Abstract
Delayed neuronal death is a hallmark of infarct development and sustained functional impairment in rodent models of focal cerebral ischemia, an experimental paradigm resembling ischemic stroke in humans. The exact molecular pathophysiology of this still enigmatic event is not only of academic interest but may hold the key for novel therapeutic strategies for human stroke. There is general understanding that acute lack of perfusion leads to rapid necrotic-oncotic cell death in the core of the ischemic infarct. In contrast, conditions associated with severely reduced, but not immediately lethal reductions of cerebral blood flow in the ischemic penumbra likely result in delayed and more programmed type of neuronal cell death. Based on results first obtained from non-neuronal cells, cysteine aspartate proteases (caspases) were described as key modulators of this process. More recently, however, it became clear that also caspase-independent mechanisms play a significant role for ischemia-induced delayed neuronal cell loss. In this chapter, we review the role of one of the first described caspase-independent cell death proteins, apoptosis-inducing factor (AIF), for post-ischemic brain damage. Our conclusion is that there is compelling evidence for a causal role of AIF in neuronal cell death following experimental stroke and other neurological disorders associated with cerebral ischemia. Hence, AIF and other, more recently described subtypes of caspase-independent cell death may provide promising targets for therapeutic interventions in cerebrovascular disease.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Every year stroke is responsible for the death of 5.5 million people and thus accounts for 10% of all deaths in industrialized countries worldwide. Despite such a high incidence and mortality, therapeutic options for stroke patients are still very limited (Lo et al. 2003). Currently, the only clinical treatment options for stroke patients are recanalization of large brain supplying arteries by local or systemic administration of recombinant tissue plasminogen activator (rtPA) and/or by mechanical removal of clots/emboli with stent retrievers (Hussain et al. 2016). A major limitation of these early therapeutic approaches, however, is that both require brain CT and MRI imaging in order to exclude hemorrhagic stroke and to localize the occluded vessel. Accordingly, rtPA lysis and mechanical recanalization can only be initiated after affected patients are admitted to a specialized center. By the time diagnostic procedures have been completed the therapeutic window for both procedures, i.e. 4.5 h after the onset of ischemia, has shortened significantly or even closed. As a result less than 10% of all stroke patients are subjected to recanalization therapy (Adams et al. 2007). The remaining 90% may only hope for spontaneous reperfusion, which in most cases, however, occurs too late to prevent penumbral cell death, the main mechanism underlying infarct outgrowth and the subsequent loss of neurological function (Molina et al. 2001). Hence, novel treatment strategies are warranted which are able to prolong neuronal survival in the ischemic penumbra, i.e., under compromised cerebral blood flow conditions.
It is well accepted that even after reperfusion cell death signaling pathways triggered by the initial ischemic event remain activated and result in additional neuronal cell death under completely normal blood flow conditions. This was first demonstrated in experimental approaches where very brief ischemic episodes were induced, i.e. 30 min of middle cerebral artery occlusion in mice or rats (MCAo), which may resemble transient ischemic attacks (TIA) in patients. Under this condition neuronal cell death may occur with a delay of up to 24 h following reperfusion (Du et al. 1996; Endres et al. 1998). Subsequently, post-reperfusion cell death was also demonstrated following more severe ischemic episodes which are associated with acute infarction and, hence, resemble acute stroke in humans. In the ischemic penumbra of mice subjected to 60 min of transient MCAo neurons die with a delay of only 3–6 h (Fig. 6.1a), i.e., also post-reperfusion cell death seems to have a clinically relevant therapeutic window. Accordingly, an optimal therapeutic approach towards the treatment of stroke should include the protection of neuronal cells during the period of compromised blood flow but also the prevention of cell death after reperfusion.
Delayed neuronal cell death in the ischemic penumbra and correlation with nuclear AIF following transient focal cerebral ischemia in mice (a) Following 60 min of middle cerebral artery occlusion (MCAo) the majority of neurons (~70%) in the ischemic penumbra, i.e. the cerebral cortex, stay alive for at least 4 h. Despite sufficient blood flow 24 h after MCAo over 90% of neurons which were viable 2 h after ischemia display altered membrane and nuclear morphology indicating cell death. (b) Correlation of neurons displaying pathological morphology with cells showing nuclear AIF (Culmsee et al. 2005). (c) In Harlequin mutant mice (HQ) which have a reduced expression of AIF protein due to a proviral insertion in the aif gene, the infarct volume, calculated on the basis of the histomorphometric data from the individual sections, showed a 43% reduction as compared to wild type littermates (n = 5, *p < 0.03) (Culmsee et al. 2005)
2 Mechanisms of Delayed Cell Death Following Focal Cerebral Ischemia
The morphological hallmarks of neuronal cell death following focal cerebral ischemia are cell shrinkage and nuclear condensation, features not present in classical necrotic cell death which is associated with cell lysis and nuclear decomposition. Cell shrinkage and nuclear condensation following cerebral ischemia are found in brain areas affected by immediate and delayed cell death. Accordingly, the mechanisms leading to ischemic cell death seem to be very similar irrespective if affected cells are located in the infarct core where blood flow is almost absent or in the ischemic penumbra where collateral blood flow may keep cells alive for several hours (Astrup et al. 1981). For many years it remained unclear how ischemia causes the morphological findings described above. Nuclear condensation is the morphological sequel of DNA damage, which usually occurs in a highly regulated manner during various forms of programmed cell death, such as apoptosis, necroptosis, or parthanatos (Andrabi et al. 2011; Galluzzi et al. 2014; Vanden Berghe et al. 2014). More than 20 years ago Linnik et al. and Charriaut-Merlangue et al. were the first to demonstrate that nuclear condensation following cerebral ischemia was the result of DNA damage and endonuclease activation (Charriaut-Marlangue et al. 1996; Linnik et al. 1995). These findings triggered intense search for the upstream signaling responsible for post-ischemic endonuclease activation which was finally believed to be the activation of caspase-3 (Namura et al. 1998). Namura and colleagues showed constitutive expression of inactive caspase-3 in neurons throughout the brain, most prominently in neuronal perikarya within piriform cortex and, most importantly, caspase-like enzyme activity in ischemic brain 30–60 min after reperfusion following 2 h MCAo. Active caspase-3 was detected in ischemic neurons at the time of reperfusion by immunohistochemistry. DNA laddering and TUNEL-positive cells as indicators of DNA fragmentation were detected 6–24 h after reperfusion (Namura et al. 1998). Further proof for the role of active caspase-3 for ischemic cell death was derived in the same year from experiments from the same laboratory using pan-caspase and caspase-3 specific peptide inhibitors. Post-ischemic neuronal cell death was prevented and neuronal function was improved when caspase activation was inhibited up to 6 h following reperfusion from 30 min MCAo (Endres et al. 1998). The ultimate mechanistic link between caspase-3 activation and post-ischemic DNA fragmentation was established by Gao and co-workers by showing that caspase-activated DNase (CAD), a molecule known to be cleaved and thereby activated by caspase-3, was responsible for post-ischemic DNA-fragmentation (Cao et al. 2001).
In consequence, many research groups focused on the upstream mechanisms of caspase-3 activation. Due to very low expression and activation levels of potentially involved molecules it turned out to be technically very challenging to identify respective mechanisms. Caspase-8, a molecule able to cleave caspase-3 in non-neuronal cells, was found to be activated following experimental stroke; however, caspase-8 was described to be activated in a population of neurons (lamina V) distinct from that where active caspase-3 was observed (lamina II/III) (Velier et al. 1999) and a direct link between caspase-8 and caspase-3 activation has not yet been demonstrated in models of cerebral ischemia. Further upstream factors in the cascade of caspase activation such as Fas/CD95 receptors and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), were found to be upregulated following MCAo, and lpr mice, which express dysfunctional Fas receptors, were protected from focal ischemic brain damage (Martin-Villalba et al. 1999). Despite these interesting findings it still remained unclear how caspase-3 was activated following cerebral ischemia until in 2001 it was demonstrated that the BH3-only Bcl-2 family Bid, which has a caspase-8 specific cleavage site, was truncated after experimental stroke (Plesnila et al. 2001). Cleaved/truncated Bid (tBid) translocates from the cytoplasm to the outer mitochondrial membrane where together with Bax it induces the formation of an oligomeric membrane pore (Zha et al. 2000), thereby releasing cytochrome c from mitochondria (Wei et al. 2000). After focal cerebral ischemia mitochondria of Bid-deficient mice released far less cytochrome c and cortical infarction was significantly reduced compared to wildtype littermates, thereby demonstrating the prominent role of mitochondria in post-ischemic cell death. This was supported by recent experiments on Bax- deficient mice, which showed a similar level of neuroprotection as Bid- deficient animals (D’Orsi et al. 2015). These data further imply that after focal cerebral ischemia caspase-3 may be activated through the mitochondrial pathway, i.e. by the mitochondrial release of cytochrome c (Fujimura et al. 2000) and apoptosome formation (Plesnila 2004; Plesnila et al. 2001; Yin et al. 2002). Not much later, however, this view was challenged by the fact that caspase-3 knock out mice, which became available at that time, showed much less neuroprotection than expected based on the anticipated prominent role of caspase-3 activation for ischemic neuronal cell death (Le et al. 2002). Together with the pronounced neuroprotective effect achieved by interactions with mitochondrial cell death signaling, (Cao et al. 2002; D’Orsi et al. 2015; Kilic et al. 2002; Martinou et al. 1994; Plesnila et al. 2001; Wiessner et al. 1999), i.e., mechanisms upstream of caspase-3 activation such as Bid and Bax activation, it became clear that alternative cell death pathways distinct from caspase-3 may be present downstream of mitochondria.
The hypothesis that caspase-independent neuronal cell death signaling exists downstream of mitochondria was also suggested by in vitro experiments showing that caspase inhibition provided only transient neuroprotection which was followed by a more delayed type of DNA-fragmentation-related cell death (see Rideout and Stefanis 2001 for review). It was Ruth Slack and her colleagues who identified a mitochondrial protein, apoptosis-inducing factor (AIF), to be one of the most potent molecular candidates for caspase-independent death in neurons (Cregan et al. 2002). AIF translocation from mitochondria to the nucleus was detected in damaged neurons in vitro in models of neuronal cell death relevant to the pathology of ischemic brain damage, such as glutamate neurotoxicity, DNA damage or oxygen-glucose deprivation, whereas neutralizing AIF antibodies, pharmacological inhibition of AIF release or AIF siRNA prevented neuronal cell death in these in vitro approaches (Becattini et al. 2006; Cao et al. 2002; Cregan et al. 2002; Culmsee et al. 2005).
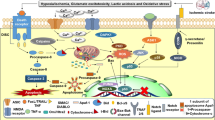
AIF is a 67 kDa flavoprotein with significant homology to bacterial and plant oxidoreductases located in the mitochondrial intramembranous space (Susin et al. 1999). Upon release from mitochondria, AIF migrates to the nucleus where it induces large-scale (~50 kbp) DNA fragmentation and cell death in a caspase-independent manner (Daugas et al. 2000; Penninger and Kroemer 2003). Recent findings in models of glutamate neurotoxicity in cultured neurons in vitro and cerebral hypoxia/ischemia in vivo suggested that AIF translocation from mitochondria to the nucleus requires Cyclophilin A (CypA), which seems to coordinate DNA binding and chromatinolysis through complex formation with histone H2AX (Artus et al., 2010; Baritaud et al., 2010; Doti et al., 2014; Zhu et al., 2007). Finally, the Dawson laboratory identified macrophage migration inhibitory factor (MIF) as the key nuclease mediating AIF-dependent DNA degradation in paradigms of parthanatos induced by oxidative stress and DNA-damage (Wang et al., 2016). Eliminating MIF’s nuclease activity exerted sustained protective effects in a model of focal cerebral ischemia, both at the level of histology and behavior.
In the brain, AIF was shown to be expressed in all so far investigated cell types, i.e. neurons and glial cells (Cao et al. 2003; Zhu et al. 2003). The expression in normal neuronal cells was confined to the mitochondria as shown by co-immunostaining with the mitochondrial marker cytochrome oxidase (Plesnila et al. 2004). Interestingly, unlike the expression pattern of many other apoptotic proteins the expression of AIF increases gradually with brain maturation and peaks in adulthood, indicating that in contrast to, e.g. caspase-3, AIF may exert its main function in adult neurons (Cao et al. 2003).
The first pathological condition where AIF was shown to play an important role for neuronal damage was cerebral hypoxia-ischemia, a model for asphyxia in newborn children. Hypoxia-ischemia in 7-day-old rats induced by ligation of the left carotid artery for 55 min together with the reduction of ambient oxygen to 7.7% in a hypoxia chamber resulted in AIF release from mitochondria and translocation to the nucleus in neurons displaying DNA fragmentation- and pyknosis (Zhu et al. 2003). Since AIF translocation was not influenced by inhibition of caspases using the pan-caspase inhibitor BAF these experiments stressed the caspase-independent manner of AIF-induced cell death. Similar findings were also observed following cardiac arrest- induced brain damage in rats, i.e., following transient global ischemia. Following 15 min of four-vessel occlusion (4-VO) AIF was found to translocate from mitochondria to the nucleus in hippocampal CA1 neurons. The temporal profile of AIF translocation coincided with the induction of large-scale DNA fragmentation (50 kbp; 24–72 h after 4-VO), a well-characterized hallmark of delayed neuronal cell death (Cao et al. 2003). In line with findings in the rodent models of transient hypoxia-ischemia in immature animals, treatment with a caspase-3 inhibitor had no effect on nuclear AIF accumulation and did not provide any long-lasting neuroprotective effects after global ischemia in adult rats (Cao et al. 2003).
At almost the same time we demonstrated the translocation of AIF from mitochondria to the nucleus following transient focal cerebral ischemia, an experimental model of ischemic stroke followed by reperfusion (Plesnila et al. 2004). Nuclear AIF was detected in single neuronal cells very early, i.e. within 1 h after 45 min of middle cerebral artery occlusion (MCAo) and peaked 24 h thereafter. The time course of AIF translocation paralleled mitochondrial cytochrome c release and apoptosis-like DNA damage as identified by hair-pin probe (HPP) staining, indicating ischemia-induced mitochondrial permeabilization and AIF-induced DNA fragmentation (Plesnila et al. 2004). Further, we showed that in the same experimental paradigm of ischemic stroke that AIF nuclear translocation was mainly found in neurons (Culmsee et al. 2005) and that the number of cells displaying pathological morphology following cerebral ischemia correlated very well (r2 = 0.99) with the number of neurons showing nuclear AIF (Fig. 6.1b).
That nuclear translocation of AIF was indeed responsible for post-ischemic cell death and not only a byproduct of the morphological changes associated with neuronal cell death was first shown in 2005. Small inhibitory RNA (siRNA)-mediated downregulation of AIF expression (−80%) in HT22 hippocampal neurons and in primary cultured neurons resulted in a significant reduction of glutamate and oxygen-glucose deprivation-induced neuronal cell death, respectively (Figs. 6.2 and 6.3). Reduction of cell death was associated with a lack of nuclear AIF translocation, thereby demonstrating that AIF plays a causal role in excitotoxic and hypoxic-hypoglycaemic cell death in vitro (Culmsee et al. 2005). In the same study we demonstrated that AIF is also relevant for post-ischemic cell death in vivo. Harlequin mutant mice carry a pro-viral insertion in the AIF-gene thereby expressing only 10–20% of normal AIF protein levels (Klein et al. 2002). These mutant mice show significantly reduced post-ischemic brain damage as compared to their wild-type littermates, which express AIF at normal levels (Culmsee et al. 2005) (Fig. 6.1c).
AIF-siRNA knockdown attenuates glutamate-induced neuronal cell death in primary cultured neurons (a) Confocal laser scanning microscope images of AIF immunoreactivity (green) were obtained after 8 h of oxygene glucose deprivation (OGD). Co-staining with DAPI (dark blue) allowed the identification of nuclear translocation of AIF (AIF/DAPI, light blue) in damaged cells. (b) Number of damaged neurons and neurons displaying nuclear AIF 4 and 8 h after reoxygenation after 4 h of oxygen—glucose deprivation. AIF translocates to the nucleus before signs of morphological neuronal damage [as determined by nuclear morphology after DAPI/Hoechst staining or propidium iodide/calcein staining] become evident (n = 4; ###p < 0.001 vs. control). (c) Primary cultured neurons were pre-treated with vehicle (lipofectamine), non-functional mutant RNA (mut-siRNA), or AIF-siRNA for 48 h before exposure to OGD for 4 h. Cell death was quantified by counting of cells with pyknotic nuclei 24 h after re-oxygenation in medium containing glucose. In AIF siRNA- treated neurons the number of cells displaying pyknotic nuclei was reduced by ~50% (n = 4; *p < 0.01 vs. control) (Culmsee et al. 2005)
AIF-siRNA preserves mitochondrial integrity and function, and cell viability. (a) Fluorescence photomicrographs show that AIF siRNA (20 nM) prevents the fission of mitochondria (stained with Mitotracker red) in glutamate-exposed (3 mM, 14 h) HT-22 cells compared to non-transfected control cells and cells transfected with scr siRNA. Scale bar 20 μm; insets show magnifications for better detection of mitochondrial morphology. (b) ATP levels from AIF siRNA transfected cells (20 nM) were protected from ATP depletion as determined 24 h after glutamate exposure (n = 6; **p\0.01 compared to glutamate treated control cells and scr siRNA; ANOVA, Scheffe’ test). (c) AIF siRNA (20 nM) prevents glutamate-induced (5 mM, 12 h) cell death in neuronal HT22 cells compared to non-transfected control cells and cells transfected with scr siRNA. (d) xCELLigence real-time measurement: HT22 cells were treated with glutamate (glut) 72 h after transfection. AIF siRNA (20 nM) shows sustained protection over time (n = 8) (Oexler et al. 2012)
Further analysis in vitro revealed that reduced AIF expression exerts preconditioning effects at the level of mitochondria, thereby preserving mitochondrial integrity and function in conditions of glutamate toxicity (Fig. 6.3) (Oexler et al. 2012). Whether such preconditioning effects at the level of mitochondria caused by reduced AIF expression levels account for protective effects against ischemic brain damage in vivo requires further investigation.
In vitro, nuclear AIF translocation was dependent on poly(ADP-ribose) polymerase 1 (PARP1) activation, as shown by using the specific PARP1 inhibitor PJ-34 (Culmsee et al. 2005). Accordingly, these results suggest that PARP1 activation is located upstream of AIF release from mitochondria and that AIF is the major factor mediating PARP1-induced cell death, findings also supported by other laboratories using different strategies to inhibit PARP, i.e. by cilostazol or gallotannin (Lee et al. 2007; Wei et al. 2007). More recently, Iduna was identified as an NMDA-receptor-induced survival protein which binds poly(ADP-ribose) polymers, thereby preventing AIF translocation to the nucleus in paradigms involving parthanatos in NMDA excitotoxicity in vitro and ischemic neuronal death in vivo (Andrabi et al. 2011). Further, activation of neuronal nitric oxide synthase (nNOS) and formation of ROS, particularly lipidperoxides, were linked to AIF-mediated neuronal cell death following experimental stroke (Li et al. 2007; Tobaben et al. 2011; Yigitkanli et al. 2017). Gene deletion of nNOS, application of a metalloporphyrin-based superoxide dismutase or inhibition of 12/15 lipidperoxidase (LOX) mimic reduced post-ischemic cell death, together with a reduction of the number of neurons displaying nuclear AIF, thereby suggesting that ROS and peroxynitrite formation may cause direct or indirect mitochondrial damage and subsequent AIF release, nuclear translocation, and large-scale DNA fragmentation (Lee et al. 2005; Li et al. 2007).
Results from our and other laboratories on the direct upstream mechanisms responsible for the release of AIF from mitochondria suggest that pro-apoptotic proteins of the bcl-2 family such as Bid interacting with regulators of mitochondrial fission such as Drp1 play an important role for this process. SiRNA-mediated knockdown and small molecule inhibitors of Bid or Drp1 fully preserved mitochondrial integrity and function, and prevented cell death, together with translocation of AIF from mitochondria to the nucleus in primary cultured neurons following oxygen-glucose deprivation and completely preserved cell and nuclear morphology following glutamate toxicity in HT22 hippocampal cells (Culmsee et al. 2005; Landshamer et al. 2008; Grohm et al. 2010, 2012). Further, the small molecular inhibitors of Drp1, MDIVI-A and MDIVI-B reduced infarct size in a model of focal cerebral ischemia in mice (Grohm et al. 2012), similar to previously reported effects of genetic Bid deletion (Plesnila et al. 2001; Yin et al. 2002)
In conclusion, the current literature suggests that AIF-mediated caspase-independent signaling pathways are of major importance for delayed neuronal cell death following experimental stroke. Caspase activation occurs during this process, however, inhibition of caspases seems to only delay and not to prevent neuronal death following focal cerebral ischemia. These findings suggest that inhibition of mitochondrial AIF release and subsequent AIF-dependent mechanisms of DNA damage may serve as novel targets for drug development aimed to mitigate cell death following stroke.
References
Adams HP Jr, del ZG, Alberts MJ, Bhatt DL, Brass L, Furlan A, Grubb RL, Higashida RT, Jauch EC, Kidwell C, Lyden PD, Morgenstern LB, Qureshi AI, Rosenwasser RH, Scott PA, Wijdicks EF (2007) Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: The American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Circulation 115:e478–e534
Andrabi SA, Kang HC, Haince JF, Lee YI, Zhang J, Chi Z, West AB, Koehler RC, Poirier GG, Dawson TM, Dawson VL (2011) Iduna protects the brain from glutamate excitotoxicity and stroke by interfering with poly(ADP-ribose) polymer-induced cell death. Nat Med 17:692–699
Artus C, Boujrad H, Bouharrour A, Brunelle MN, Hoos S, Yuste VJ, Lenormand P, Rousselle JC, Namane A, England P, Lorenzo HK, Susin SA (2010) AIF promotes chromatinolysis and caspase-independent programmed necrosis by interacting with histone H2AX. EMBO J 29:1585–1599
Astrup J, Siesjo BK, Symon L (1981) Thresholds in cerebral ischemia—the ischemic penumbra. Stroke 12:723–725
Baritaud M, Boujrad H, Lorenzo HK, Krantic S, Susin SA (2010) Histone H2AX: the missing link in AIF-mediated caspase-independent programmed necrosis. Cell Cycle 9:3166–3173
Becattini B, Culmsee C, Leone M, Zhai D, Zhang X, Crowell KJ, Rega MF, Landshamer S, Reed JC, Plesnila N, Pellecchia M (2006) Structure-activity relationships by interligand NOE-based design and synthesis of antiapoptotic compounds targeting Bid. Proc Natl Acad Sci U S A 103:12602–12606
Cao G, Pei W, Lan J, Stetler RA, Luo Y, Nagayama T, Graham SH, Yin XM, Simon RP, Chen J (2001) Caspase-activated DNase/DNA fragmentation factor 40 mediates apoptotic DNA fragmentation in transient cerebral ischemia and in neuronal cultures. J Neurosci 21:4678–4690
Cao G, Pei W, Ge H, Liang Q, Luo Y, Sharp FR, Lu A, Ran R, Graham SH, Chen J (2002) In vivo delivery of a Bcl-xL fusion protein containing the TAT protein transduction domain protects against ischemic brain injury and neuronal apoptosis. J Neurosci 22:5423–5431
Cao G, Clark RS, Pei W, Yin W, Zhang F, Sun FY, Graham SH, Chen J (2003) Translocation of apoptosis-inducing factor in vulnerable neurons after transient cerebral ischemia and in neuronal cultures after oxygen-glucose deprivation. J Cereb Blood Flow Metab 23:1137–1150
Charriaut-Marlangue C, Margaill I, Represa A, Popovici T, Plotkine M, Ben Ari Y (1996) Apoptosis and necrosis after reversible focal ischemia: an in situ DNA fragmentation analysis. J Cereb Blood Flow Metab 16:186–194
Cregan SP, Fortin A, MacLaurin JG, Callaghan SM, Cecconi F, Yu SW, Dawson TM, Dawson VL, Park DS, Kroemer G, Slack RS (2002) Apoptosis-inducing factor is involved in the regulation of caspase-independent neuronal cell death. J Cell Biol 158:507–517
Culmsee C, Zhu C, Landshamer S, Becattini B, Wagner E, Pellecchia M, Blomgren K, Plesnila N (2005) Apoptosis-inducing factor triggered by poly(ADP-ribose) polymerase and Bid mediates neuronal cell death after oxygen-glucose deprivation and focal cerebral ischemia. J Neurosci 25:10262–10272
Daugas E, Nochy D, Ravagnan L, Loeffler M, Susin SA, Zamzami N, Kroemer G (2000) Apoptosis-inducing factor (AIF): a ubiquitous mitochondrial oxidoreductase involved in apoptosis. FEBS Lett 476:118–123
D’Orsi B, Kilbride SM, Chen G, Perez AS, Bonner HP, Pfeiffer S, Plesnila N, Engel T, Henshall DC, Dussmann H, Prehn JH (2015) Bax regulates neuronal Ca2+ homeostasis. J Neurosci 35:1706–1722
Doti N, Reuther C, Scognamiglio PL, Dolga AM, Plesnila N, Ruvo M, Culmsee C (2014) Inhibition of the AIF/CypA complex protects against intrinsic death pathways induced by oxidative stress. Cell Death Dis 5:e993
Du C, Hu R, Csernansky CA, Hsu CY, Choi DW (1996) Very delayed infarction after mild focal cerebral ischemia: a role for apoptosis? J Cereb Blood Flow Metab 16:195–201
Endres M, Namura S, Shimizu-Sasamata M, Waeber C, Zhang L, Gomez-Isla T, Hyman BT, Moskowitz MA (1998) Attenuation of delayed neuronal death after mild focal ischemia in mice by inhibition of the caspase family. J Cereb Blood Flow Metab 18:238–247
Fujimura M, Morita-Fujimura Y, Noshita N, Sugawara T, Kawase M, Chan PH (2000) The cytosolic antioxidant copper/zinc-superoxide dismutase prevents the early release of mitochondrial cytochrome c in ischemic brain after transient focal cerebral ischemia in mice. J Neurosci 20:2817–2824
Galluzzi L, Kepp O, Krautwald S, Kroemer G, Linkermann A (2014) Molecular mechanisms of regulated necrosis. Semin Cell Dev Biol 35:24–32. https://doi.org/10.1016/j.semcdb.2014.02.006. Epub 2014 Feb 26
Grohm J, Plesnila N, Culmsee C (2010) Bid mediates fission, membrane permeabilization and peri-nuclear accumulation of mitochondria as a prerequisite for oxidative neuronal cell death. Brain Behav Immun 24:831–838
Grohm J, Kim SW, Mamrak U, Tobaben S, Cassidy-Stone A, Nunnari J, Plesnila N, Culmsee C (2012) Inhibition of Drp1 provides neuroprotection in vitro and in vivo. Cell Death Differ 19:1446–1458
Hussain M, Moussavi M, Korya D, Mehta S, Brar J, Chahal H, Qureshi I, Mehta T, Ahmad J, Zaidat OO, Kirmani JF (2016) Systematic review and pooled analyses of recent Neurointerventional randomized controlled trials: setting a new standard of care for acute ischemic stroke treatment after 20 years. Interv Neurol 5:39–50
Kilic E, Dietz GP, Hermann DM, Bahr M (2002) Intravenous TAT-Bcl-Xl is protective after middle cerebral artery occlusion in mice. Ann Neurol 52:617–622
Klein JA, Longo-Guess CM, Rossmann MP, Seburn KL, Hurd RE, Frankel WN, Bronson RT, Ackerman SL (2002) The harlequin mouse mutation downregulates apoptosis-inducing factor. Nature 419:367–374
Landshamer S, Hoehn M, Barth N, Duvezin-Caubet S, Schwake G, Tobaben S, Kazhdan I, Becattini B, Zahler S, Vollmar A, Pellecchia M, Plesnila N, Wagner E, Culmsee C (2008) Bid-induced release of AIF from mitochondria causes immediate neuronal cell death. Cell Death Differ 15:1553–1563
Le DA, Wu Y, Huang Z, Matsushita K, Plesnila N, Augustinack JC, Hyman BT, Yuan J, Kuida K, Flavell RA, Moskowitz MA (2002) Caspase activation and neuroprotection in caspase-3- deficient mice after in vivo cerebral ischemia and in vitro oxygen glucose deprivation. Proc Natl Acad Sci U S A 99:15188–15193
Lee BI, Chan PH, Kim GW (2005) Metalloporphyrin-based superoxide dismutase mimic attenuates the nuclear translocation of apoptosis-inducing factor and the subsequent DNA fragmentation after permanent focal cerebral ischemia in mice. Stroke 36:2712–2717
Lee JH, Park SY, Shin HK, Kim CD, Lee WS, Hong KW (2007) Poly(ADP-ribose) polymerase inhibition by cilostazol is implicated in the neuroprotective effect against focal cerebral ischemic infarct in rat. Brain Res 1152:182–190. Epub 2007
Li X, Nemoto M, Xu Z, Yu SW, Shimoji M, Andrabi SA, Haince JF, Poirier GG, Dawson TM, Dawson VL, Koehler RC (2007) Influence of duration of focal cerebral ischemia and neuronal nitric oxide synthase on translocation of apoptosis-inducing factor to the nucleus. Neuroscience 144:56–65
Linnik MD, Miller JA, Sprinkle-Cavallo J, Mason PJ, Thompson FY, Montgomery LR, Schroeder KK (1995) Apoptotic DNA fragmentation in the rat cerebral cortex induced by permanent middle cerebral artery occlusion. Brain Res Mol Brain Res 32:116–124
Lo EH, Dalkara T, Moskowitz MA (2003) Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci 4:399–415
Martinou JC, Dubois-Dauphin M, Staple JK, Rodriguez I, Frankowski H, Missotten M, Albertini P, Talabot D, Catsicas S, Pietra C (1994) Overexpression of BCL-2 in transgenic mice protects neurons from naturally occurring cell death and experimental ischemia. Neuron 13:1017–1030
Martin-Villalba A, Herr I, Jeremias I, Hahne M, Brandt R, Vogel J, Schenkel J, Herdegen T, Debatin KM (1999) CD95 ligand (Fas-L/APO-1L) and tumor necrosis factor-related apoptosis-inducing ligand mediate ischemia-induced apoptosis in neurons. J Neurosci 19:3809–3817
Molina CA, Montaner J, Abilleira S, Ibarra B, Romero F, Arenillas JF, Alvarez-Sabin J (2001) Timing of spontaneous recanalization and risk of hemorrhagic transformation in acute cardioembolic stroke. Stroke 32:1079–1084
Namura S, Zhu J, Fink K, Endres M, Srinivasan A, Tomaselli KJ, Yuan J, Moskowitz MA (1998) Activation and cleavage of caspase-3 in apoptosis induced by experimental cerebral ischemia. J Neurosci 18:3659–3668
Oexler EM, Dolga A, Culmsee C (2012) AIF depletion provides neuroprotection through a preconditioning effect. Apoptosis 17:1027–1038
Penninger JM, Kroemer G (2003) Mitochondria, AIF and caspases-rivaling for cell death execution. Nat Cell Biol 5:97–99
Plesnila N (2004) Role of mitochondrial proteins for neuronal cell death after focal cerebral ischemia. Acta Neurochir Suppl 89:15–19
Plesnila N, Zinkel S, Le DA, Amin-Hanjani S, Wu Y, Qiu J, Chiarugi A, Thomas SS, Kohane DS, Korsmeyer SJ, Moskowitz MA (2001) BID mediates neuronal cell death after oxygen/ glucose deprivation and focal cerebral ischemia. Proc Natl Acad Sci U S A 98:15318–15323
Plesnila N, Zhu C, Culmsee C, Groger M, Moskowitz MA, Blomgren K (2004) Nuclear translocation of apoptosis-inducing factor after focal cerebral ischemia. J Cereb Blood Flow Metab 24:458–466
Rideout HJ, Stefanis L (2001) Caspase inhibition: a potential therapeutic strategy in neurological diseases. Histol Histopathol 16:895–908
Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM, Mangion J, Jacotot E, Costantini P, Loeffler M, Larochette N, Goodlett DR, Aebersold R, Siderovski DP, Penninger JM, Kroemer G (1999) Molecular characterization of mitochondrial apoptosis-inducing factor. Nature 397:441–446
Tobaben S, Grohm J, Seiler A, Conrad M, Plesnila N, Culmsee C (2011) Bid-mediated mitochondrial damage is a key mechanism in glutamate-induced oxidative stress and AIF-dependent cell death in immortalized HT-22 hippocampal neurons. Cell Death Differ 18:282–292
Vanden Berghe T, Linkermann A, Jouan-Lanhouet S, Walczak H, Vandenabeele P (2014) Regulated necrosis: the expanding network of non-apoptotic cell death pathways. Nat Rev Mol Cell Biol 15:135–147
Velier JJ, Ellison JA, Kikly KK, Spera PA, Barone FC, Feuerstein GZ (1999) Caspase-8 and caspase-3 are expressed by different populations of cortical neurons undergoing delayed cell death after focal stroke in the rat. J Neurosci 19:5932–5941
Wang Y, An R, Umanah GK, Park H, Nambiar K, Eacker SM, Kim B, Bao L, Harraz MM, Chang C, Chen R, Wang JE, Kam TI, Jeong JS, Xie Z, Neifert S, Qian J, Andrabi SA, Blackshaw S, Zhu H, Song H, Ming GL, Dawson VL, Dawson TM (2016) A nuclease that mediates cell death induced by DNA damage and poly(ADP-ribose) polymerase-1. Science 354(6308)
Wei MC, Lindsten T, Mootha VK, Weiler S, Gross A, Ashiya M, Thompson CB, Korsmeyer SJ (2000) tBID, a membrane-targeted death ligand, oligomerizes BAK to release cytochrome c. Genes Dev 14:2060–2071
Wei G, Wang D, Lu H, Parmentier S, Wang Q, Panter SS, Frey WH, Ying W (2007) Intranasal administration of a PARG inhibitor profoundly decreases ischemic brain injury. Front Biosci 12:4986–4996
Wiessner C, Allegrini PR, Rupalla K, Sauer D, Oltersdorf T, McGregor AL, Bischoff S, Bottiger BW, van der PH (1999) Neuron-specific transgene expression of Bcl-XL but not Bcl-2 genes reduced lesion size after permanent middle cerebral artery occlusion in mice. Neurosci Lett 268:119–122
Yigitkanli K, Zheng Y, Pekcec A, Lo EH, van Leyen K (2017) Increased 12/15-Lipoxygenase leads to widespread brain injury following global cerebral ischemia. Transl Stroke Res 8:194–202
Yin XM, Luo Y, Cao G, Bai L, Pei W, Kuharsky DK, Chen J (2002) Bid-mediated mitochondrial pathway is critical to ischemic neuronal apoptosis and focal cerebral ischemia. J Biol Chem 277:42074–42081
Zha J, Weiler S, Oh KJ, Wei MC, Korsmeyer SJ (2000) Posttranslational N-myristoylation of BID as a molecular switch for targeting mitochondria and apoptosis. Science 290:1761–1765
Zhu C, Qiu L, Wang X, Hallin U, Cande C, Kroemer G, Hagberg H, Blomgren K (2003) Involvement of apoptosis-inducing factor in neuronal death after hypoxia-ischemia in the neonatal rat brain. J Neurochem 86:306–317
Zhu C, Wang X, Huang Z, Qiu L, Xu F, Vahsen N, Nilsson M, Eriksson PS, Hagberg H, Culmsee C, Plesnila N, Kroemer G, Blomgren K (2007) Apoptosis-inducing factor is a major contributor to neuronal loss induced by neonatal cerebral hypoxia-ischemia. Cell Death Differ 14:775–784
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Plesnila, N., Culmsee, C. (2018). Involvement of Apoptosis-Inducing Factor (AIF) in Neuronal Cell Death Following Cerebral Ischemia. In: Fujikawa, D. (eds) Acute Neuronal Injury. Springer, Cham. https://doi.org/10.1007/978-3-319-77495-4_6
Download citation
DOI: https://doi.org/10.1007/978-3-319-77495-4_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-77494-7
Online ISBN: 978-3-319-77495-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)