functions. Not all mRNA molecules are translated because of post-transcriptional processing and destruction. Unlike DNA microarrays, proteomic microarrays provide the direct information on protein profiles. The antibody microarrays contain the predetermined set of selected antibodies against hundreds of cellular proteins involved in signal transduction, apoptosis, epigenetic regulation, vesicular transport, cytoskeleton, etc. [47, 56,57,58,59,60].
During first post-stoke hours the phosphorylation and activation of different signaling proteins and transcription factors such as ERK1/2, MEK3/6, JNK, p38, c-Jun, c-Myc, ATF, CREB, Elk, STAT-1, and PTEN, which control different signaling pathways and metabolic processes, were observed in the ischemic penumbra after MCAO in the rat brain [43, 46, 49, 61,62,63]. The up-regulation of immediate-early proteins c-Fos, c-Jun, and Jun B, which form the transcription factor AP-1, was observed in the ischemic penumbra 1 h after stroke in the mouse brain [64]. Proteins STAT3, IκBα, and c-Jun were up-regulated at 4 h after photothrombotic stroke in the rat brain [65]. Activation of the mTOR signaling pathway in ischemic penumbra was demonstrated after cerebral ischemia–reperfusion injury in rats. Expression of mTOR, Akt, S6K significantly increased in the penumbra but decreased in the infarction core at 1, 6 and 24 h after MCAO. The expression of cleaved caspase-9 and caspase-3 elevated both in the ischemic penumbra and in the ischemic core, whereas the levels of phosphorylated ULK1, Beclin1, and LC3-II decreased at 24 h after MCAO. These data showed the implementation of neuronal apoptosis and simultaneous inhibition of autophagy in the penumbra. mTOR inhibitor rapamycin reduced neuronal apoptosis, decreased the infarct volume and activated autophagy [66]. Some novel anti-stroke proteins have been recently identified. An acute-phase protein orosomucoid-2 (ORM2) that present in the normal mice brain was significantly over-expressed in the ischemic penumbra after MCAO. Its administration at 4.5 or 6 h after MCAO reduced the infarct volume and neurological deficit. In the ischemic penumbra it reduced inflammation, suppressed oxidative stress, and inhibited apoptosis by decreasing caspase-3 activity [67]. The newly identified molecular N-myc downstream-regulated gene 2 (Ndrg2) was over-expressed in the penumbra astrocytes at 2, 4, and 12 h after reperfusion and peaked at 24 h after MCAO in rats. NDRG2 protein was translocated from the cytoplasm to the nucleus and co-localized with apoptotic TUNEL-positive cells. In the cultured astrocytes NDRG2 silencing inhibited ROS production, reduced the level of cleaved caspase-3, and decreased the percentage of apoptotic cells, whereas its over-expression had the opposite effects. Therefore, NDRG2 was involved in stroke-induced apoptosis of astrocytes [68, 69].
The antibody microarrays evaluate changes in the expression of several hundreds of pre-selected signaling and neuronal proteins in the ischemic brain. This approach was used in the study of biochemical changes in the ischemic penumbra caused by photothrombotic stroke in the rat cerebral cortex. Several dozens of up-, or down-regulated proteins involved in pro- or anti-apoptotic processes were identified at 1–24 h after PTS (Tables 2, 3) [47, 70, 71].
Pro-apoptotic proteins
The interesting and important consequence of photothrombotic stroke in the rat cerebral cortex was the concerted up-regulation of various pro-apoptotic proteins that initiate and perform diverse apoptotic processes (Tables 2, 3). Their list included:
-
Proteins that execute both caspase-dependent and caspase-independent apoptosis pathways: caspases 3, 6 and 7, Smac/DIABLO, apoptosis inducing factor AIF, and PSR, a sensor of apoptotic cells;
-
Signaling proteins that initiate or regulate different pro-apoptotic pathways: Bcl-10, MAP kinases p38 and JNK, DYRK1A;
-
Transcription factors that control the expression of apoptotic proteins: E2F1, p53, c-Myc and GADD153;
-
Diverse multifunctional proteins that, in addition to other functions, can stimulate apoptosis in specific situations: glutamate receptor NMDAR2a, neurotrophin receptor p75, Par4, glutamate decarboxylase GAD65/67, and inflammatory caspase 11 [47, 70].
The earliest events observed in the ischemic penumbra at 1 h after PTS included over-expression of caspase 3 and its active form, Smac/DIABLO, Bcl-10, c-Myc, p53, Par4, GADD153, and DYRK1A. Other apoptosis-associated proteins such as E2F1, p75, p38, JNK, GAD65/67, NMDAR2a, and PSR were up-regulated later, at 4 h after PTS (Table 2). All signaling pathways and transcription factors that initiate and regulate the expression of various pro- and anti-apoptotic proteins are not completely discovered yet. However, one can suspect some of them.
Transcription factor E2F1 is one of the key players that determine the cell fate. It controls the expression of various genes that regulate DNA synthesis and repair, cell cycle and apoptosis [72, 73]. E2F1 stimulates apoptosis when the cell cycle is impaired or suppressed that is typical for neurons [74,75,76]. Its synthesis is controlled by MAP kinase p38 and transcription factor c-Myc [77]. E2F1 induces expression of various pro-apoptotic proteins such as caspases 3, 7, 8 and 9, Smac/DIABLO, Apaf-1, BH3-only proteins of the Bcl-2 family, p53 and p73 [75, 76]. The over-expression of E2F1, p53, c-Myc, p38, Smac/DIABLO, Bcl-x, caspases 3, 6 and 7 was observed in the PTS-induced penumbra (Table 2) [47]. This is in agreement with the involvement of E2F1 [78] and p53 [79] in the response of the penumbra tissue to transient stroke in rats. Cerebral ischemia leads to up-regulation of caspases 3, 6 and 7 [80]. Oppositely, the inhibition of the E2F1/p53 pathway prevents neuronal apoptosis [74]. E2F1 can stimulate stress-induced apoptosis through the pro-apoptotic ASK1/JNK pathway. It transcriptionally regulates ASK1, the ROS-sensitive JNK activator [81,82,83]. MAP kinase JNK, the stress-induced apoptosis promoter, was also up-regulated in the penumbra after PTS (Table 2) [47]. JNK also increases the pro-apoptotic activity of p53 [84]. Another MAP kinase p38 that regulates the expression of pro-apoptotic transcription factors E2F1 and p53 was also up-regulated in the PTS-induced penumbra (Table 2). The effect of p38 on E2F1 expression might be mediated by MAPKAPK2 (mitogen-activated protein kinase (MAPK)-activated protein kinase 2) [85, 86], which was up-regulated in the penumbra as well (Table 3).
Apoptosis promoter p53 is a master regulator of numerous cell functions. It controls the transcription of hundreds target genes involved in regulation of DNA repair, cell cycle arrest, metabolism, mRNA translation, apoptosis and autophagy [87]. There are two pro-apoptotic pathways of p53 activity: mitochondrial and transcriptional. Transcriptional activity of p53 is inhibited by pifithrin-α, whereas the mitochondrial pathway is inhibited by pifithrin-μ. The mitochondrial effect of p53 occurs early, within first 30 min after hypoxia/ischemia [88]. It is more important than the transcriptional pathway, because the neuroprotective efficiency of pifithrin-μ is higher than that of pifithrin-α. Pifithrin-μ suppresses TUNEL staining, release of cytochrome c and Smac/Diablo, activation of caspase-3, expression of other pro-apoptotic proteins Noxa and PUMA in the ischemic brain [89, 90]. It improves the functional outcome after ischemia [89]. In contrast, pifithrin-α [90], or deletion of the p53 gene [91] caused neuroprotection through suppression of the transcriptional effect of p53. p53 controls the expression of p21WAF-1, MDM2 and caspase 6 that were up-regulated in the PTS-induced penumbra (Table 3) [71]. The anti-apoptotic protein p21Waf-1 is up-regulated upon DNA damage; it inhibits p53-mediated apoptosis and arrests the cell cycle to allow DNA reparation [92]. Its up-regulation in the PTS-induced penumbra (Table 3) was in agreement with over-expression of p21WAF-1 mRNA in the rat brain neurons after transient MCAO [93]. Another p53 antagonist MDM2 stimulates its proteolysis and thereby suppresses its transcriptional activity. Over-expression of MDM2 mediated recovery of the damaged neurons after MCAO in rats [94]. Photothrombotic stroke also upregulated p63, a p53 antagonist (Table 3) [47]. This was consistent with the ischemia-induced expression of p63 and rapid inhibition of apoptosis in cortical neurons [95]. Apparently, neuronal survival, death and aging depend on the balance between the levels and activities of p53, p63, and p73 [96].
Transcription factor c-Myc is a master regulator of numerous target genes. It activates (or sometimes represses) 10–15% of all genes involved in regulation of energy metabolism, protein synthesis, tumorigenesis, cell cycle and apoptosis. It functions at both transcriptional and epigenetic levels [97,98,99]. Its over-expression predisposes cells to apoptosis. c-Myc stimulates the expression of p53 and E2F1 [98]. Its apoptosis-regulating activity is in part mediated by the p53/Mdm2/Arf pathway [100]. The up-regulation of c-Myc in the PTS-induced penumbra (Table 2) also potentiated apoptosis.
The dual specificity tyrosine-phosphorylation-regulated kinase 1A (DYRK1A) phosphorylates different transcription factors: FKHR, NFAT, STAT3, and CREB. Under oxidative stress it stimulates the pro-apoptotic pathway ASK1/JNK [101]. In the adult brain the over-expression of DYRK1A impairs various signaling pathways and induces loss of neurons during aging, Alzheimer, Parkinson and Huntington diseases [102]. The over-expression of DYRK1A in the PTS-induced penumbra (Table 2) could be pro-apoptotic.
Among PTS-induced multifunctional proteins that, except other functions, can initiate apoptosis in specific situations, the level of Par4 (prostate apoptosis response 4) in the penumbra increased by 1.5–2 times at 1–24 h after PTS (Table 2) [47, 70]. Similarly, the up-regulation of Par4 was observed in the rat hippocampal and striatum neurons after transient forebrain ischemia [103]. Its pro-apoptotic effect was associated with the inhibition of NF-κB and down-regulation of Bcl-2, IAP, and calbindin. The ischemic induction of Par4, p53 and Bcl-2 family proteins leads to mitochondrial dysfunction and subsequent release of pro-apoptotic proteins [103, 104].
The neurotrophin receptor p75 promotes neuronal apoptosis upon binding of neurotrophic factors. It competes for neurotrophins with the pro-survival receptors Trk A, TrkB, and TrkC [105]. Its over-expression indicated the domination of pro-apoptotic tendency in the penumbra (Table 2). Similarly, MCAO induced the up-regulation of p75 in the penumbra [105, 106].
The over-expression of transcription factor GADD153 (also known as CHOP) is a hallmark of ER stress. It is poorly expressed in normal cells, but DNA damage or cell cycle arrest activate it. When misfolded proteins accumulate, GADD153 regulates the expression of specific genes and causes down-regulation of Bcl-2, depletion of glutathione, ROS production, suppression of proliferation and apoptosis [107,108,109]. GADD153 was up-regulated after focal cerebral ischemia [107, 109, 110]. Its over-expression in the penumbra after photothrombotic stroke (Table 2) could also stimulate apoptosis.
The physiological function of glutamate decarboxylase GAD65/67 is the conversion of l-glutamate into GABA. However, its over-expression may cause apoptosis [111]. Possibly, the up-regulation of GAD65/67 in the penumbra after PTS [47] or MCAO [112] was another one apoptosis-stimulating factor in the rat brain.
PTS also increased the level of inflammatory caspase 11 in the ischemic penumbra (Table 2). Caspase 11 can mediate apoptosis of astrocytes and microglia [113]. Therefore, its up-regulation displays the glial response to spreading injury and apoptosis in the ischemic brain. The overturning of phosphatidylserines to the outer side of the plasma membrane is an “eat-me” signal for recognition and phagocytic elimination of apoptotic cells [114]. Apparently, the PTS-induced over-expression of the phosphatidylserine receptor PSR (Table 2) was directed to removal of apoptotic cells.
Anti-apoptotic proteins
Simultaneously with apoptotic processes, diverse anti-apoptotic proteins were up-regulated in the photothrombotic penumbra. Some of them were over-expressed early, 1 h after PTS, whereas others were up-regulated later, at 4 h (Table 3) [47, 70]. The 1.5-fold over-expression of phosphothreonines (Table 3) indicated the activity of serine/threonine protein kinases. Phosphothreonines are rare in the normal cerebral tissue, but their level markedly increases due to protein kinase activity after brain damage. For example, protein kinases ERK1/2 phosphorylate different transcription factors and cytoskeleton proteins, and thus, regulate cell mobility, proliferation and survival. The up-regulation of ERK1 was observed in the PTS-induced penumbra [47], or after permanent MCAO in rats [115]. Raf1, which activates ERK [116], was also up-regulated after PTS (Table 3).
Protein kinase Bα (Akt), a component of the PI-3 kinase/Akt signaling pathway, regulates cell metabolism, proliferation, and survival. Its anti-apoptotic activity is associated with the inhibition of pro-apoptotic glycogen synthase kinase 3β (GSK-3β). The up-regulation of protein kinase Bα and the down-regulation of GSK-3 in the PTS-induced penumbra (Table 3) could be involved in protection of penumbra cells. These data correlate with the ischemia-induced over-expression of Akt mRNA and Akt phosphorylation in the rat brain [115, 117, 118].
The up-regulation of estrogen and EGF receptors stimulates the signaling pathways Raf1/ERK and PI3-kinase/Akt. Over-expression of both receptors was observed in the PTS-induced penumbra (Table 3). This was apparently aimed at neuroprotection. Estrogen is known to induce anti-apoptotic proteins Bcl-2 and Bcl-xL, which protect brain from ischemic stroke [119].
The up-regulation of protein phosphatase 1α and MAP kinase phosphatase-1 (MKP-1), which dephosphorylate cellular proteins and stop their activation, could be also involved in neuroprotection in the PTS-induced penumbra [47]. It was shown that MKP-1 stops the stimulation of pro-apoptotic proteins JNK and p38 after MCAO in the mouse brain that contributes to endogenous neuroprotection. Inhibition of MKP-1 exacerbated stroke-induced brain damage [120, 121].
Ca2+ overload to 10−4–10−3 M induces apoptosis or necrosis, but at lesser concentrations (about 10−6 M) Ca2+ mediates neuroprotective processes [26,27,28,29]. Ca2+/calmodulin complex regulates numerous functions such as production and release of neurotransmitters, cytoskeleton remodeling, axonal transport, gene expression, cell survival, learning and memory. Calmodulin and calmodulin-dependent kinases, CaMKII and CaMKIV, are abundant in the mammalian brain. Nevertheless, their levels in the ischemic penumbra increased more after photothrombotic stroke (Table 3). CaMKII-mediated neuroprotection is associated with phosphorylation and inhibition of some pro-apoptotic proteins such as NO synthase and GSK-3β. It also phosphorylates NMDA and AMPA glutamate receptors. This increases Ca2+ influx and aggravates excitotoxicity. Inhibitors of CaMKII protect the brain from ischemic damage [122]. CaMKIV inhibits excitotoxicity through activation of the anti-apoptotic pathway PI3K/Akt, and phosphorylation of CREB, which mediates the expression of some anti-apoptotic genes [123, 124].
Calreticulin regulates calcium homeostasis and controls protein folding in ER. It modulates the expression and activity of p53, and thus regulates apoptosis [125]. At the early stroke stages calreticulin inhibits neuronal apoptosis in the ischemic penumbra [126]. The PTS-induced up-regulation of Ca2+-dependent kinase ERK5 in the ischemic penumbra (Table 3) also contributed to neuroprotection. ERK5 is activated by oxidative stress. It mediates the neuroprotection effect of neurotrophic factors [127].
Other Ca2+-dependent signaling proteins such as phospholipase Cγ (PLCγ) and protein kinase C (PKC) were down-regulated in the PTS-induced penumbra (Table 3). Under damage of the plasma membrane, or activation of tyrosine kinase receptors, PLCγ1 cleaves membrane phospholipids and releases inositol-1,4,5-triphosphate, which stimulates Ca2+ release from ER, and diacylglycerol, which activates protein kinase C (PKC). PKC regulates cell homeostasis, synaptic transmission, proliferation, and apoptosis [128]. PKC inhibitors protect neurons from ischemic [129] and excitotoxic damage [130] that indicated the involvement of PKC in neurodegeneration. The observed down-regulation of PKC (Table 3) could rescue cells in the penumbra.
Cell survival and apoptosis are also controlled by the Wnt/β-catenin and Notch signaling pathways [131, 132]. β-catenin links the intracellular actin cytoskeleton to N-cadherin, which is responsible for adhesive cell–cell contacts. Excessive cytoplasmic β-catenin binds to the axin1/APC/GSK-3β complex. Axin1 facilitates phosphorylation of β-catenin by kinase GSK-3β that leads to its ubiquitin-dependent degradation. The binding of extracellular Wnt to its receptor stimulates decay of this complex and release of β-catenin, which moves into the nucleus and induces expression of genes responsible for cell survival. Ischemia-induced inhibition of the Wnt signaling pathway increases neuronal death [133]. The ubiquitous protein FRAT1 mediates dissociation of the axin1/GSK-3β complex and prevents degradation of β-catenin [134]. Over-expression of FRAT1 inhibits GSK-3β and rescues neurons from ischemic death [135]. In the PTS-induced penumbra axin1 and GSK-3 were down-regulated (Table 3) that could reduce cell death. However, stronger, twofold down-regulation of FRAT1 (Table 3) possibly aggravated neurodegeneration.
The Notch signaling pathway controls differentiation, maturation and survival of neuronal and glial cells, learning and memory. The up-regulation of Notch antagonist Numb in the ischemic penumbra stimulated apoptosis [136]. The down-regulation of Numb in the PTS-induced penumbra (Table 3) could contribute to cell survival.
Conclusions and future perspectives
There are two basic features of cell death regulation in the ischemic penumbra (Fig. 2):
-
Simultaneous and concerted up-regulation of various proteins that execute or regulate the implementation of the apoptotic program, and the proteins normally involved in diverse cellular functions, but capable to induce apoptosis in specific situations. Therefore, diverse apoptosis pathways are induced simultaneously from different initial positions.
-
Both apoptotic and anti-apoptotic proteins are over-expressed in the penumbra. The cell fate in the penumbra is determined by the balance between these opposite tendencies.
How do the primary injurious factors that spread from the infarction core (glutamate-induced Ca2+ overload, K+-mediated depolarization, mitochondria damage, ROS, NO, and acidosis) induce protein expression in the ischemic penumbra? Which signaling pathways and transcription factors regulate the expression of different pro- and anti-apoptotic genes? One can suggest they first induce some immediate early signaling proteins (sensors) such as Ca2+ and ROS sensors (HIF1, ASK1, protein phosphatases and other thiol-containing enzymes, Ca2+/calmodulin-dependent proteins, and so on). Then stress-sensitive protein kinases JNK, p38, c-Myc, etc. stimulate transcription factors such as E2F1, p53, GADD153, CREB, NF-κB to produce pro-survival and pro-apoptotic proteins.
Excessive intracellular Ca2+ can enter the neuronal nucleus and activate the expression of specific genes depending on the penetration path. Ca2+ influx through synaptic MNDA receptors can induce the calcium wave, which propagates to the nucleus. Nuclear calcium stimulates the CaMKIV/CREB pathway that induces expression of genes involved in the regulation of physiological functions: metabolism, synaptic transmission, and cell survival. In contrast, hyperactivation of extrasynaptic NMDA receptors under stress or ischemia induces pathogenic Ca2+ overload and expression of genes associated with apoptosis and neurodegeneration. In this case CREB may be functionally inactivated by rapid dephosphorylation and following degradation. Activation of these receptors causes nuclear accumulation of histone deacetylases HDAC4 and HDAC5 that globally repress transcription. It also stimulates the nuclear accumulation of pro-apoptotic transcription factor FOXO3A. Additionally, Ca2+ overload rapidly decreases the mitochondrial membrane potential [123, 137, 138].
ROS-sensitive transcription factors such as AP-1, HIF-1α, NF-κB, and STAT-1α trigger expression of genes associated either with progressive neural dysfunction and death, or with cell survival [32]. Their effects depend on the damage degree and cellular context. For example, during transient global ischemia, NF-κB is briefly activated in surviving neurons. However, its prolonged activation induces production of death-associated proteins in the neurons, which are destined to die [139]. The oxidative DNA damage leads to activation of protein kinase ATM (ataxia telangiectasia-mutated) and reparation enzymes. ATM phosphorylates numerous proteins including p53, histone H2AX, and others. The pathway ATM/E2F1/p53 orchestrates neuronal apoptosis [74]. Nitric oxide produced by ischemic cells diffuses to surrounding tissue. NO influences transcription factors that regulate gene expression through guanylate cyclase/cGMP/protein kinase G/CREB cascade. NO controls the activity of transcription factors NF-κB, N-Myc, c-Fos and c-Jun that regulate cell survival [140]. Global regulation of transcriptional processes at the epigenetic level is involved in the phenomenon of ischemic tolerance and post-stroke recovery [141,142,143].
The simultaneous expression of different pro- and anti-apoptotic proteins in the ischemic penumbra may occur either in same, or in different cell types: neurons, glia, vessels, blood elements, or connective tissue, which may be further subdivided by several subtypes. Neuroglial interactions play a significant role in mutual regulation of survival and death of neurons and glial cells [144,145,146,147,148,149]. Moreover, the complex neurons/glia/microvessels is currently considered as a “neurovascular unit”, in which any cell types interact and participate in either tissue salvage, or demise [147,148,149]. Fragmented apoptotic cells or bodies are cleared by microglia and astrocytes. This contributes to delayed neuron loss and decrease in the infarct volume after stroke [149].
Proteomic experiments provide the preliminary data on the involvement of various proteins in the development of ischemic penumbra. The next step is the detailed study of signaling and transcriptional mechanisms that control protein expression in different cell types in the ischemic penumbra. There are a number of problems to be addressed in future:
-
What cell type: neurons, glia, or vascular endothelium is most sensitive to ischemic damage? What is the role of intercellular interactions in the neurovascular units in the ischemic penumbra?
-
What molecular mechanisms underlie the ischemic/reperfusion damage to different cellular subsystems: nucleus, membranes, mitochondria, endoplasmic reticulum, cytoskeleton, vesicular transport, etc.?
-
Which signaling pathways and transcription factors control the expression of proteins involved in the regulation of cell survival and death in the ischemic penumbra?
-
What is the temporal dynamics and spatial distribution of processes involved in the responses of different penumbra cells to ischemia/reperfusion? When various pharmacological modulators should be applied?
-
Is the multitarget therapy more promising for stroke treatment? What proteins and organelles should be affected, and what is the temporal sequence of their application?
Some proteins mentioned above may serve as novel molecular targets for anti-stroke therapy, and their inhibitors or activators may be potential anti-stroke neuroprotectors.
References
Iadecola C, Anrather J (2011) Stroke research at a crossroad: asking the brain for directions. Nat Neurosci 14:1363–1368. https://doi.org/10.1038/nn.2953
Hankey GJ (2017) Stroke. Lancet 389:641–654. https://doi.org/10.1016/S0140-6736(16)30962-X
Mehta SL, Manhas N, Raghubir R (2007) Molecular targets in cerebral ischemia for developing novel therapeutics. Brain Res Rev 4:34–66. https://doi.org/10.1016/j.brainresrev.2006.11.003
Puyal J, Ginet V, Clarke PG (2013) Multiple interacting cell death mechanisms in the mediation of excitotoxicity and ischemic brain damage: a challenge for neuroprotection. Prog Neurobiol 105:24–48. https://doi.org/10.1016/j.pneurobio.2013.03.002
Majid A (2014) Neuroprotection in stroke: past, present, and future. ISRN Neurol. https://doi.org/10.1155/2014/515716
Min J, Farooq MU, Gorelick PB (2013) Neuroprotective agents in ischemic stroke: past failures and future opportunities. Clin Investig 3:1167–1177. https://doi.org/10.4155/cli.13.91
Karsy M, Brock A, Guan J, Taussky P, Kalani MY, Park MS (2017) Neuroprotective strategies and the underlying molecular basis of cerebrovascular stroke. Neurosurg Focus 42:E3. https://doi.org/10.3171/2017.1.FOCUS16522
Auriel E, Bornstein NM (2010) Neuroprotection in acute ischemic stroke—current status. J Cell Mol Med 14:2200–2202. https://doi.org/10.1111/j.1582-4934.2010.01135.x
Rajah GB, Ding Y (2017) Experimental neuroprotection in ischemic stroke: a concise review. Neurosurg Focus 42:E2. https://doi.org/10.3171/2017.1.FOCUS16497
Broughton BR, Reutens DC, Sobey CG (2009) Apoptotic mechanisms after cerebral ischemia. Stroke 40:e331–e339. https://doi.org/10.1161/STROKEAHA.108.531632
Onténiente B, Couriaud C, Braudeau J, Benchoua A, Guégan C (2003) The mechanisms of cell death in focal cerebral ischemia highlight neuroprotective perspectives by anti-caspase therapy. Biochem Pharmacol 66:1643–1649
Yao H, Takasawa R, Fukuda K, Shiokawa D, Sadanaga-Akiyoshi F, Ibayashi S, Tanuma S, Uchimura H (2001) DNA fragmentation in ischemic core and penumbra in focal cerebral ischemia in rats. Brain Res Mol Brain Res 91:112–118
Benchoua A, Guégan C, Couriaud C, Hosseini H, Sampaïo N, Morin D, Onténiente B (2001) Specific caspase pathways are activated in the two stages of cerebral infarction. J Neurosci 21:7127–7134
Wei L, Ying DJ, Cui L, Langsdorf J, Yu SP (2004) Necrosis, apoptosis and hybrid death in the cortex and thalamus after barrel cortex ischemia in rats. Brain Res 1022:54–61
Fricker M, Tolkovsky AM, Borutaite V, Coleman M, Brown GC (2018) Neuronal cell death. Physiol Rev 98:813–880. https://doi.org/10.1152/physrev.00011.2017
Fluri F, Schuhmann MK, Kleinschnitz C (2015) Animal models of ischemic stroke and their application in clinical research. Drug Des Dev Ther 9:3445–3454. https://doi.org/10.2147/DDDT.S56071
Mergenthaler P, Meisel A (2012) Do stroke models model stroke? Dis Model Mech 5:718–725. https://doi.org/10.1242/dmm.010033
Sommer CJ (2017) Ischemic stroke: experimental models and reality. Acta Neuropathol 133:245–261. https://doi.org/10.1007/s00401-017-1667-0
Krafft PR, Bailey EL, Lekic T, Rolland WB, Altay O, Tang J, Wardlaw JM, Zhang JH, Sudlow CL (2012) Etiology of stroke and choice of models. Int J Stroke 7:398–406. https://doi.org/10.1111/j.1747-4949.2012.00838.x
Uzdensky AB (2017) Photothrombotic stroke as a model of ischemic stroke. Transl Stroke Res. https://doi.org/10.1007/s12975-017-0593-8
Watson BD, Dietrich WD, Busto R, Wachtel MS, Ginsberg MD (1985) Induction of reproducible brain infarction by photochemically initiated thrombosis. Ann Neurol 17:497–504
Yang S, Liu K, Ding H, Gao H, Zheng X, Ding Z, Xu K, Li P (2018) Longitudinal in vivo intrinsic optical imaging of cortical blood perfusion and tissue damage in focal photothrombosis stroke model. J Cereb Blood Flow Metab. https://doi.org/10.1177/0271678x18762636
Pevsner PH, Eichenbaum JW, Miller DC, Pivawer G, Eichenbaum KD, Stern A, Zakian KL, Koutcher JA (2001) A photothrombotic model of small early ischemic infarcts in the rat brain with histologic and MRI correlation. J Pharmacol Toxicol Methods 45:227–233
Choi DW (1992) Excitotoxic cell death. J Neurobiol 23:1261–1276
Lau A, Tymianski M (2010) Glutamate receptors, neurotoxicity and neurodegeneration. Pflugers Arch 460:525–542. https://doi.org/10.1007/s00424-010-0809-1
Arundine M, Tymianski M (2003) Molecular mechanisms of calcium-dependent neurodegeneration in excitotoxicity. Cell Calcium 34:325–337
Berliocchi L, Bano D, Nicotera P (2005) Ca2+ signals and death programmes in neurons. Philos Trans R Soc Lond B Biol Sci 360:2255–2258. https://doi.org/10.1098/rstb.2005.1765
Brini M, Calì T, Ottolini D, Carafoli E (2014) Neuronal calcium signaling: function and dysfunction. Cell Mol Life Sci 71:2787–2814. https://doi.org/10.1007/s00018-013-1550-7
Clapham DE (2007) Calcium signaling. Cell 131:1047–1058. https://doi.org/10.1016/j.cell.2007.11.028
Abramov AY, Scorziello A, Duchen MR (2007) Three distinct mechanisms generate oxygen free radicals in neurons and contribute to cell death during anoxia and reoxygenation. J Neurosci 27:1129–1138. https://doi.org/10.1523/JNEUROSCI.4468-06.2007
Allen CL, Bayraktutan U (2009) Oxidative stress and its role in the pathogenesis of ischemic stroke. Int J Stroke 4:461–470. https://doi.org/10.1111/j.1747-4949.2009.00387.x
Chen H, Yoshioka H, Kim GS, Jung JE, Okami N, Sakata H, Maier CM, Narasimhan P, Goeders CE, Chan PH (2011) Oxidative stress in ischemic brain damage: mechanisms of cell death and potential molecular targets for neuroprotection. Antioxid Redox Signal 14:1505–1517. https://doi.org/10.1089/ars.2010.3576
Garry PS, Ezra M, Rowland MJ, Westbrook J, Pattinson KT (2015) The role of the nitric oxide pathway in brain injury and its treatment–from bench to bedside. Exp Neurol 263:235–243. https://doi.org/10.1016/j.expneurol.2014.10.017
Rami A, Bechmann I, Stehle JH (2008) Exploiting endogenous anti-apoptotic proteins for novel therapeutic strategies in cerebral ischemia. Prog Neurobiol 85:273–296. https://doi.org/10.1016/j.pneurobio.2008.04.003
Guo MF, Yu JZ, Ma CG (2011) Mechanisms related to neuron injury and death in cerebral hypoxic ischaemia. Folia Neuropathol 49:78–87
Irving EA, Bamford M (2002) Role of mitogen- and stress-activated kinases in ischemic injury. J Cereb Blood Flow Metab 22:631–647
Shen HM, Liu ZG (2006) JNK signaling pathway is a key modulator in cell death mediated by reactive oxygen and nitrogen species. Free Radic Biol Med 40:928–939. https://doi.org/10.1016/j.freeradbiomed.2005.10.056
Colasanti M, Suzuki H (2000) The dual personality of NO. Trends Pharmacol Sci 21:249–252
Liu C, Zhang K, Shen H, Yao X, Sun Q, Chen G (2018) Necroptosis: a novel manner of cell death, associated with stroke (Review). Int J Mol Med 41:624–630. https://doi.org/10.3892/ijmm.2017.3279
Hribljan V, Lisjak D, Petrović DJ, Mitrečić D (2019) Necroptosis is one of the modalities of cell death accompanying ischemic brain stroke: from pathogenesis to therapeutic possibilities. Croat Med J 60:121–126
Linnik MD (1996) Role of apoptosis in acute neurodegenerative disorders. Restor Neurol Neurosci 9:219–225. https://doi.org/10.3233/RNN-1996-9404
Radak D, Katsiki N, Resanovic I, Jovanovic A, Sudar-Milovanovic E, Zafirovic S, Mousad SA, Isenovic ER (2017) Apoptosis and acute brain ischemia in ischemic stroke. Curr Vasc Pharmacol 15:115–122. https://doi.org/10.2174/1570161115666161104095522
Ferrer I (2006) Apoptosis: future targets for neuroprotective strategies. 21. Cerebrovasc Dis 21(Suppl 2):9–20. https://doi.org/10.1159/000091699
Nakka VP, Gusain A, Mehta SL, Raghubir R (2008) Molecular mechanisms of apoptosis in cerebral ischemia: multiple neuroprotective opportunities. Mol Neurobiol 37:7–38
Ferrer I, Friguls B, Dalfó E, Justicia C, Planas AM (2003) Caspase-dependent and caspase-independent signalling of apoptosis in the penumbra following middle cerebral artery occlusion in the adult rat. Neuropathol Appl Neurobiol 29:472–481
Krupinski J, Slevin M, Marti E, Catena E, Rubio F, Gaffney J (2003) Time-course phosphorylation of the mitogen activated protein (MAP) kinase group of signalling proteins and related molecules following middle cerebral artery occlusion (MCAO) in rats. Neuropathol Appl Neurobiol 29:144–158
Demyanenko S, Uzdensky A (2017) Profiling of signaling proteins in penumbra after focal photothrombotic infarct in the rat brain cortex. Mol Neurobiol 54:6839–6856. https://doi.org/10.1007/s12035-016-0191-x
Krupinski J, Lopez E, Marti E, Ferrer I (2000) Expression of caspases and their substrates in the rat model of focal cerebral ischemia. Neurobiol Dis 7:332–342
Li F, Omori N, Sato K, Jin G, Nagano I, Manabe Y, Shoji M, Abe K (2002) Coordinate expression of survival p-ERK and proapoptotic cytochrome c signals in rat brain neurons after transient MCAO. Brain Res 958:83–88
Ferrer I, Planas AM (2003) Signaling of cell death and cell survival following focal cerebral ischemia: life and death struggle in the penumbra. J Neuropathol Exp Neurol 62:329–339
Althaus J, Siegelin MD, Dehghani F, Cilenti L, Zervos AS, Rami A (2007) The serine protease Omi/HtrA2 is involved in XIAP cleavage and in neuronal cell death following focal cerebral ischemia/reperfusion. Neurochem Int 50:172–180. https://doi.org/10.1016/j.neuint.2006.07.018
Saito A, Hayashi T, Okuno S, Ferrand-Drake M, Chan PH (2003) Interaction between XIAP and Smac/DIABLO in the mouse brain after transient focal cerebral ischemia. J Cereb Blood Flow Metab 23:1010–1019
Nishioka T, Nakase H, Nakamura M, Konishi N, Sakaki T (2006) Sequential and spatial profiles of apoptosis in ischemic penumbra after two-vein occlusion in rats. J Neurosurg 104:938–944. https://doi.org/10.3171/jns.2006.104.6.938
Jin K, Mao XO, Eshoo MW, Nagayama T, Minami M, Simon RP, Greenberg DA (2001) Microarray analysis of hippocampal gene expression in global cerebral ischemia. Ann Neurol 50:93–103
Lu A, Tang Y, Ran R, Clark JF, Aronow BJ, Sharp FR (2003) Genomics of the periinfarction cortex after focal cerebral ischemia. J Cereb Blood Flow Metab 23:786–810
Kopf E, Zharhary D (2007) Antibody arrays—An emerging tool in cancer proteomics. Int J Biochem Cell Biol 39:1305–1317. https://doi.org/10.1016/j.biocel.2007.04.029
Borrebaeck CA, Wingren C (2014) Antibody array generation and use. Methods Mol Biol 1131:563–571. https://doi.org/10.1007/978-1-62703-992-5_36
Cretich M, Damin F, Chiari M (2014) Protein microarray technology: how far off is routine diagnostics? Analyst 139:528–542. https://doi.org/10.1039/c3an01619f
Solier C, Langen H (2014) Antibody-based proteomics and biomarker research—current status and limitations. Proteomics 14:774–783. https://doi.org/10.1002/pmic.201300334
Wingren C (2016) Antibody-based proteomics. Adv Exp Med Biol 926:163–179. https://doi.org/10.1007/978-3-319-42316-6-11
Ferrer I, Friguls B, Dalfó E, Planas AM (2003) Early modifications in the expression of mitogen-activated protein kinase (MAPK/ERK), stress-activated kinases SAPK/JNK and p38, and their phosphorylated substrates following focal cerebral ischemia. Acta Neuropathol 105:425–437
Li J, Li Y, Ogle M, Zhou X, Song M, Yu SP, Wei L (2010) DL-3-n-butylphthalide prevents neuronal cell death after focal cerebral ischemia in mice via the JNK pathway. Brain Res 1359:216–226. https://doi.org/10.1016/j.brainres.2010.08.061
Omori N, Jin G, Li F, Zhang WR, Wang SJ, Hamakawa Y, Nagano I, Manabe Y, Shoji M, Abe K (2002) Enhanced phosphorylation of PTEN in rat brain after transient middle cerebral artery occlusion. Brain Res 954:317–322
Hata R, Maeda K, Hermann D, Mies G, Hossmann KA (2000) Dynamics of regional brain metabolism and gene expression after middle cerebral artery occlusion in mice. J Cereb Blood Flow Metab 20:306–315
Küry P, Schroeter M, Jander S (2004) Transcriptional response to circumscribed cortical brain ischemia: spatiotemporal patterns in ischemic vs. remote non-ischemic cortex. Eur J Neurosci 19:1708–1720. https://doi.org/10.1111/j.1460-9568.2004.03226.x
Wu M, Zhang H, Kai J, Zhu F, Dong J, Xu Z, Wong M, Zeng LH (2017) Rapamycin prevents cerebral stroke by modulating apoptosis and autophagy in penumbra in rats. Ann Clin Transl Neurol 5:138–146. https://doi.org/10.1002/acn3.507
Wan JJ, Wang PY, Zhang Y, Qin Z, Sun Y, Hu BH, Su DF, Xu DP, Liu X (2019) Role of acute-phase protein ORM in a mice model of ischemic stroke. J Cell Physiol. https://doi.org/10.1002/jcp.28653
Li Y, Shen L, Cai L, Wang Q, Hou W, Wang F, Zeng Y, Zhao G, Yao L, Xiong L (2011) Spatial-temporal expression of NDRG2 in rat brain after focal cerebral ischemia and reperfusion. Brain Res 1382:252–258. https://doi.org/10.1016/j.brainres.2011.01.023
Ma YL, Zhang LX, Liu GL, Fan Y, Peng Y, Hou WG (2017) N-Myc downstream-regulated gene 2 (Ndrg2) is involved in ischemia-hypoxia-induced astrocyte apoptosis: a novel target for stroke therapy. Mol Neurobiol 54:3286–3299. https://doi.org/10.1007/s12035-016-9814-5
Demyanenko SV, Panchenko SN, Uzdensky AB (2015) Expression of neuronal and signaling proteins in penumbra around a photothrombotic infarction core in rat cerebral cortex. Biochemistry (Moscow) 80:790–799. https://doi.org/10.1134/S0006297915060152
Uzdensky A, Demyanenko S, Fedorenko G, Lapteva T, Fedorenko A (2017) Photothrombotic infarct in the rat brain cortex: protein profile and morphological changes in penumbra. Mol Neurobiol 54:4172–4188. https://doi.org/10.1007/s12035-016-9964-5
Engelmann D, Pützer BM (2010) Translating DNA damage into cancer cell death-A roadmap for E2F1 apoptotic signalling and opportunities for new drug combinations to overcome chemoresistance. Drug Resist Updat 13:119–131. https://doi.org/10.1016/j.drup.2010.06.001
Meng P, Ghosh R (2014) Transcription addiction: can we garner the Yin and Yang functions of E2F1 for cancer therapy? Cell Death Dis 5:e1360. https://doi.org/10.1038/cddis.2014.326
Camins A, Verdaguer E, Folch J, Beas-Zarate C, Canudas AM, Pallàs M (2007) Inhibition of ataxia telangiectasia-p53-E2F-1 pathway in neurons as a target for the prevention of neuronal apoptosis. Curr Drug Metab 8:709–715
Folch J, Junyent F, Verdaguer E, Auladell C, Pizarro JG, Beas-Zarate C, Pallàs M, Camins A (2012) Role of cell cycle re-entry in neurons: a common apoptotic mechanism of neuronal cell death. Neurotox Res 22:195–207. https://doi.org/10.1007/s12640-011-9277-4
Raimundo N, Song L, Shutt TE, McKay SE, Cotney J, Guan MX, Gilliland TC, Hohuan D, Santos-Sacchi J, Shadel GS (2012) Mitochondrial stress engages E2F1 apoptotic signaling to cause deafness. Cell 148:716–726. https://doi.org/10.1016/j.cell.2011.12.027
Bretones G, Delgado MD, León J (2015) Myc and cell cycle control. Biochim Biophys Acta 1849:506–516. https://doi.org/10.1016/j.bbagrm.2014.03.013
MacManus JP, Jian M, Preston E, Rasquinha I, Webster J, Zurakowski B (2003) Absence of the transcription factor E2F1 attenuates brain injury and improves behavior after focal ischemia in mice. J Cereb Blood Flow Metab 23:1020–1028. https://doi.org/10.1097/01.WCB.0000084249.20114.FA
Li Y, Chopp M, Zhang ZG, Zaloga C, Niewenhuis L, Gautam S (1994) p53-immunoreactive protein and p53 mRNA expression after transient middle cerebral artery occlusion in rats. Stroke 25:849–855
Prunell GF, Arboleda VA, Troy CM (2005) Caspase function in neuronal death: delineation of the role of caspases in ischemia. Curr Drug Targets CNS Neurol Disord 4:51–61
Tan J, Zhuang L, Jiang X, Yang KK, Karuturi KM, Yu Q (2006) Apoptosis signal-regulating kinase 1 is a direct target of E2F1 and contributes to histone deacetylase inhibitor-induced apoptosis through positive feedback regulation of E2F1 apoptotic activity. J Biol Chem 281:10508–10515. https://doi.org/10.1074/jbc.M512719200
Bashari D, Hacohen D, Ginsberg D (2011) JNK activation is regulated by E2F and promotes E2F1-induced apoptosis. Cell Signal 23:65–70. https://doi.org/10.1016/j.cellsig.2010.08.004
Haim Y, Blüher M, Konrad D, Goldstein N, Klöting N, Harman-Boehm I, Kirshtein B, Ginsberg D, Tarnovscki T, Gepner Y, Shai I, Rudich A (2017) ASK1 (MAP3K5) is transcriptionally upregulated by E2F1 in adipose tissue in obesity, molecularly defining a human dys-metabolic obese phenotype. Mol Metab 6:725–736. https://doi.org/10.1016/j.molmet.2017.05.003
Gao Y, Signore AP, Yin W, Cao G, Yin X, Sun F, Luo Y, Graham SH, Chen J (2005) Neuroprotection against focal ischemic brain injury by inhibition of c-Jun N-terminal kinase and attenuation of the mitochondrial apoptosis-signaling pathway. J Cereb Blood Flow Metab 25:694–712. https://doi.org/10.1038/sj.jcbfm.9600062
de Olano N, Koo CY, Monteiro LJ, Pinto PH, Gomes AR, Aligue R, Lam EW (2012) The p38 MAPK-MK2 axis regulates E2F1 and FOXM1 expression after epirubicin treatment. Mol Cancer Res 10:1189–1202. https://doi.org/10.1158/1541-7786.MCR-11-0559
Hou ST, Xie X, Baggley A, Park DS, Chen G, Walker T (2002) Activation of the Rb/E2F1 pathway by the nonproliferative p38 MAPK during Fas (APO1/CD95)-mediated neuronal apoptosis. J Biol Chem 277:48764–48770
Fisher OM, Lord SJ, Falkenback D, Clemons NJ, Eslick GD, Lord RV (2017) The prognostic value of TP53 mutations in oesophageal adenocarcinoma: a systematic review and meta-analysis. Gut 66:399–410. https://doi.org/10.1136/gutjnl-2015-310888
Nijboer CH, Heijnen CJ, Groenendaal F, May MJ, van Bel F, Kavelaars A (2008) A dual role of the NF-kappaB pathway in neonatal hypoxic-ischemic brain damage. Stroke 39:2578–2586. https://doi.org/10.1161/STROKEAHA.108.516401
Nijboer CH, Heijnen CJ, van der Kooij MA, Zijlstra J, van Velthoven CT, Culmsee C, van Bel F, Hagberg H, Kavelaars A (2011) Targeting the p53 pathway to protect the neonatal ischemic brain. Ann Neurol 70:255–264. https://doi.org/10.1002/ana.22413
Leker RR, Aharonowiz M, Greig NH, Ovadia H (2004) The role of p53-induced apoptosis in cerebral ischemia: effects of the p53 inhibitor pifithrin alpha. Exp Neurol 187:478–486. https://doi.org/10.1016/j.expneurol.2004.01.030
Morrison RS, Wenzel HJ, Kinoshita Y, Robbins CA, Donehower LA, Schwartzkroin PA (1996) Loss of the p53 tumor suppressor gene protects neurons from kainate-induced cell death. J Neurosci 16:1337–1345
Cmielová J, Rezáčová M (2011) p21Cip1/Waf1 protein and its function based on a subcellular localization. J Cell Biochem 112:3502–3506. https://doi.org/10.1002/jcb.23296
van Lookeren Campagne M, Gill R (1998) Increased expression of cyclin G1 and p21WAF1/CIP1 in neurons following transient forebrain ischemia: comparison with early DNA damage. J Neurosci Res 53:279–296
Tu Y, Hou ST, Huang Z, Robertson GS, MacManus JP (1998) Increased Mdm2 expression in rat brain after transient middle cerebral artery occlusion. J Cereb Blood Flow Metab 18:658–669
Bui T, Sequeira J, Wen TC, Sola A, Higashi Y, Kondoh H, Genetta T (2009) ZEB1 links p63 and p73 in a novel neuronal survival pathway rapidly induced in response to cortical ischemia. PLoS ONE 4:e4373. https://doi.org/10.1371/journal.pone.0004373
Fatt MP, Cancino GI, Miller FD, Kaplan DR (2014) p63 and p73 coordinate p53 function to determine the balance between survival, cell death, and senescence in adult neural precursor cells. Cell Death Differ 21:1546–1559. https://doi.org/10.1038/cdd.2014.61
Dang CV, O’Donnell KA, Zeller KI, Nguyen T, Osthus RC, Li F (2006) The c-Myc target gene network. Semin Cancer Biol 16:253–264. https://doi.org/10.1016/j.semcancer.2006.07.014
Pelengaris S, Khan M, Evan G (2002) c-MYC: more than just a matter of life and death. Nat Rev Cancer 2:764–776. https://doi.org/10.1038/nrc904
Poole CJ, van Riggelen J (2017) MYC-Master Regulator of the cancer epigenome and transcriptome. Genes (Basel). https://doi.org/10.3390/genes8050142
Gustafson WC, Weiss WA (2010) Myc proteins as therapeutic targets. Oncogene 29:1249–1259. https://doi.org/10.1038/onc.2009.512
Choi HK, Chung KC (2011) DYRK1A positively stimulates ASK1-JNK signaling pathway during apoptotic cell death. Exp Neurobiol 20:35–44. https://doi.org/10.5607/en.2011.20.1.35
Wegiel J, Gong CX, Hwang YW (2011) The role of DYRK1A in neurodegenerative diseases. FEBS J 278:236–245. https://doi.org/10.1111/j.1742-4658.2010.07955.x
Culmsee C, Zhu Y, Krieglstein J, Mattson MP (2001) Evidence for the involvement of Par-4 in ischemic neuron cell death. J Cereb Blood Flow Metab 21:334–343
Culmsee C, Landshamer S (2006) Molecular insights into mechanisms of the cell death program: role in the progression of neurodegenerative disorders. Curr Alzheimer Res 3:269–283
Roux PP, Barker PA (2002) Neurotrophin signaling through the p75 neurotrophin receptor. Prog Neurobiol 67:203–233
Angelo MF, Aviles-Reyes RX, Villarreal A, Barker P, Reines AG, Ramos AJ (2009) p75 NTR expression is induced in isolated neurons of the penumbra after ischemia by cortical devascularization. J Neurosci Res 87:1892–1903. https://doi.org/10.1002/jnr.21993
Onoue S, Kumon Y, Igase K, Ohnishi T, Sakanaka M (2005) Growth arrest and DNA damage-inducible gene 153 increases transiently in the thalamus following focal cerebral infarction. Brain Res Mol Brain Res 134:189–197. https://doi.org/10.1016/j.molbrainres.2004.10.029
Oyadomari S, Mori M (2004) Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ 11:381–389. https://doi.org/10.1038/sj.cdd.4401373
Paschen W, Gissel C, Linden T, Althausen S, Doutheil J (1998) Activation of gadd153 expression through transient cerebral ischemia: evidence that ischemia causes endoplasmic reticulum dysfunction. Brain Res Mol Brain Res 60:115–122
Hayashi T, Saito A, Okuno S, Ferrand-Drake M, Doddand RL, Chan PK (2005) Damage to the endoplasmic reticulum and activation of apoptotic machinery by oxidative stress in ischemic neurons. J Cereb Blood Flow Metab 25:41–53. https://doi.org/10.1038/sj.jcbfm.9600005
Jaenisch N, Popp A, Guenther M, Schnabel J, Witte OW, Frahm C (2014) Pro-apoptotic function of GABA-related transcripts following stroke. Neurobiol Dis 70:237–244. https://doi.org/10.1016/j.nbd.2014.06.015
Schmidt-Kastner R, Zhang B, Belayev L, Khoutorova L, Amin R, Busto R, Ginsberg MD (2002) DNA microarray analysis of cortical gene expression during early recirculation after focal brain ischemia in rat. Brain Res Mol Brain Res 108:81–93
Suk K (2005) Role of caspases in activation-induced cell death of neuroglial. Curr Enzyme Inhib 1:43–50
Li MO, Sarkisian MR, Mehal WZ, Rakic P, Flavell RA (2003) Phosphatidylserine receptor is required for clearance of apoptotic cells. Science 302:1560–1563. https://doi.org/10.1126/science.1087621
Kitagawa H, Warita H, Sasaki C, Zhang WR, Sakai K, Shiro Y, Mitsumoto Y, Mori T, Abe K (1999) Immunoreactive Akt, PI3-K and ERK protein kinase expression in ischemic rat brain. Neurosci Lett 274:45–48
Wu HW, Li HF, Wu XY, Zhao J, Guo J (2008) Reactive oxygen species mediate ERK activation through different Raf-1-dependent signaling pathways following cerebral ischemia. Neurosci Lett 432:83–87. https://doi.org/10.1016/j.neulet.2007.11.073
Zhao H, Sapolsky RM, Steinberg GK (2006) Phosphoinositide-3-kinase/Akt survival signal pathways are implicated in neuronal survival after stroke. Mol Neurobiol 34:249–270
Liu BN, Han BX, Liu F (2014) Neuroprotective effect of pAkt and HIF-1α on ischemia rats. Asian Pac J Trop Med 7:221–225. https://doi.org/10.1016/S1995-7645(14)60025-0
Hurn PD, Macrae IM (2000) Estrogen as a neuroprotectant in stroke. J Cereb Blood Flow Metab 20:631–652
Koga S, Kojima S, Kishimoto T, Kuwabara S, Yamaguchi A (2012) Over-expression of map kinase phosphatase-1 (MKP-1) suppresses neuronal death through regulating JNK signaling in hypoxia/re-oxygenation. Brain Res 1436:137–146. https://doi.org/10.1016/j.brainres.2011.12.004
Liu L, Doran S, Xu Y, Manwani B, Ritzel R, Benashski S, McCullough L, Li J (2014) Inhibition of mitogen-activated protein kinase phosphatase-1 (MKP-1) increases experimental stroke injury. Exp Neurol 261:404–411. https://doi.org/10.1016/j.expneurol.2014.05.009
Coultrap SJ, Vest RS, Ashpole NM, Hudmon A, Bayer KU (2011) CaMKII in cerebral ischemia. Acta Pharmacol Sin 32:861–872. https://doi.org/10.1038/aps.2011.68
Bading H (2013) Nuclear calcium signalling in the regulation of brain function. Nat Rev Neurosci 14:593–608. https://doi.org/10.1038/nrn3531
Bell KF, Bent RJ, Meese-Tamuri S, Ali A, Forder JP, Aarts MM (2013) Calmodulin kinase IV-dependent CREB activation is required for neuroprotection via NMDA receptor-PSD95 disruption. J Neurochem 126:274–287. https://doi.org/10.1111/jnc.12176
Chen B, Wu Z, Xu J, Xu Y (2015) Calreticulin binds to Fas ligand and inhibits neuronal cell apoptosis induced by ischemia-reperfusion injury. Biomed Res Int 2015:895284. https://doi.org/10.1155/2015/895284
Mesaeli N, Phillipson C (2004) Impaired p53 expression, function, and nuclear localization in calreticulin-deficient cells. Mol Biol Cell 15:1862–1870. https://doi.org/10.1091/mbc.E03-04-0251
Su C, Sun F, Cunningham RL, Rybalchenko N, Singh M (2014) ERK5/KLF4 signaling as a common mediator of the neuroprotective effects of both nerve growth factor and hydrogen peroxide preconditioning. Age 36:9685. https://doi.org/10.1007/s11357-014-9685-5
Bright R, Mochly-Rosen D (2005) The role of protein kinase C in cerebral ischemic stroke. Stroke 36:2781–2790
Hara H, Onodera H, Yoshidomi M, Matsuda Y, Kogure K (1990) Staurosporine, a novel protein kinase C inhibitor, prevents postischemic neuronal damage in the gerbil and rat. J Cereb Blood Flow Metab 10:646–653
Felipo V, Miñana MD, Grisolía S (1993) Inhibitors of protein kinase C prevent the toxicity of glutamate in primary neuronal cultures. Brain Res 604:192–196
Li F, Chong ZZ, Maiese K (2005) Vital elements of the Wnt-Frizzled signaling pathway in the nervous system. Curr Neurovasc Res 2:331–340
Mathieu P, Adami PV, Morelli L (2013) Notch signaling in the pathologic adult brain. Biomol Concepts 4:465–476. https://doi.org/10.1515/bmc-2013-0006
Mastroiacovo F, Busceti CL, Biagioni F, Moyanova SG, Meisler MH, Battaglia G, Caricasole A, Bruno V, Nicoletti F (2009) Induction of the Wnt antagonist, Dickkopf-1, contributes to the development of neuronal death in models of brain focal ischemia. J Cereb Blood Flow Metab 29:264–276. https://doi.org/10.1038/jcbfm.2008.111
Culbert AA, Brown MJ, Frame S, Hagen T, Cross DA, Bax B, Reith AD (2001) GSK-3 inhibition by adenoviral FRAT1 overexpression is neuroprotective and induces Tau dephosphorylation and beta-catenin stabilisation without elevation of glycogen synthase activity. FEBS Lett 507:288–294
Russell JC, Kishimoto K, O’Driscoll C, Hossain MA (2011) Neuronal pentraxin 1 induction in hypoxic-ischemic neuronal death is regulated via a glycogen synthase kinase-3α/β dependent mechanism. Cell Signal 23:673–682. https://doi.org/10.1016/j.cellsig.2010.11.021
Ma M, Wang X, Ding X, Teng J, Shao F, Zhang J (2013) Numb/Notch signaling plays an important role in cerebral ischemia-induced apoptosis. Neurochem Res 38:254–261. https://doi.org/10.1007/s11064-012-0914-y
Bading H (2017) Therapeutic targeting of the pathological triad of extrasynaptic NMDA receptor signaling in neurodegenerations. J Exp Med 214:569–578. https://doi.org/10.1084/jem.20161673
Hardingham GE, Bading H (2010) Synaptic versus extrasynaptic NMDA receptor signalling: implications for neurodegenerative disorders. Nat Rev Neurosci 11:682–696. https://doi.org/10.1038/nrn2911
Clemens JA (2000) Cerebral ischemia: gene activation, neuronal injury, and the protective role of antioxidants. Free Rad Biol Med 28:1526–1531
Contestabile A (2008) Regulation of transcription factors by nitric oxide in neurons and in neural-derived tumor cells. Prog Neurobiol 84:317–328. https://doi.org/10.1016/j.pneurobio.2008.01.002
Demyanenko S, Neginskaya M, Berezhnaya E (2017) Expression of class I histone deacetylases in ipsilateral and contralateral hemispheres after the focal photothrombotic infarction in the mouse brain. Transl Stroke Res. https://doi.org/10.1007/s12975-017-0595-6
Hu Z, Zhong B, Tan J, Chen C, Lei Q, Zeng L (2017) The emerging role of epigenetics in cerebral ischemia. Mol Neurobiol 54:1887–1905. https://doi.org/10.1007/s12035-016-9788-3
Schweizer S, Meisel A, Märschenz S (2013) Epigenetic mechanisms in cerebral ischemia. J Cereb Blood Flow Metab 33:1335–1346. https://doi.org/10.1038/jcbfm.2013.93
Verkhratsky A, Butt A (eds) (2013) Glial physiology and pathophysiology. Wiley, New Jersey
Rego AC, Malva J (2007) Interaction between neurons and glia in aging and disease. Springer, Berlin
Kettenmann H, Ransom BA (2012) Neuroglia. Oxford University Press, Oxford
del Zoppo GJ (2009) Relationship of neurovascular elements to neuron injury during ischemia. Cerebrovasc Dis 27(Suppl 1):65–76. https://doi.org/10.1159/000200442
Dirnagl U (2012) Pathobiology of injury after stroke: the neurovascular unit and beyond. Ann N Y Acad Sci 1268:21–25. https://doi.org/10.1111/j.1749-6632.2012.06691.x
Puig B, Brenna S, Magnus T (2018) Molecular communication of a dying neuron in stroke. Int J Mol Sci 9:19. https://doi.org/10.3390/ijms19092834
Acknowledgements
The work was supported by the Russian Science Foundation (Grant # 18-15-00110).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Uzdensky, A.B. Apoptosis regulation in the penumbra after ischemic stroke: expression of pro- and antiapoptotic proteins. Apoptosis 24, 687–702 (2019). https://doi.org/10.1007/s10495-019-01556-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-019-01556-6