Abstract
In response to herbivory by spider mites (Tetranychus urticae), lima bean plants produced significantly greater quantities of extrafloral nectar (EFN) than intact conspecific plants. Moreover, EFN amounts of infested plants depended on exposure to odor of infested neighbor plants. Two d after spider mite infestation, a test plant produced more EFN when exposed prior to infestation to volatiles from infested neighbor plants than when exposed to volatiles from uninfested conspecific plants. However, this effect was only detectable 2 d after spider mite infestation and vanished 4 d after infestation. These results suggest that EFN production is enhanced during the earlier stages of damage by T. urticae in response to previous exposure to volatiles from infested neighbor plants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Volatiles emitted from infested plants (hereafter called “VIP”) have several ecological functions. One of the well-studied ecological functions of VIP is to mediate interactions between plants and carnivorous natural enemies of the herbivores (for review, see Dicke and Vet, 1999). Furthermore, VIP have been found to mediate interactions between infested plants and neighboring intact plants. When intact plants were exposed to volatiles emitted by neighboring infested plants, the level of defense in the intact plants against herbivores increased (Dicke et al., 1990; Arimura et al., 2000; Bruin and Sabelis, 2001; Choh et al., 2004). We have been studying such plant–plant interactions in a system consisting of lima bean plants (Phaseolus lunatus), herbivorous mites (Tetranychus urticae), and predatory mites (Phytoseiulus persimilis).
Intact lima bean plants attract the predatory mite, P. persimilis when they have been exposed to volatiles emitted from conspecific plants infested by its prey, T. urticae (Dicke et al., 1990). Furthermore, in this tritrophic system, the exposed intact lima bean plants increase: (1) their direct defenses against spider mites (Arimura et al., 2000), and (2) the production of herbivore-induced volatiles that attract P. persimilis (indirect defense) (Choh et al., 2004). These enhanced defenses are called “priming,” which has intensively been studied in plant–pathogen interactions (Conrath et al., 2002). In addition, we recently reported that intact lima bean plants increased the secretion of extrafloral nectar (EFN) when they were exposed to VIP, and that this consequently increased the residence time of P. persimilis on the exposed plants (Choh et al., 2006; Kost and Heil, 2006). We also found that unexposed lima bean plants increase EFN production when they are infested by the herbivorous mite, T. urticae (herbivore-induced EFN; Choh et al., 2006). We hypothesize that such an herbivore-induced EFN production was also primed by previous exposure to VIP. We tested the hypothesis by changing the duration of the infestation.
Methods and Materials
Plants and Mites
Lima bean plants were grown in soil in a greenhouse at 25 ± 2°C, 60–70% r.h. under a photoperiod of 16:8 hr (L:D). We used plants grown for 4–6 d after germination.
T. urticae were obtained from the Laboratory of Ecological Information, Graduate School of Agriculture, Kyoto University, and were reared on lima bean plants in a climate-controlled room (25 ± 2°C, 60–70% r.h., 16:8 hr L:D).
Exposure of Plants to Volatiles
For exposure of plants to VIP, we used six acrylic 60 × 60 × 60 cm cages with two 30 × 30 cm windows on opposite sides of the cage. Three cages were used for the experiment, and another three for control. The windows were covered with 225 μm nylon gauze, and airflow within the cage was below detection level. The cage had a 30 × 30 cm sliding door at the front. As odor sources, we used 8 plants that had been infested with 60 adult T. urticae females per plant for 1 d. The number of the odor source plants was determined on the basis of our preliminary experiments to ensure plant exposure to sufficient amounts of volatiles. Eight uninfested plants were used as control odor sources. Two uninfested plants were placed into a cage with the odor source plants (see Choh et al., 2006 for details of the setup) and exposed to either VIP or uninfested plant volatiles (UPV) for 10 d in a climate-controlled room (25 ± 2°C, 60–70% r.h., 16:8 hr L:D). All plants were placed in plastic containers (12 cm diam, 9 cm high) filled with water to prevent the migration of T. urticae from infested plants to uninfested plants. The distance between the exposed plants and the odor source plants was 25 cm. Both infested and uninfested plants had 2–3 unfolded trifoliate leaves 10 d after exposure. We used newly cleaned cages for each experimental replicate. Under these experimental conditions, we visually inspected the exposed plants for signs of infestation and found that no spider mites invaded the exposed plants.
Measurement of EFN
After exposure of plants to VIP and UPV for 10 d, most of the EFN that had already been secreted was removed from VIP- and UPV-exposed plants with 5 μl capillaries (Ringcaps®, Hirschmann Laborgeräte GmbH, Germany). Measuring the length of nectar in the capillary enables the direct and precise quantification of the collected nectar volume. One of two exposed plants in a cage was used for the T. urticae infestation experiment, and the other was used as an intact (uninfested) control. We placed 30 T. urticae on the two primary leaves (total 60 T. urticae per plant) of the VIP- or UPV-exposed plants. As a control, no T. urticae were placed on VIP- and UPV-exposed plants. Thus, EFN quantities were measured in four sets of plants: (1) plants exposed to VIP and infested later, (2) plants exposed to VIP and kept uninfested later, (3) plants exposed to UPV and infested later, (4) plants exposed to UPV and kept uninfested later.
The amounts of newly secreted EFN were measured 2 d after infestation with T. urticae (herbivore-induced EFN). After removal of most of the induced EFN, we kept the plants for another 2 d under the same conditions, and then the secreted EFN was measured on the fourth day of infestation. Similarly, EFN secretion on intact plants (constitutively produced EFN) was measured 2 and 4 d after exposure to plant volatiles. We used 20 plants per treatment. The collection was always carried out between 10 and 12 a.m. in case the secretion of nectar showed a diel pattern (Raine et al., 2002). The experiments were conducted in a climate-controlled room (25 ± 2°C, 60–70% r.h., 16:8 hr L:D.). The amounts of secreted EFN per plant were analyzed by using a t-test and a Tukey-Kramer's test.
Results and Discussion
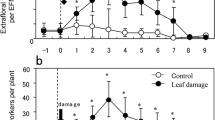
EFN secretion of intact lima bean plants was increased when measured directly after a 10-d exposure to volatiles from infested plants (intact UPV-exposed plants: 0.928 ± 0.053 μl; intact VIP-exposed plants: 1.38 ± 0.113 μl; P < 0.001, t-test). These findings corroborated those of our previous study (Choh et al., 2006). When measuring EFN quantities 2 and 4 d after cessation of a 10 d exposure to plant volatiles, no differences were observed between intact VIP- and UPV-exposed plants (P > 0.05, Tukey-Kramer's test; Fig. 1a and b). Results indicate that increased EFN secretion of intact plants in response to exposure to VIP (Choh et al., 2006) did not continue once exposure was terminated. Compared with intact UPV-exposed plants, UPV-exposed plants infested by T. urticae did not significantly increase EFN secretion 2 d after infestation (P > 0.05, Tukey-Kramer's test; Fig. 1a). However, UPV-exposed plants infested with T. urticae secreted significantly more EFN during days 3 and 4 of infestation when compared with intact UPV-exposed plants (P < 0.05, Tukey-Kramer's test; Fig. 1b).
Quantities of extrafloral nectar (EFN) (mean ± SE) secreted by lima bean plants obtained from following treatments: intact UPV-exposed plants, intact VIP-exposed plants, infested UPV-exposed plants, and infested VIP-exposed plants. UPV = uninfested plant volatiles; VIP = volatiles from infested plants. The duration of plant volatile exposure was 10 d. Immediately after exposure, test plants were infested with T. urticae, and controls were kept uninfested (intact). Quantities of EFN were measured (a) 2 d and (b) 4 d after cessation of plant volatile exposure. Different letters above bars indicate significant differences among treatments within each time period by Tukey-Kramer's test (P < 0.05).
VIP-exposed plants infested for 2 d by T. urticae secreted significantly more EFN than the other three treatments (P < 0.05, Tukey-Kramer's test; Fig. 1a). Although VIP-exposed plants infested by T. urticae for the following 2 d (third and fourth days of damage) secreted more EFN than VIP- and UPV-exposed uninfested plants, these differences were not statistically significant (P > 0.05, Tukey-Kramer's test; Fig. 1b). Similarly, EFN secretion did not differ significantly between VIP- and UPV-exposed plants that were infested by T. urticae for the following 2 d (i.e., 4 d after damage) (P > 0.05, Tukey-Kramer's test; Fig. 1b).
The enhanced EFN secretion observed in the first 2 d may be adaptive to the plants, as previous studies have already demonstrated that increased EFN secretion by lima bean plants can be used for indirect defense (Kost and Heil, 2005; Choh et al., 2006). Together with our previous study (Choh et al., 2004), we showed priming effects of plant–plant interaction in lima bean plants: VIP exposure not only resulted in increased VIP emission (Choh et al., 2004), but also in increased herbivore-induced EFN secretion (P < 0.05, Tukey-Kramer's test; Fig. 1a). This protects against attack by herbivores, thus benefiting lima bean plants in nature (Heil, 2004).
References
Arimura, G., Ozawa, R., Shimoda, T., Nishioka, T., Boland, W., and Takabayashi, J. 2000. Herbivory-induced volatiles elicit defence genes in lima bean leaves. Nature 406:512–515.
Bruin, J. and Sabelis, M. W. 2001. Meta-analysis of laboratory experiments on plant–plant information transfer. Biochem. Syst. Ecol. 29:1089–1102.
Choh, Y., Shimoda, T., Ozawa, R., Dicke, M., and Takabayashi, J. 2004. Exposure of lima bean leaves to volatiles from herbivore-induced conspecific plants results in emission of carnivore attractants: Active or passive process? J. Chem. Ecol. 30:1305–1317.
Choh, Y., Kugimiya, S., and Takabayashi, J. 2006. Induced production of extrafloral nectar in intact lima bean plants in response to volatiles from spider mite-infested conspecific plants as a possible indirect defense against spider mites. Oecologia 147:455–460.
Conrath, U., Pieterse, C. M. J., and Mauch-Mani, B. 2002. Priming in plant–pathogen interactions. Trends Plant Sci. 7:210–216.
Dicke, M. and Vet, L. E. M. 1999. Plant–carnivore interactions: evolutionary and ecological consequences for plant, herbivore and carnivore, pp. 483–520, in H. Olff, V. K. Brown, and R. H. Drent (eds.). Herbivores: Between Plants and Predators. Blackwell Science, Oxford.
Dicke, M., Sabelis, M. W., Takabayashi, J., Bruin, J., and Posthumus, M. A. 1990. Plant strategies of manipulating predator-prey interactions through allelochemicals: prospects for application in pest control. J. Chem. Ecol. 16:3091–3118.
Heil, M. 2004. Induction of two indirect defences benefits lima bean (Phaseolus lunatus, Fabaceae) in nature. J. Ecol. 92:527–536.
Kost, C. and Heil, M. 2005. Increased availability of extrafloral nectar reduces herbivory in Lima bean plants (Phaseolus lunatus, Fabaceae). Basic Appl. Ecol. 6:237–248.
Kost, C. and Heil, M. 2006. Herbivore-induced plant volatiles induce an indirect defence in neighbouring plants. J. Ecol. 94:619–628.
Raine, N. E., Willmer, P., and Stone, G. N. 2002. Spatial structuring and floral avoidance behavior prevent ant-pollinator conflict in Mexican ant–acacia. Ecology 83:3086–3096.
Acknowledgment
This study was supported by CREST of Japan Science and Technology (JST) Corporation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Choh, Y., Takabayashi, J. Herbivore-Induced Extrafloral Nectar Production in Lima Bean Plants Enhanced by Previous Exposure to Volatiles from Infested Conspecifics. J Chem Ecol 32, 2073–2077 (2006). https://doi.org/10.1007/s10886-006-9130-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-006-9130-z