Abstract
Plants show defensive responses after exposure to volatiles from neighbouring plants infested by herbivores. When a plant’s neighbours host only species of herbivores that do not feed on the plant itself, the plant can conserve energy by maintaining a low defence level. An intriguing question is whether plants respond differently to volatiles from plants infested by herbivores that pose greater or lesser degrees of danger. We examined the secretion of extrafloral nectar (EFN) in lima bean plants exposed to volatiles from cabbage plants infested by common cutworm, two-spotted spider mites, or diamondback moth larvae. Although the first two herbivore species feed on lima bean plants, diamondback moth larvae do not. As a control, lima bean plants were exposed to volatiles from uninfested cabbage plants. Only when exposed to volatiles from cabbage plants infested by spider mites did lima bean plants significantly increase their EFN secretion compared with the control. Increased EFN secretion can function as an indirect defence by supplying the natural enemies of herbivores with an alternative food source. Of the three herbivore species, spider mites were the most likely to move from cabbage plants to lima bean plants and presumably posed the greatest threat. Although chemical analyses showed differences among treatments in volatiles produced by herbivore-infested cabbage plants, which compounds or blends triggered the increased secretion of EFN by lima bean plants remains unclear. Thus, our results show that plants may tune their defence levels according to herbivore risk level.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plants emit specific blends of volatiles when infested by herbivores (Turlings et al. 1995; Takabayashi and Dicke 1996; Dicke 1999; Heil 2008; Dicke et al. 2009; Dicke and Baldwin 2010). The volatiles are known to attract carnivores (Turlings et al. 1995; Takabayashi and Dicke 1996; Dicke 1999; Heil 2008), repel herbivores (Pallini et al. 1997; De Moraes et al. 2001; Kessler and Baldwin 2001; Heil 2008), and mediate communication between plants (Bruin et al. 1995; Baldwin et al. 2006; Heil 2008; Heil and Karban 2010). For example, uninfested plants increase their defence levels when exposed to volatiles from herbivore-infested conspecific plants (Dicke et al. 1990; Arimura et al. 2000; Dolch and Tscharntke 2000; Choh et al. 2004a, 2006; Heil and Kost 2006; Kost and Heil 2006; Frost et al. 2007). Furthermore, the volatiles can signal within an individual plant; plants increase resistance in undamaged parts when exposed to volatiles from damaged parts of themselves (Frost et al. 2007; Heil and Silva Bueno 2007; Karban and Shiojiri 2009).
Volatiles from herbivore-infested plants can indicate the presence of specific herbivores. Because plants emit different blends of volatiles when infested by different herbivore species (De Moraes et al. 1998; Turlings et al. 1998; Ozawa et al. 2000; Shiojiri et al. 2001; de Boer et al. 2004; Bruinsma et al. 2009), the volatiles may indicate which species of herbivore has attacked. Although natural enemies of herbivores are known to discriminate among volatile blends from plants infested by different herbivore species (Turlings et al. 1995; Takabayashi and Dicke 1996; Dicke 1999; Heil 2008; Dicke et al. 2009; Dicke and Baldwin 2010), whether plants discriminate similarly has not been tested. Recently, Kikuta et al. (2011) reported that seedlings of Chrysanthemum cinerariaefolium induced biosynthesis of the natural insecticide pyrethrin only when exposed to a specific concentration of volatiles from mechanically-damaged conspecifics. These results showed that plants sometimes but do not always increase their defence levels in response to volatiles from herbivore-infested plants.
Although plants that are exposed to volatiles from herbivore-infested neighbours would benefit by responding defensively (Dicke et al. 1990; Arimura et al. 2000; Dolch and Tscharntke 2000; Karban et al. 2000; Choh et al. 2004a, 2006; Heil and Kost 2006; Kost and Heil 2006; Frost et al. 2007), the same responses would be costly if herbivores were absent (Baldwin 1998). Furthermore, herbivores do not use all plant species as food sources. Hence, plants should prime their defences only when they are likely to be attacked. In this study, we tested whether plants have the ability to recognize herbivore species based on plant volatile profiles.
Lima bean plants and their herbivores are a well-studied system of plant communication (Dicke et al. 1990; Bruin et al. 1992; Arimura et al. 2000; Choh et al. 2004a, 2006; Choh and Takabayashi 2006; Heil and Kost 2006; Kost and Heil 2006; Heil and Silva Bueno 2007). When exposed to volatiles from conspecifics infested by herbivores, lima bean plants increased secretion of extrafloral nectar (EFN), which can function as an indirect defence against herbivores by encouraging the natural enemies of herbivores to remain on the plants by offering an alternative food source (Choh et al. 2006; Kost and Heil 2006). We examined EFN secretion in lima bean plants exposed to volatiles from cabbage plants infested by the herbivorous mite Tetranychus urticae, the common cutworm Spodoptera litura, and diamondback moth Plutella xylostella larvae. The system we studied here was not natural, but could occur in agricultural fields. Lima beans are host plants for S. litura larvae and T. urticae, but not for P. xylostella larvae, suggesting that lima bean plants need not defend against P. xylostella larvae. Although the host range of an herbivore could indicate herbivory risk, we actually tested the likelihood of herbivore migration between cabbage and lima bean plants as an indicator of herbivory risk for lima bean plants. If lima bean plants recognize herbivore species using volatiles from herbivore-infested cabbage plants, EFN secretion levels in the exposed plants would be expected to differ depending on the herbivore species attacking the cabbage plants. Furthermore, we investigated volatile emissions from herbivore-infested cabbage plants to identify key compounds that were involved in changes in EFN secretion in lima bean plants.
Materials and methods
Plants and herbivores
Lima bean plants (Phaseolus lunatus cv. Pole Sieva) were grown in soil in a greenhouse at 25 ± 2 °C and 60–70 % relative humidity (r.h.), under a 16:8 h light:dark (L:D) photoperiod. We used plants grown for 4–6 days after germination. Cabbage plants (Brassica oleracea cv. Shikidori) were grown in soil under the same conditions as lima bean plants. We used plants with three expanded leaves that had been grown from seeds for 3 weeks.
Diamondback moth (P. xylostella) larvae are specialist herbivores of crucifer plants. They were collected from crucifer crops in a field in Kyoto City (35°N, 136°E), Japan in 2006 and kept on crucifer plants (Brassica campestris cv. Rakuten) as food. Common cutworm (S. litura) was obtained from a culture maintained at the National Institute of Agrobiological Sciences in Tsukuba, Ibaraki, Japan in 2006. These insects were reared on an artificial diet (Insecta LF; Nihon Nousan Kogyo Ltd, Yokohama, Japan). Herbivorous mites (T. urticae) were obtained from the Laboratory of Ecological Information, Graduate School of Agriculture, Kyoto University, in 2002 and reared on lima bean plants. Although S. litura larvae and T. urticae feed on both cabbage and lima bean plants, P. xylostella larvae feed only on cabbage plants. None of these herbivore species uses extrafloral nectar as an alternative food source. We maintained cultures of all three herbivore species in climate-controlled rooms (25 ± 2 °C, 50–70 % r.h, 16:8 h L:D). All experiments were conducted in a climate-controlled room (25 ± 2 °C, 60–70 % r.h, 16:8; L:D).
Exposure of lima bean plants to volatiles
To expose lima bean plants to volatiles from herbivore-infested cabbage plants, we used four acrylic 60 × 60 × 60 cm cages, each with two 30 × 30 cm windows on opposite sides of the cage. The windows were covered with 225 μm mesh nylon gauze, and airflow within the cage was below detectable levels. The cage had a 30 × 30 cm sliding door at the front. We placed either three second-instar larvae of P. xylostella, three second-instar larvae of S. litura, or 60 adult female T. urticae on a cabbage plant. Because P. xylostella larvae stop feeding and pupate 6–7 days after introduction to host plants (Choh et al. 2008), we replaced the larvae with another three second instar larvae 5 days after the first introduction to subject cabbage plants to continuous damage by the larvae. The overlap of P. xylostella generations on an individual plant meant that young larvae continued feeding when older ones pupated. As odour sources, we used eight cabbage plants per treatment, with a treatment being infestation with one species of herbivore as described above. Eight uninfested cabbage plants were used as the control.
One uninfested lima bean plant was placed in a cage with the odour source plants (see Choh et al. 2006 for setup details) and exposed to volatiles from the cabbage plants for 10 days. All plants were placed in plastic containers (12 cm diameter, 9 cm height) filled with water to prevent the migration of herbivores from the cabbage plants to the lima bean plant. The lima bean plant was positioned 25 cm from the cabbage plants. We used newly-cleaned cages for each of 36 replicates per treatment. We visually inspected lima bean plants for signs of infestation and found that no herbivores had invaded them.
Measurement of EFN
After 10 days of exposure to volatiles, the volume of EFN secreted by lima bean plants was measured with 5 μl capillaries (Ringcaps®, Hirschmann Laborgeräte GmbH & Co. KG, Eberstadt, Germany). The length of nectar in the capillary was a direct and precise quantification of the nectar volume. The EFN collection was always carried out between 10 am and midday in case nectar secretion showed a diel pattern (Raine et al. 2002). Although several studies, particularly field experiments, measured EFN concentrations with a refractometer as an indicator of EFN secretion (Heil and Kost 2006; Kost and Heil 2006; Heil and Silva Bueno 2007), we previously confirmed that the EFN concentration in lima bean plants did not change, irrespective of herbivore damage or exposure to volatiles under climate-controlled conditions (Choh 2006). Therefore, we measured only the volume (not the concentration) of EFN secreted by a plant, as in previous studies (Wäckers and Wunderlin 1999; Wäckers et al. 2001; Choh and Takabayashi 2006; Choh et al. 2006). The EFN volume per plant was analysed using Tukey–Kramer test when ANOVA supported a significant difference.
Migration of herbivores from cabbage to lima bean
To test the risks posed by herbivores to neighbouring plants, we examined the migration of herbivores from cabbage to lima bean plants under laboratory conditions. We placed a cut stem of a cabbage plant and a primary leaf of a lima bean plant 8 cm apart in a plastic container (12 × 16 cm; 4 cm deep). Water-saturated cotton wool was attached to the stem and petiole, respectively. Three individuals of either of adult female T. urticae, second-instar larvae of P. xylostella, or first-instar larvae of S. litura were placed on the cabbage plant. Under these conditions, the herbivores could walk between plants. The location of the herbivore species was checked either 3 or 10 days after they had been placed on the cabbage. We used new set-ups for each time point (3 or 10 days) so as not to disturb the migration behaviour of the herbivores. We repeated the experiment 16 times per treatment, and compared the number of migrated individuals among herbivore species with a Mann–Whitney U test.
Performance of herbivores on plants
We examined the performance of herbivores on the two plant species as a potential factor affecting the likelihood of migration between lima bean and cabbage plants. To measure the performance of S. litura larvae on cabbage and lima bean, we placed a second-instar larva of S. litura on a leaf patch (4 × 4 cm) that was cut from a cabbage leaf or from a primary leaf of a lima bean plant. The leaf patches were placed on wet cotton wool in Petri dishes. We offered fresh leaf daily to avoid larval starvation and measured the duration of the larval stage for 30 days after introduction. We repeated the experiment 15 times. Data were compared using a log-rank test.
To measure the performance of T. urticae on cabbage and lima bean plants, we placed an individual adult female of T. urticae that had been randomly selected from the culture on a leaf patch (1 × 1 cm) cut from a cabbage leaf or a primary leaf of a lima bean plant. The leaf patches were kept on water-saturated cotton in Petri dishes. We repeated the above experiment 12 times per treatment. The numbers of eggs laid by the mites over 3 days were compared using a Mann–Whitney U test.
Because P. xylostella larvae feed only on crucifer plants, we did not test the performance of P. xylostella larvae on either plant.
Chemical analysis
To chemically analyse the volatiles, we prepared four groups of cabbage plants (i.e., uninfested plants, P. xylostella-infested plants, S. litura-infested plants, and T. urticae-infested plants) as described above. Headspace volatiles were collected 4–8 h after the lights were turned on and 1, 3, 5, 8, and 10 days after the introduction of herbivores. One plant of each treatment was placed in a 2-l glass bottle that had two nozzles. Cabbage cotyledons are easily broken, and the bottle was narrow, so there was a risk that they would be accidentally damaged when the plants were put into the bottle. Therefore, we removed the cotyledons before introducing the herbivores to ensure that all plants avoided accident. Although removing the cotyledons may have affected volatile emissions, all plants were treated identically, thereby controlling for the effect.
One nozzle was connected to an air cylinder and the other to a glass tube packed with Tenax TA adsorbent (100 mg, mesh 20/35; GL Science, Tokyo, Japan). Pure air from the cylinder was drawn into the glass bottle, and volatile compounds from the headspace of the bottle were collected with Tenax TA for 2 h at a flow rate of 100 ml/min. The collected volatiles were analysed by GC–MS (GC: Agilent 6890 with HP-5MS capillary column: 30 m long, 0.25 mm I.D. and 0.25 μm film thickness; MS: Agilent 5973 mass selective detector, 70 eV; Agilent, Santa Clara, CA, USA) equipped with a thermal desorption cold trap injector (TCT; CP4010, Chrompack, The Netherlands). Headspace volatiles collected on Tenax-TA were released in the TCT thermo-desorption unit at 220 °C for 8 min with helium as the carrier gas. The desorbed compounds were collected in the TCT cold trap unit (SIL5CB-coated fused silica capillary) at −130 °C. Flash heating of the cold trap unit provided sharp injection of the compounds into the capillary column of the gas chromatograph to which the cold trap unit was connected. The oven temperature of the GC was programmed to rise from 40 °C (5-min. hold) to 280 °C at 15 °C/min. The headspace volatiles were identified using the database (Wiley 7 N, Agilent) and by comparing their retention times with those of authentic compounds. Peak areas of authentic compounds were used for quantification. Because authentic α-thujene was not commercially available, this compound was quantified against a standard curve of sabinene, which has a similar structure. Therefore, its quantification should be considered tentative. The collections were replicated eight times. Volatile emissions were statistically compared among treatments at each time point using the Tukey–Kramer test when ANOVA supported significant differences.
Results
EFN secretion
The amount of EFN secreted by lima bean plants differed significantly among the four cabbage plant treatments (F 3,140 = 5.12, P = 0.0022, ANOVA). Although lima bean plants exposed to volatiles from cabbage plants infested by P. xylostella or S. litura larvae did not secrete more EFN compared with those exposed to control cabbage plants (Fig. 1, P > 0.05, Tukey–Kramer test), the plants secreted significantly more EFN when exposed to volatiles from cabbage plants infested by T. urticae (Fig. 1, P < 0.05, Tukey–Kramer test).
Quantities of extrafloral nectar (EFN) (mean + SE of 36 replicates per treatment) secreted by lima bean plants exposed to volatiles from uninfested cabbage plants (control) and cabbage plants infested with Plutella xylostella larvae, Spodoptera litura larvae, or female Tetranychus urticae adults. Different letters above the bars indicate significant differences among treatments by Tukey–Kramer’s test (P < 0.05)
Migration of herbivores from cabbage to lima bean plants
All P. xylostella larvae remained on the cabbage plant at three and 10 days after release (Fig. 2). Therefore, these data were excluded from the statistical analysis. There was a significant difference in the migration rate between adult female T. urticae and S. litura larvae at 3 days (Fig. 2, U = 8.5, P < 0.0001, Mann–Whitney U test) and 10 days (Fig. 2, U = 17.0, P < 0.0001, Mann–Whitney U test) after release. The migration rate of T. urticae was much higher than that of S. litura larvae (Fig. 2).
Proportions (mean + SE of 16 replicates) of three individuals of Plutella xylostella larvae, Spodoptera litura larvae, and adult female Tetranychus urticae that migrated from cabbage to lima bean plants. No P. xylostella larvae migrated to lima bean plants during the experiments. Asterisks indicate significant differences in the migration rates between T. urticae and S. litura by Mann–Whitney U test (P < 0.0001)
Performance of herbivores on plants
There was no difference in the duration of the S. litura larval stage when fed cabbage or lima bean leaves (cabbage: 21.1 ± 1.2, lima bean: 20.7 ± 0.7, χ2 = 1.18, P = 0.28, logrank test). The number of eggs laid by T. urticae on cabbage leaves was significantly lower than that on lima bean leaves (cabbage: 14.9 ± 1.9, lima bean: 28 ± 1.5, U = 6.5, P < 0.001, Mann–Whitney U test).
Volatiles from herbivore-infested cabbage plants
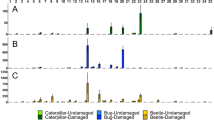
Although there were no significant differences among treatments in most compounds emitted by cabbage plants at day 1, T. urticae-infested cabbage plants emitted more (Z)-3-hexenyl acetate than did other treatments (Fig. 3a). Herbivore-infested cabbage plants had higher volatile emissions than uninfested cabbage plants from 3 to 10 days after the infestation (Fig. 3b–e). At day 3, herbivore-infested cabbage plants did not increase emission of (Z)-3-hexenyl acetate and (E,E)-α-farnesene relative to uninfested plants (Fig. 3b). Spodoptera litura- and P. xylostella-infested cabbage plants emitted significantly more of eight and three compounds, respectively, than uninfested cabbage plants (Fig. 3b). At day 5, S. litura- and T. urticae-infested cabbage plants each emitted significantly more of nine of the ten compounds than uninfested cabbage plants (Fig. 3c). Plutella xylostella-infested plants emitted more of two compounds than uninfested cabbage plants (Fig. 3c). At day 8, herbivore-infested cabbage plants emitted larger amounts of most of the ten compounds than uninfested plants, irrespective of herbivore species (Fig. 3d). At day 10, S. litura-infested cabbage plants emitted larger amounts of most compounds than uninfested plants; only sabinene levels were not increased in this (or any) treatment (Fig. 3e). (Z)-3-Hexenyl acetate, a green leaf volatile produced by plants in response to mechanical damage, is reported to induce an increase in EFN secretion in uninfested lima bean plants (Kost and Heil 2006; Heil et al. 2008). Compared with uninfested cabbage plants, T. urticae- and S. litura-infested cabbage plants emitted larger amounts of (Z)-3-hexenyl acetate on days 1, 5, and 8 and days 8 and 10 respectively (Fig. 3).
Gas chromatography-mass spectrometry analyses of headspace volatiles from uninfested cabbage plants and cabbage plants infested by Plutella xylostella larvae, Spodoptera litura larvae, and Tetranychus urticae for 1 (a), 3 (b), 5 (c), 8 (d), and 10 (e) days. The amounts of volatile compounds (mean + SE of eight replicates) are shown for volatile emission from plants. The compounds identified were: 1 α-thujene, 2 α-pinene, 3 sabinene, 4 β-myrcene, 5 (Z)-3-hexenyl acetate, 6 α-terpinene, 7 limonene, 8 1,8-cineole, 9 γ-terpinene, and 10 (E,E)-α-farnesene. Different letters above the bars indicate significant differences among treatments by Tukey–Kramer’s test (P < 0.05)
Discussion
Lima bean plants increased their secretion of EFN when exposed to volatiles from herbivore-infested conspecifics (Choh et al. 2006; Kost and Heil 2006). These findings suggested that plants recognize the presence of herbivores on their neighbours. In this study, there were no significant differences in EFN secretion by lima bean plants when exposed to volatiles from cabbage plants being attacked by any of three different herbivore species, suggesting that lima bean plants do not discriminate among herbivore species based on volatiles from infested neighbours. Interestingly, lima bean plants secreted more EFN when exposed to volatiles from cabbage plants infested by T. urticae compared with lima bean plants exposed to volatiles from uninfested cabbage plants. These results suggest that lima bean plants could distinguish between volatiles from uninfested and T. urticae-infested cabbage plants, but not among volatiles from uninfested cabbage plants and cabbage plants infested by two other herbivore species.
Volatiles from herbivore-infested plants are known to differ by herbivore species (Geervliet et al. 1997; De Moraes et al. 1998; Turlings et al. 1998; Ozawa et al. 2000; Shiojiri et al. 2001, 2010; de Boer et al. 2004; Fatouros et al. 2005; Bruinsma et al. 2009). In fact, our cabbage plants emitted different blends of volatiles when infested by different herbivore species. Whether damage by T. urticae is equivalent to damage by the other two species is unclear, but differences in feeding mode and in elicitors in the oral secretions of the three herbivore species may induce different blends of volatiles in cabbage plants, independent of the amount of damage.
Herbivore-infested cabbage plants increased their emission of (Z)-3-hexenyl acetate, which induced an increase in EFN production in lima bean plants exposed to the compound (Kost and Heil 2006; Heil et al. 2008). Although lima bean plants exposed to volatiles from T. urticae-infested cabbage plants secreted larger amounts of EFN than lima bean plants exposed to volatiles from uninfested cabbage plants, T. urticae-infested cabbage plants did not emit larger amounts of (Z)-3-hexenyl acetate than uninfested cabbage plants throughout the period of exposure. Ethylene, which was not detectable by the analytical method used in this study, may be involved in odour-mediated plant communication (Arimura et al. 2000, 2002; Tscharntke et al. 2001) and may have affected our results. Further studies are needed to clarify which volatile compounds from herbivore-infested cabbage plants were involved in the increased EFN secretions of lima bean plants.
Spodoptera litura larvae and T. urticae both have broad host ranges and could threaten lima bean plants neighbouring their host plants. However, our migration experiments showed that T. urticae were more likely to attack neighbouring lima bean plants than were S. litura larvae. These results may be partly explained by the unsuitability of cabbage plants as hosts for T. urticae. Because predatory mites that prey on T. urticae use EFN as an alternative food source (van Rijn and Tanigoshi 1999; Choh et al. 2006), increased EFN secretion in lima bean plants could function as an indirect defence by increasing the residence time of predatory mites (Choh et al. 2006).
When exposed to volatiles from T. urticae-infested leaves, lima bean leaves increased their production of endogenous jasmonic acid, a phytohormone involved in plant defence responses against herbivores (Arimura et al. 2002). In fact, jasmonic acid is involved in induced resistance against T. urticae (Li et al. 2002; Choh et al. 2004b), in the emission of carnivore attractants (Dicke et al. 1999; Ozawa et al. 2000; Gols et al. 2003), and in the secretion of EFN (Heil 2004) in lima bean plants. Jasmonic acid-induced defence responses are reported to benefit plants under herbivore attack, but to be costly in the absence of herbivory (Baldwin 1998). If plants responded to all volatiles from herbivore-infested neighbours, their responses would be wasteful in cases when herbivores did not attack. To best allocate their resources, plants may have evolved the ability to tune their defences against the herbivores that are most likely to attack them. This study offered the new perspective that a plant may evaluate the threat of herbivores on neighbouring plants via airborne cues. To clarify the ecological importance of plants’ ability to discriminate herbivores on nearby plants, studies in natural systems are essential.
References
Arimura G, Ozawa R, Shimoda T, Nishioka T, Boland W, Takabayashi J (2000) Herbivory-induced volatiles elicit defence genes in lima bean leaves. Nature 406:512–515
Arimura G, Ozawa R, Nishioka T, Boland W, Koch T, Kühnemann F, Takabayashi J (2002) Herbivore-induced volatiles induce the emission of ethylene in neighboring lima bean plants. Plant J 29:87–98
Baldwin IT (1998) Jasmonate-induced responses are costly but benefit plants under attack in native populations. Proc Nat Acad Sci USA 95:8113–8118
Baldwin IT, Halitschke R, Paschold A, von Dahl CC, Preston CA (2006) Volatile signalling in plant-plant interactions: “talking trees” in the genomics era. Science 311:812–815
Bruin J, Dicke M, Sabelis MW (1992) Plants are better protected against spider-mites after exposure to volatiles from infested conspecifics. Experientia 48:525–529
Bruin J, Sabelis MW, Dicke M (1995) Do plants tap SOS signals from their infested neighbours? Trends Ecol Evol 10:167–170
Bruinsma M, Posthumus MA, Mumm R, Mueller MJ, van Loon JJA, Dicke M (2009) Jasmonic acid-induced volatiles of Brassica oleracea attract parasitoids: effects of time and dose, and comparison with induction by herbivores. J Exp Bot 60:2575–2587
Choh Y (2006) Plant-plant interactions mediated by herbivore-induced plant volatiles. Kyoto University, PhD dissertation
Choh Y, Takabayashi J (2006) Herbivore-induced extrafloral nectar production in lima bean plants enhanced by previous exposure to volatiles from infested conspecifics. J Chem Ecol 32:2073–2077
Choh Y, Shimoda T, Ozawa R, Dicke M, Takabayashi J (2004a) Exposure of lima bean leaves to volatiles from herbivore-induced conspecific plants results in emission of carnivore attractants: active or passive process? J Chem Ecol 30:1305–1317
Choh Y, Ozawa R, Takabayashi J (2004b) Effects of exogenous jasmonic acid and benzo (1,2,3) thiadazole-7-carbothionic acid S-methyl ester (BTH), a functional analogue of salicylic acid, on the eggs production of a herbivorous mite Tetranychus urticae (Acari: Tetranychidae). Appl Entomol Zool 39:311–314
Choh Y, Kugimiya S, Takabayashi J (2006) Induced production of extrafloral nectar in intact lima bean plants in response to volatiles from spider mite-infested conspecific plants as a possible indirect defense against spider mites. Oecologia 147:455–460
Choh Y, Uefune M, Takabayashi J (2008) Diamondback moth females oviposit more on plants infested by non-parasitized than by parasitized conspecifics. Ecol Entomol 33:565–568
de Boer JG, Posthumus MA, Dicke M (2004) Identification of volatiles that are used in discrimination between plants infested with prey or nonprey herbivores by a predatory mite. J Chem Ecol 30:2215–2230
De Moraes CM, Lewis WJ, Paré PW, Alborn HT, Tumlinson JH (1998) Herbivore-infested plants selectively attract parasitoids. Nature 393:570–573
De Moraes CM, Mescher C, Tumlinson JH (2001) Caterpillar-induced nocturnal plant volatiles repel conspecific females. Nature 410:577–580
Dicke M (1999) Specificity of herbivore-induced plant volatiles. In: Insect-plant interactions and induced plant defence (Novartis Foundation). Wiley, Chichester, pp 43–54
Dicke M, Baldwin IT (2010) Evolutionary context for herbivore-induced plant volatiles: beyond the ‘cry for help’. Trends Plant Sci 15:167–175
Dicke M, Sabelis MW, Takabayashi J, Bruin J, Posthumus MA (1990) Plant strategies of manipulating predator-prey interactions through allelochemicals: prospects for application in pest control. J Chem Ecol 16:3091–3118
Dicke M, Gols R, Ludeking D, Posthumus MA (1999) Jasmonic and herbivory differentially induce carnivore-attractanting plant volatiles in lima bean plants. J Chem Ecol 25:1907–1922
Dicke M, van Loon JJA, Soler R (2009) Chemical complexity of volatiles from plants induced by multiple attacks. Nat Chem Biol 5:317–324
Dolch R, Tscharntke T (2000) Defoliation of alders (Alnus glutinosa) affects herbivory by leaf beetles on undamaged neighbours. Oecologia 125:504–511
Fatouros NE, van Loon JJA, Hordijk KA, Smid HM, Dicke M (2005) Herbivore-induced plant volatiles mediate in-flight host discrimination by parasitoids. J Chem Ecol 31:2033–2047
Frost CJ, Appel HM, Carlson JE, de Moraes CM, Mescher MC, Schultz JC (2007) Within-plant signalling via volatiles overcomes vascular constraints on systemic signalling and primes responses against herbivores. Ecol Lett 10:490–498
Geervliet JBF, Posthumus MA, Vet LEM, Dicke M (1997) Comparative analysis of headspace volatiles from different caterpillar-infested or uninfested food plants of Pieris species. J Chem Ecol 23:2935–2954
Gols R, Roosjen M, Dijkman H, Dicke M (2003) Induction of direct and indirect plant responses by jasmonic acid, low spider mite densities, or a combination of jasmonic acid treatment and spider mite infestation. J Chem Ecol 29:2651–2666
Heil M (2004) Induction of two indirect defences benefits Lima bean (Phaseolus lunatus, Fabaceae) in nature. J Ecol 92:527–536
Heil M (2008) Indirect defence via tritrophic interactions. New Phytol 178:41–61
Heil M, Karban R (2010) Explaining evolution of plant communication by airborne signals. Trends Ecol Evol 25:137–144
Heil M, Kost C (2006) Priming of indirect defences. Ecol Lett 9:813–817
Heil M, Silva Bueno JC (2007) Within-plant signalling by volatiles leads to induction and priming of an indirect plant defense in nature. Proc Nat Acad Sci USA 104:5467–5472
Heil M, Lion U, Boland W (2008) Defense-inducing volatiles: in search of active motif. J Chem Ecol 34:601–604
Karban R, Shiojiri K (2009) Self recognition affects plant communication and defense. Ecol Lett 12:502–506
Karban R, Baldwin IT, Baxter IJ, Laue G, Felton GW (2000) Communication between plants: induced resistance in wild tobacco plants following clipping of neighboring sagebrush. Oecologia 125:66–71
Kessler A, Baldwin IT (2001) Defensive function of herbivore-induced plant volatile emission innature. Science 291:2141–2144
Kikuta Y, Ueda H, Nakayama K, Katsuda Y, Ozawa R, Takabayashi J, Matsuda K (2011) Specific regulation of pyrethrin biosynthesis in Chrysanthemum cinerariaefolium by a blend of volatiles emitted from artificially damaged conspecific plants. Plant Cell Physiol 52:588–596
Kost C, Heil M (2006) Herbivore-induced plant volatiles induce an indirect defence in neighbouring plants. J Ecol 94:619–628
Li C, Williams MM, Loh YT, Lee GI, Howe GA (2002) Resistance of cultivated tomato to cell content-feeding herbivores is regulated by the octadecanoid-signaling pathway. Plant Physiol 130:494–503
Ozawa R, Arimura G, Takabayashi J, Shimoda T, Nishioka T (2000) Involvement of jasmonate- and salicylate-related signaling pathways for the production of specific herbivore-induced volatiles in plants. Plant Cell Physiol 41:391–398
Pallini A, Janssen A, Sabelis MW (1997) Odour-mediated responses of phytophagous mites to conspecific and heterospecific competitors. Oecologia 110:179–185
Raine NE, Willmer P, Stone GN (2002) Spatial structuring and floral avoidance behavior prevent ant-pollinator conflict in Mexican ant-acacia. Ecology 83:3086–3096
Shiojiri K, Takabayashi J, Yano S, Takafuji A (2001) Infochemically mediated tritrophic interaction webs on cabbage plants. Popul Ecol 43:23–29
Shiojiri K, Ozawa R, Kugimiya S, Uefune M, van Wijk M, Sabelis MW, Takabayashi J (2010) Herbivore-specific, density-dependent induction of plant volatiles: honest or “cry wolf” signals? PLoS ONE 5:1–11
Takabayashi J, Dicke M (1996) Plant-carnivore mutualism through herbivore-induced carnivore attractants. Trends Plant Sci 1:109–113
Tscharntke T, Thiessen S, Dolch R, Boland W (2001) Herbivory, induced resistance, and interplant signals transfer in Alnus glutinosa. Bioche Syst Ecol 29:1024–1047
Turlings TCJ, Loughrin JH, McCall PJ, Röse USR, Lewis WJ, Tumlinson JH (1995) How caterpillar-damaged plants protect themselves by attracting parasitic wasps. Proc Nat Acad Sci USA 92:4169–4174
Turlings TCJ, Bernasconi M, Bertossa R, Bigler F, Caloz G, Dorn S (1998) The induction of volatile emissions in maize by three herbivore species with different feeding habitats: possible consequences for their natural enemies. Biol Cont 11:122–129
Van Rijn PCJ, Tanigoshi LK (1999) The contribution of extrafloral nectar to survival and reproduction of the predatory mite Iphiseius degenerans and Ricinus communis. Exp Appl Acarol 23:281–296
Wäckers FL, Wunderlin R (1999) Induction of cotton extrafloral nectar production in response to herbivory does not require a herbivore-specific elicitor. Entomol Exp Appl 91:149–154
Wäckers FL, Zuber D, Wunderlin R, Keller F (2001) The effect of herbivory on temporal and spatial dynamics of foliar nectar production in cotton and castor. Ann Bot 87:365–370
Acknowledgments
This research was financially supported by the Global Center of Excellence Program “Formation of a Strategic Base for Biodiversity and Evolutionary Research: from Genome to Ecosystem” of the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan; a Grant-in-Aid for Scientific Research (S) from MEXT, Japan (No. 19101009), and a Core-to-Core project from the Japan Science and Technology Agency.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Choh, Y., Ozawa, R. & Takabayashi, J. Do plants use airborne cues to recognize herbivores on their neighbours?. Exp Appl Acarol 59, 263–273 (2013). https://doi.org/10.1007/s10493-012-9616-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-012-9616-z