Abstract
Herbivore feeding induces plants to emit volatiles that are detectable and reliable cues for foraging parasitoids, which allows them to perform oriented host searching. We investigated whether these plant volatiles play a role in avoiding parasitoid competition by discriminating parasitized from unparasitized hosts in flight. In a wind tunnel set-up, we used mechanically damaged plants treated with regurgitant containing elicitors to simulate and standardize herbivore feeding. The solitary parasitoid Cotesia rubecula discriminated among volatile blends from Brussels sprouts plants treated with regurgitant of unparasitized Pieris rapae or P. brassicae caterpillars over blends emitted by plants treated with regurgitant of parasitized caterpillars. The gregarious Cotesia glomerata discriminated between volatiles induced by regurgitant from parasitized and unparasitized caterpillars of its major host species, P. brassicae. Gas chromatography-mass spectrometry analysis of headspace odors revealed that cabbage plants treated with regurgitant of parasitized P. brassicae caterpillars emitted lower amounts of volatiles than plants treated with unparasitized caterpillars. We demonstrate (1) that parasitoids can detect, in flight, whether their hosts contain competitors, and (2) that plants reduce the production of specific herbivore-induced volatiles after a successful recruitment of their bodyguards. As the induced volatiles bear biosynthetic and ecological costs to plants, downregulation of their production has adaptive value. These findings add a new level of intricacy to plant–parasitoid interactions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Female parasitoids of herbivorous insects have to search for hosts to lay their eggs in or on them. Host searching is guided by volatile synomones that are mostly produced by the host plants after induction caused by herbivore feeding (Dicke, 1999; Shiojiri et al., 2001; Hilker and Meiners, 2002; Turlings et al., 2002; Steidle and van Loon, 2003). The ability to distinguish unparasitized from parasitized hosts and to reject the latter for oviposition (i.e., host discrimination) is widespread among hymenopteran parasitoids (van Lenteren, 1981; Vinson, 1985; van Alphen and Visser, 1990). The reproductive success of endoparasitoids is dependent on the quality and size of the hosts, because hosts provide a limited nutritional resource for their offspring (Godfray, 1994). Oviposition on or into already parasitized hosts by a conspecific parasitoid (i.e., superparasitism) can lead to competition with deleterious consequences such as mortality or reduced fitness. Therefore, avoiding superparasitism can have several advantages, including the optimization of foraging efficiency (van Lenteren, 1981; Godfray, 1994).
So far, host discrimination by parasitoids is known to be mediated by marking pheromones deposited by the ovipositing female either on or in the host, or within the explored patch. In addition, physiological changes in the host hemolymph induced by the parasitoid progeny (van Lenteren, 1981; Nufio and Papaj, 2001) or physical changes of the host surface acting as external markers (Takasu and Hirose, 1988) may provide cues for host discrimination. Marking pheromones are usually perceived through contact chemoreception (van Lenteren, 1981), although some examples of olfactory perception are known as well (Sheehan et al., 1993; van Giessen et al., 1993; van Baaren and Nenon, 1996).
Herbivore-induced volatiles are emitted in considerable quantities by plants, and the blend induced by herbivory differs in a qualitative and/or quantitative sense from the volatile blends of undamaged or mechanically damaged plants (Turlings et al., 1995; Dicke and Vet, 1999; Shiojiri et al., 2000). The emitted blend can be specific for the plant species, the herbivore species that damages the plant, and even for a specific stage of the herbivore (Turlings et al., 1995; Takabayashi and Dicke, 1996; Dicke and Vet, 1999). Based on the high detectability and reliability of herbivore-induced plant volatiles, parasitoids are able to use these cues to locate their hosts from a distance (Vet and Dicke, 1992). However, it is still unknown whether foraging females are able to distinguish unparasitized from parasitized hosts at a distance via olfactory cues produced by their host plant. Yet, this ability would save them foraging time and would reduce risks associated with host defense. We investigated this for a tritrophic system consisting of Brassica plants, Pieris caterpillars, and Cotesia parasitoids.
Brussels sprouts plants are known to emit a blend of volatiles when infested by Pieris brassicae L. (Lepidoptera: Pieridae) or P. rapae L. caterpillars. These induced volatiles attract Cotesia rubecula Marshall (Hymenoptera: Braconidae), a solitary endoparasitoid of the small cabbage white P. rapae and Cotesia glomerata L., a gregarious parasitoid of both Pieris species (Agelopoulos and Keller, 1994; Geervliet et al., 1994, 1998; Mattiacci et al., 1995, 2001). Differences between headspace volatiles collected from undamaged and Pieris-infested Brussels sprouts plants are extensively documented (Blaakmeer et al., 1994; Mattiacci et al., 1995, 2001; Smid et al., 2002). Coupled gas chromatography-electroantennography (GC-EAG) analysis has revealed that 20 of the headspace volatiles elicit a response in the antennae of C. rubecula and C. glomerata (Smid et al., 2002). The application of regurgitant of P. brassicae larvae or the enzyme β-glucosidase into a mechanical wound elicits induction of similar volatile blends in cabbage plants as in herbivory, showing that the plants recognize infestation through an elicitor in the oral secretion of Pieris caterpillars (Mattiacci et al., 1995).
In the present study, we investigate (1) whether females of the two Cotesia species prefer, in flight, induced odors emitted by plants fed upon by unparasitized caterpillars or treated with their regurgitant over odors emitted by plants fed upon by parasitized caterpillars or treated with their regurgitant, and (2) whether the induced plant odor blends differ, thereby providing a chemical basis for this discrimination behavior in the two wasp species.
Methods and Materials
Plants and Insects
Brussels sprouts plants (Brassica oleracea var. gemmifera cv. Cyrus) were reared in a greenhouse (18 ± 2°C, 70% RH, 16L:8D). Plants (8 to 12 wk old) having ca. 14–16 leaves were used for the experiments.
P. rapae and P. brassicae were reared on Brussels sprouts plants in a climate room (21 ± 1°C, 50–70% RH, 16L:8D). The parasitoid wasps C. rubecula and C. glomerata were reared on P. rapae and P. brassicae larvae, respectively, feeding on Brussels sprouts plants under greenhouse conditions (see above).
For bioassay experiments, C. rubecula and C. glomerata pupae were collected and reared in gauze cages in a climate room (23 ± 1°C, 50–70% RH, 16L: 8D). Once eclosed, the wasps were provided with water and honey. They had no contact with plant material or caterpillars before the initiation of the bioassays. They are referred to as naive wasps.
Regurgitant Collection
Regurgitant was collected from unparasitized and parasitized fifth instar larvae of P. brassicae, and from unparasitized and parasitized fourth instar P. rapae larvae 24 hr before the bioassay or headspace collection. Regurgitant droplets were collected with a 5-μl glass capillary tube after gently squeezing the caterpillars with forceps. About 5–10 caterpillars of each group (parasitized and unparasitized larvae) were used to collect 50 μl regurgitant, which was immediately put in separate vials on ice. Larvae that had been exposed to oviposition by female wasps were dissected before the regurgitant was pooled to ensure that they were indeed parasitized.
Plant Treatments
For the bioassay with P. rapae caterpillars, 30 first instars were offered to females of C. rubecula. Each caterpillar was observed to be stung by a wasp, and subsequently was reared on cabbage. Another 30 unparasitized L1 caterpillars were reared on cabbage under identical circumstances. After 4 d, 15 unparasitized larvae were placed on a Brussels sprouts plant. Another 15 larvae that were supposed to be parasitized were placed on a different plant. After 48 hr, the plants were used in the bioassay to investigate parasitoid odor preference. Parasitized caterpillars were reared until the last instar to examine whether they had indeed been parasitized and to assess the degree of parasitization.
In bioassays during which caterpillar regurgitant was used, each plant was damaged on the fifth fully expanded leaf, and for headspace collection on the fifth and sixth leaves from the top by using a pattern wheel. The wheel was rolled over the leaf surface on each side of the midrib, 10 lines in parallel, and 10 lines perpendicularly to the midrib creating a ca. 8 cm2 field with punctures being distributed over about 1/3 of the leaf surface. Per artificially damaged leaf, 25 μl of regurgitant, freshly collected from either parasitized or unparasitized caterpillars, were applied with a micropipette and distributed with a brush. Afterwards, the plants were kept in a climate room (23 ± 1°C, 50–70% RH) for 18 to 24 hr until the bioassay or headspace analysis. After the induction period, only the treated leaves were used for both behavioral bioassays and headspace analysis.
Bioassays
Parasitoid odor preference experiments were conducted in a wind tunnel set-up (25 ± 5°C, 50–70% r.h., 0.7 klux) described by Geervliet et al. (1994), with a wind speed of 0.2 m/s. For the bioassays, only the treated leaves that were of the same physiological age and size were used and excised from the induced plants just prior to the experiment. In this way, a highly standardized set of odor sources was offered to the parasitoids in a choice situation (Mattiacci et al., 1994, 1995). The petioles were inserted in vials with water, and the two leaves acting as odor sources were placed at the upwind end of the wind tunnel, ca. 14 cm apart. Experiments were carried out on 9–12 d, spread out over 2–3 mo for each treatment. For each of these days, new plants and new wasps were used. Three different plant treatments were tested in a two-choice flight experiment in the wind tunnel: (1) cabbage leaves infested with either parasitized or unparasitized second instars of P. rapae, (2) artificially damaged cabbage leaves treated with regurgitant collected from either unparasitized (UNPAR) or parasitized (PAR) P. rapae, and (3) artificially damaged cabbage leaves treated with regurgitant from either unparasitized or parasitized P. brassicae caterpillars. Two to 6-d-old, naive female wasps of each species were used. Each wasp was individually released on a microscope slide with a small part of a leaf from a caterpillar-damaged cabbage plant from which first instars had been removed just prior to the bioassay. For each Cotesia species, damage done by the Pieris species under test was used. This priming served to increase the responsiveness of the wasps during the bioassay. The microscope slide was placed in the middle of the release cylinder, which was 60 cm downwind from the odor sources.
After release, the behavior of the wasp was observed. Only flights that resulted in the first landing on one of the odor sources were recorded as response. All landings on other parts of the wind tunnel besides the release cylinder or odor source were recorded as no response. If the wasp remained longer than 10 min in or on the release cylinder, it was recorded as no response and discarded. During the bioassay, plant positions were changed from left to right and vice versa after every second wasp. Females of the two wasp species were alternately tested for every treatment with a maximum number of 15 females per species and day. The two species were tested on the same day to expose them to similar conditions. On each observation day, one leaf from one plant of each treatment was tested.
Collection of Headspace Volatiles
Headspace collection was performed at 23 ± 1°C, 50–70% r.h. and approximately 10 klux; nine replicate plants were used per treatment. Volatiles from the treatments to be compared were collected on the same day to minimize variation among plant and caterpillar batches as well as day-to-day variation. Four replicates were obtained from the same batch of 10- to 12-wk-old plants within 9 d in September 2002, and five replicates were obtained from the same batch of ca. 10- to 12-wk-old plants within 11 d in April 2003. First, incoming air was led through a 5-l glass jar for 1 hr, at a flow rate of 300 ml/min to clean the system. Then, two leaves of the same plant induced by regurgitant from unparasitized P. brassicae caterpillars, and two leaves from a different plant induced by regurgitant of parasitized caterpillars were excised and immediately placed into separate jars with their petioles in a vial with water. They were purged for a further 1 hr. During the third hour, the headspace was collected using traps (10 cm long), packed with 200 mg Tenax TA (Markes, Pontyclun, UK; 60–80 mesh) connected to the air outlet.
GC-MS Analysis of Headspace Samples
Volatiles were desorbed from the Tenax traps using an automated thermodesorption unit (model Unity, Markes) at 200°C for 10 min (He flow 30 ml/min) and focused in an electrically cooled (−10°C) sorbent trap to allow for narrow starting bands. Volatiles were injected into the GC in splitless mode by ballistic heating of the cold trap for 3 min to 270°C. After separation by capillary gas chromatography (column: 30 m × 0.32 mm ID RTX-5 Silms, film thickness 0.33 μm; temperature program: 40–95°C at 3°C/min, then to 165°C at 2°C/min, and finally to 250°C at 15°C/min), volatiles were introduced into a Finnigan quadrupole mass spectrometer (Model Trace) operating at 70 eV in EI ionization mode. Mass spectra were recorded with full scan mode (33–300 AMU, 3 scans/sec). Compounds were identified using deconvolution software (AMDIS) in combination with Nist 98 and Wiley 7th edition spectral libraries, and by comparing their linear retention indices with those of authentic references from our own library.
Other identifications were made by comparison of mass spectra and linear retention indices (Ruther, 2000; Adams, 2001), by interpolating homologous series, and/or by using reference substances. The identified peaks were integrated by Xcalibur software (Finnigan). To minimize any interference by coeluting compounds, specific ions were selected for each compound of interest. Generally, these ions were identical with the model ions indicated by AMDIS.
Statistical Analysis
The null hypothesis (H0) was that there is no difference in the probability of wasps flying to the UNPAR treatment or PAR treatment. The alternative hypothesis (H1) was that the wasps used herbivore-induced plant volatiles in host-discrimination, and thus, that they preferred volatiles from the UNPAR treatment over those from the PAR treatment. The choices of the wasps in the two-choice olfactometer conform to a binomial distribution. Therefore, choices of the wasps between two odor sources within a bioassay were analyzed by using a one-sided binomial test (Siegel, 1956). Responsiveness among the choice tests between the different treatments was analyzed with 2 × 2 contingency tables using chi-square statistics.
Amounts of volatiles trapped were analyzed on the basis of peak area, as determined in the GC-MS analysis. For the headspace analyses, samples of both treatments collected on the same day were analyzed as paired data. The Wilcoxon’s matched-pair signed rank test was employed to test whether the peak area per compound differed between leaves treated with regurgitant collected from parasitized and unparasitized caterpillars. A sign test was used to determine whether the number of compounds that were emitted in larger amounts differed from a 50:50 distribution over the two treatments.
Results
Parasitoid Behavior
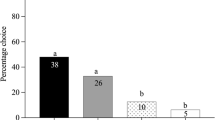
Females of C. rubecula landed more often on leaves infested with unparasitized larvae of P. rapae than on leaves infested with parasitized caterpillars (Figure 1a; P = 0.01). Only those combinations of leaves were taken into account for which the parasitization rate after dissection was found to be minimally 50%.
Response of C. rubecula to cabbage leaves (a) exposed to parasitized (PAR) and unparasitized (UNPAR) second instar larvae of P. rapae, (b) artificially damaged and treated with regurgitant (REG) of PAR and UNPAR fourth instar P. rapae larvae, and (c) artificially damaged and treated with REG from PAR and UNPAR fifth instar P. brassicae larvae in a two-choice set-up. Asterisks indicate significant differences within a choice test: *P < 0.05, **P < 0.01 (binomial test). The bars show the percentages of wasps landing on either odor source. The numbers show the number of wasps responding; the numbers in brackets show the total number of wasps tested. All wasps were released on a leaf damaged by first instar larvae either of UNPAR P. rapae (a/b) or UNPAR P. brassicae (c).
Because the amount of leaf surface area removed from leaves fed upon by the unparasitized caterpillars was about 20% larger than from leaves fed upon by parasitized caterpillars, and the amount of ad libitum feeding could not be manipulated, we conducted subsequent bioassays with leaves to which a standardized amount of artificial damage and regurgitant were applied. In bioassays with regurgitant of P. rapae larvae, females of C. rubecula preferred to land on leaves treated with regurgitant of unparasitized caterpillars (Figure 1b; P = 0.039). Also, when leaves treated with regurgitant of P. brassicae were offered, C. rubecula females landed more often on the UNPAR treatment than on the PAR treatment (Figure 1c; P = 0.045).
C. glomerata wasps did not discriminate between artificially damaged cabbage leaves treated with regurgitant collected from either unparasitized or parasitized P. rapae larvae (Figure 2a). However, in tests using leaves treated with regurgitant of P. brassicae larvae, C. glomerata females showed a clear preference for landing on leaves treated with regurgitant of unparasitized larvae (Figure 2b; P = 0.002). Additionally, the wasps’ responsiveness was higher in the tests with leaves treated with P. brassicae regurgitant than in tests with regurgitant of P. rapae larvae (P < 0.001, χ2 = 17.2; df = 1).
Response of C. glomerata to artificially damaged cabbage leaves treated with regurgitant (REG) from parasitized (PAR) or unparasitized (UNPAR) Pieris spp. caterpillars of (a) 4th instar P. rapae larvae and (b) fifth instar P. brassicae larvae, and in a two-choice set-up. Asterisks indicate significant differences within a choice test: **P < 0.01, n.s. not significant (binomial test). The numbers show the number of wasps responding; the numbers in brackets show the total number of wasps tested. All wasps were released on a leaf damaged by first instar larvae either of UNPAR (a) P. brassicae or (b) P. rapae.
Headspace Analysis
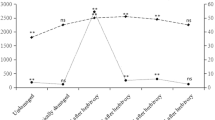
We focused our headspace analysis on those compounds that elicited electrophysiological responses from antennal olfactory receptors of the two Cotesia species (Smid et al., 2002). The plants treated with regurgitant of parasitized or unparasitized P. brassicae larvae emitted a volatile blend that consisted of green leaf volatiles, aldehydes, alcohols and esters, terpenes, and methyl salicylate. Averaged over nine replications, 19 out of the 22 compounds were emitted in larger amounts in the UNPAR treatment than in the PAR treatment (P < 0.001, sign test). In addition, higher amounts of the major terpenoids limonene (P = 0.05), α-thujene (P = 0.04), and 1,8-cineole (P = 0.01; two-sided Wilcoxon’s matched pair signed rank test) were released in the UNPAR treatment (Figure 3). Thus, the quantitative profile of the emitted volatiles changes, and three volatiles are emitted in significantly lower amounts when cabbage plants are induced by regurgitant of parasitized P. brassicae compared to cabbage plants induced by regurgitant of unparasitized P. brassicae.
Comparison of the volatile blends emitted by cabbage plants artificially damaged and treated with regurgitant of unparasitized (UNPAR) or parasitized (PAR) caterpillars of P. brassicae for 22 compounds expressed as mean peak area (in arbitrary units) and the standard error. For each compound, the mean of nine peak areas of the UNPAR treatments and nine peak areas of the PAR treatments are shown. Asterisks indicate significant differences: *P < 0.05, **P < 0.01 (Wilcoxon’s matched pairs signed rank test).
Discussion
The results show that C. rubecula females without oviposition experience discriminated suitable hosts, i.e., hosts that were not parasitized, from parasitized hosts in flight by using host-induced plant volatiles. Females of C. glomerata discriminated between host-induced plant volatiles induced by unparasitized and parasitized larvae in the case of their preferred host P. brassicae. As a result, the wasps save time compared to wasps that first have to land and explore a patch to detect nonvolatile marking substances left by previous parasitoid visitors. Additionally, C. glomerata females reduce the risk of being bitten or coming in contact with the regurgitant of P. brassicae caterpillars. P. brassicae caterpillars, unlike P. rapae caterpillars, vigilantly defend themselves and can seriously harm their parasitoids (Brodeur et al., 1996).
Especially for a solitary endoparasitoid such as C. rubecula, host discrimination is crucial because supernumerary larvae compete to the extent that only a single parasitoid can emerge (Salt, 1961). The older larva usually eliminates the younger. The extent of the disadvantage depends critically on the time that elapses between the two ovipositions. Thus, in cases with a limited number of hosts present, parasitoids should be more apt to attack recently parasitized hosts in which their larvae have a higher probability to survive. Internal markers released by the egg or physiological changes in the host caused by parasitism become more noticeable with time (van Lenteren, 1976; Godfray, 1994 and references therein).
In gregarious parasitoids such as C. glomerata, scramble competition may occur, i.e., no aggressive behavior toward other parasitoid larvae or physiological suppression occurs, and the parasitoids develop as long as food is available. Superparasitism can be an adaptive strategy under some conditions, such as when wasps are faced with a limited number of hosts for a longer period. However, an increase in clutch size is often associated with a decrease in size of surviving offspring and a reduced fitness of the emerging adults (e.g., Harvey et al., 1993; Potting et al., 1997).
Naive females of C. glomerata discriminate between parasitized and unparasitized hosts in close-range tests (Ikawa and Suzuki, 1982; Le Masurier, 1990). Experienced C. glomerata wasps regulate the number of eggs laid in response to the number of available suitable hosts (Ikawa and Suzuki, 1982). We used females experienced with a particular plant–host complex (as described before) to assess whether they discriminated in flight. Therefore, it remains unclear if the response was innate or triggered by the short odor experience just prior to the release in the wind tunnel. Other studies have shown that a single experience with a plant–host complex has no influence on the behavior of either C. glomerata or C. rubecula toward the offered plant–host complexes in the wind tunnel (Geervliet et al., 1998). However, in our case, the brief experience resulted in a higher response of C. glomerata that flew to one of the odor sources in tests with plants treated with regurgitant of P. brassicae.
When we offered leaves induced with regurgitant of P. rapae, a high proportion of females of our Dutch strain of C. glomerata were unresponsive, and those that did fly toward the plants did not discriminate. In the Netherlands, C. glomerata specializes on P. brassicae, which has been ascribed to the fact that C. rubecula outcompetes C. glomerata in P. rapae larvae (Geervliet et al., 2000). The solitary endoparasitoid C. rubecula always wins the competition for the same host against the gregarious C. glomerata (Laing and Corrigan, 1987). Our results suggest that the priming of C. glomerata wasps with leaf damage of first instar P. rapae caterpillars that we practiced just prior to the bioassay induced a low response and a failure to discriminate between plants treated with regurgitant of unparasitized or parasitized P. rapae larvae.
To the best of our knowledge, this is the first report in which elicitation of herbivore-induced plant volatile production, by controlling the amount of mechanical leaf damage combined with the application of regurgitant, has been shown to result in a reduced emission of specific plant volatiles when elicitors originate from parasitized caterpillars. Souissi et al. (1998) reported that unparasitized mealybug-infested plants or unparasitized mealybugs are more attractive to walking Apoanagyrus lopezi (Hymenoptera: Encyrtidae) endoparasitoids than plants infested with parasitized mealybugs or parasitized mealybugs alone. The amount of herbivore feeding was not controlled in their study, and it was not checked whether the plant was the source of the attractants. Sheehan et al. (1993) showed that females of Microplitis croceipes (Hymenoptera: Braconidae) were able to discriminate in flight between previously visited and unvisited sites in a plant patch, even in the absence of the host. In this case, herbivore-induced plant volatiles were not tested, and it was assumed that a chemical marking deposited on the substrate served as the cue for discrimination.
Presently, it is still unknown for any parasitoid species exactly which compounds are involved as synomones (Dicke and van Loon, 2000). Previous work on volatiles emitted by a different Brussels sprouts cultivar (Blaakmeer et al., 1994) showed differences between intact and caterpillar-damaged plants for hexyl acetate, (Z)-3-hexenyl acetate, sabinene, and 1,8-cineole. These compounds were also increased in the headspace of plants treated with regurgitant of unparasitized P. brassicae caterpillars compared to treatments with regurgitant of parasitized P. brassicae caterpillars. The headspace composition documented here also overlaps with that described by Smid et al. (2002), who studied yet another Brussels sprouts cultivar and used feeding first instar caterpillars during headspace collection. An obvious difference is that benzyl cyanide, a breakdown product of glucosinolates (the taxonomically characteristic secondary plant metabolites of Brassicaceous plants), is lacking in our samples. It was recently established (Wittstock et al., 2004) that this compound is released from the feces of Pieris caterpillars, which explains its absence from our samples. Limonene elicits an olfactory response in the antennae of both Cotesia species (Smid et al., 2002). The fact that the reduced emission of three volatiles correlated with discriminatory behavior that were shown to elicit electrophysiological activity suggests that the quantity of these compounds, possibly relative to those of other compounds, provides information to the parasitoids. We used leaves that were detached just prior to the experiments. For maize, it has been shown that excision of leaves may influence the emission rate of certain components in some treatments at some periods of the day, without affecting the overall effect of treatment (Schmelz et al., 2001). In our experiments, the treatments were maximally standardized with regard to leaf physiological age and size, and leaves from both treatments were excised to investigate locally emitted volatiles only.
Studies on the interrelationship of Cotesia larvae with their hosts show drastic effects of parasitism on the caterpillars’ physiology such as changes in hemolymph protein titer and composition (reviewed by Beckage, 1993). Application of β-glucosidase, a component of P. brassicae regurgitant, to artificially damaged plants resulted in the release of a volatile blend similar to that of leaves treated with regurgitant (Mattiacci et al., 1995). Quantitative differences in headspace composition resulting from treating plants with regurgitant obtained from either parasitized or unparasitized caterpillars might be attributable to a reduced amount of β-glucosidase in regurgitant of parasitized P. brassicae.
Parasitization of P. rapae by C. rubecula has been shown to increase fitness of the brassicaceous plant Arabidopsis thaliana (van Loon et al., 2000). In the case of C. glomerata, which preferentially parasitizes the gregariously feeding P. brassicae, only a suppression of specific synomones, as documented here, would benefit the wasp, because for this parasitoid–host combination an overall reduction in the amount of volatiles released could signal a lower but still sufficient number of available unparasitized hosts rather than parasitized hosts. As we controlled the amount of mechanical damage and the volume of elicitor-containing regurgitant, we can exclude the involvement of other stress factors, such as viral, bacterial, or fungal pathogens, that might have interfered with the feeding performance of the caterpillars and consequently with induced synomone emission by the plants.
The induced volatiles bear biosynthetic and ecological costs (Dicke and Sabelis, 1989; Dicke and van Loon, 2000; Hoballah et al., 2004). Many of the compounds are produced through de novo biosynthesis (Paré and Tumlinson, 1997). Ecological costs in terms of attraction of herbivores can be much higher than the biosynthetic costs (Dicke and Vet, 1999). Thus, downregulation of induced volatile production is likely to be advantageous for plants.
In summary, we have shown that parasitoids can use herbivore-induced plant volatiles for host discrimination in flight, a crucial ability to enhance their reproductive success. Besides the fact that plant synomones are reliable and detectable indicators of the presence and identity of herbivores, we document that parasitoids can use them as a source of information on host suitability. Consequently, parasitoids can save energy and time in finding suitable hosts. From the plant’s perspective, our study has uncovered an advantage through a reduced production of a subset of herbivore-induced volatiles, after having successfully recruited their bodyguards.
References
R. P. Adams (2001) Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy Allured Publishing Corporation Carol Stream
N. G. Agelopoulos M. A. Keller (1994) ArticleTitlePlant–natural enemy association in the tritrophic system, Cotesia rubecula–Pieris rapae–Brassicaceae (Cruciferae): II. Preference of C. rubecula for landing and searching J. Chem. Ecol. 20 1735–1748 Occurrence Handle10.1007/BF02059895
N. E. Beckage (1993) Games parasites play: The dynamic roles of proteins and peptides in the relationship between parasite and host N. E. Beckage S. N. Thompson B. A. Federici (Eds) Parasites and Pathogens of Insects Academic Press San Diego 25–57
A. Blaakmeer J. B. F. Geervliet J. J. A. Loon Particlevan M. A. Posthumus T. A. Beek Particlevan Æ. Groot Particlede (1994) ArticleTitleComparative headspace analysis of cabbage plants damaged by two species of Pieris caterpillars: consequences for in-flight host location by Cotesia parasitoids Entomol. Exp. Appl. 73 175–182
J. Brodeur J. B. F. Geervliet L. E. M. Vet (1996) ArticleTitleThe role of host species, age and defensive behaviour on ovipositional decisions in a solitary specialist and gregarious generalist parasitoid (Cotesia species) Entomol. Exp. Appl. 81 132 Occurrence Handle10.1007/BF00192137
M. Dicke (1999) ArticleTitleAre herbivore-induced plant volatiles reliable indicators of herbivore identity to foraging carnivorous arthropods? Entomol. Exp. Appl. 91 131–142 Occurrence Handle10.1023/A:1003608019062
M. Dicke M. W. Sabelis (1989) Does it pay plants to advertize for bodyguards? Towards a cost–benefit analysis of induced synomone production H. Lambers M. L. Cambridge H. Konings T. L. Pons (Eds) Causes and Consequences of Variation in Growth Rate and Productivity of Higher Plants SPB Publishing The Hague 341–358
M. Dicke J. J. A. Loon Particlevan (2000) ArticleTitleMultitrophic effects of herbivore-induced plant volatiles in an evolutionary context Entomol. Exp. Appl. 97 237–249 Occurrence Handle10.1023/A:1004111624780
M. Dicke L. E. M. Vet (1999) Plant–carnivore interactions: Evolutionary and ecological consequences for plant, herbivore and carnivore H. Olff V. K. Brown R. H. Drent (Eds) Herbivores: Between Plants and Predators Blackwell Science Oxford 483–520
J. B. F. Geervliet L. E. M. Vet M. Dicke (1994) ArticleTitleVolatiles from damaged plants as major cues in long-range host-searching by the specialist parasitoid Cotesia rubescula Entomol. Exp. Appl. 73 289–297
J. B. F. Geervliet A. I. Vreugdenhil M. Dicke L. E. M. Vet (1998) ArticleTitleLearning to discriminate between infochemicals from different plant–host complexes by the parasitoids Cotesia glomerata and C. rubecula Entomol. Exp. Appl. 86 241–252 Occurrence Handle10.1023/A:1003186706517
J. B. F. Geervliet M. S. W. Verdel S. Henk J. Schaub M. Dicke L. E. M. Vet (2000) ArticleTitleCoexistence and niche segregation by field populations of the parasitoids Cotesia glomerata and C. rubecula in the Netherlands: predicting field performance from laboratory data Oecologia 124 55–63 Occurrence Handle10.1007/s004420050024
H. C. J. Godfray (1994) Parasitoids—Behavioral and Evolutionary Ecology Princeton University Press Princeton
J. A. Harvey I. F. Harvey D. J. Thompson (1993) ArticleTitleThe effect of superparasitism on development of the solitary wasp parasitiod Venturia canescens (Hymenoptera: Ichneumonidae) Ecol. Entomol. 18 203–208
M. Hilker T. Meiners (2002) ArticleTitleInduction of plant responses towards oviposition and feeding of herbivorous arthropods: a comparison Entomol. Exp. Appl. 104 181–192 Occurrence Handle10.1023/A:1021232319226
M. E. Hoballah T. G. Kollner J. Degenhardt T. C. J. Turlings (2004) ArticleTitleCosts of induced volatile production in maize Oikos 105 168–180 Occurrence Handle10.1111/j.0030-1299.2004.12831.x
T. Ikawa Y. Suzuki (1982) ArticleTitleOvipositional experience of the gregarious parasitoid, Apanteles glomeratus (Hymenoptera: Braconidae), influencing her discrimination of the host larvae, Pieris rapae crucivora Appl. Entomol. Zool. 17 119–126
J. E. Laing J. E. Corrigan (1987) ArticleTitleIntrinsic competition between the gregarious parasite, Cotesia glomeratus and the solitary parasite, Cotesia rubecula (Hymenoptera: Braconidae) for their host, Artogeia rapae (Lepidoptera: Pieridae) Entomophaga 32 493–501
A. D. Masurier ParticleLe (1990) ArticleTitleHost discrimination by Cotesia (=Apanteles) glomerata parasitising Pieris rapae Entomol. Exp. Appl. 54 65–72 Occurrence Handle10.1007/BF00353988
L. Mattiacci M. Dicke M. A. Posthumus (1994) ArticleTitleInduction of parasitoid attracting synomone in Brussels sprouts plants by feeding of Pieris brassicae larvae: Role of mechanical damage and herbivore elicitor J. Chem. Ecol. 20 2229–2247 Occurrence Handle10.1007/BF02033199
L. Mattiacci M. Dicke M. A. Posthumus (1995) ArticleTitleβ-Glucosidase: An elicitor of the herbivore-induced plant odor that attracts host-searching parasitic wasps Proc. Natl. Acad. Sci. USA 92 2036–2040 Occurrence Handle11607516
L. Mattiacci S. Rudelli B. Ambühl Rocca S. Genini S. Dorn (2001) ArticleTitleSystemically-induced response of cabbage plants against a specialist herbivore, Pieris brassicae Chemoecology 11 167–173
C. R. Nufio D. R. Papaj (2001) ArticleTitleHost marking behavior in phytophagous insects and parasitoids Entomol. Exp. Appl. 99 273–293 Occurrence Handle10.1023/A:1019204817341
P. W. Paré J. H. Tumlinson (1997) ArticleTitleInduced synthesis of plant volatiles Nature 385 30–31 Occurrence Handle10.1038/385030a0
R. P. J. Potting H. M. Snellen L. E. M. Vet (1997) ArticleTitleFitness consequences of superparasitism and mechanism of host discrimination in the stemborer parasitoid, Cotesia flavipes Entomol. Exp. Appl. 82 341–348 Occurrence Handle10.1023/A:1002960625981
J. Ruther (2000) ArticleTitleRetention index database for identification of general green leaf volatiles in plants by coupled capillary gas chromatography-mass spectrometry J. Chromatogr. A 890 313–319 Occurrence Handle10.1016/S0021-9673(00)00618-X Occurrence Handle11009035
G. Salt (1961) ArticleTitleCompetition among insect parasitoids Symp. Soc. Exp. Biol. 15 96–119
E. A. Schmelz H. T. Alborn J. H. Tumlinson (2001) ArticleTitleThe influence of intact-plant and excised-leaf bioassay designs of volicitin- and jasmonic acid-induced sesquiterpene volatile release in Zea mays Planta 214 171–179 Occurrence Handle11800380
W. Sheehan F. L. Wäckers W. J. Lewis (1993) ArticleTitleDiscrimination of previously searched, host-free sites by Micoplitis croceipes (Hymenoptera: Braconidae) J. Insect Behav. 6 323–331 Occurrence Handle10.1007/BF01048113
K. Shiojiri J. Takabayashi S. Yano A. Takafuji (2000) ArticleTitleHerbivore-species-specific interactions between crucifer plants and parasitic wasps (Hymenoptera: Braconidae) that are mediated by infochemicals present in areas damaged by herbivores Appl. Entomol. Zool. 35 519–524 Occurrence Handle10.1303/aez.2000.519
K. Shiojiri J. Takabayashi S. Yano A. Takafuji (2001) ArticleTitleInfochemically mediated tritrophic interaction webs on cabbage plants Popul. Ecol. 43 23–29
S. Siegel (1956) Nonparametric Statistics for the Behavioral Sciences McGraw-Hill Kogakusha Tokyo
H. M. Smid J. J. A. Loon Particlevan M. A. Posthumus L. E. M. Vet (2002) ArticleTitleGC-EAG-analysis of volatiles from Brussels sprouts plants damaged by two species of Pieris caterpillars: olfactory respective range of a specialist and generalist parasitoid wasp species Chemoecology 12 169–176
R. Souissi J.-P. Nenon B. Rü ParticleLe (1998) ArticleTitleOlfactory responses of parasitoid Apoanagyrus lopezi to odor of plants, mealybugs, and plant–mealybug complexes J. Chem. Ecol. 24 37–48 Occurrence Handle10.1023/A:1022332711331
J. L. M. Steidle J. J. A. Loon Particlevan (2003) ArticleTitleDietary specialization and infochemical use in carnivorous arthropods: testing a concept Entomol. Exp. Appl. 108 133–148 Occurrence Handle10.1046/j.1570-7458.2003.00080.x
J. Takabayashi M. Dicke (1996) ArticleTitlePlant–carnivore mutualism through herbivore-induced carnivore attractants Trends Plant Sci. 1 109–113 Occurrence Handle10.1016/S1360-1385(96)90004-7
K. Takasu Y. Hirose (1988) ArticleTitleHost discrimination in the parasitoid Ooencyrtus nezarae: The role the egg stalk as an external marker Entomol. Exp. Appl. 47 47–48 Occurrence Handle10.1007/BF00186714
T. C. J. Turlings J. H. Loughrin P. J. McCall U. S. R. Röse W. J. Lewis J. H. Tumlinson (1995) ArticleTitleHow caterpillar-damaged plants protect themselves by attracting parasitic wasps Proc. Natl. Acad. Sci. USA 92 4169–4174 Occurrence Handle7753779
T. C. J. Turlings S. Gouinguené T. Degen M. E. Fritzsche Hoballah (2002) The chemical ecology of plant–caterpillar–parasitoid interactions T. Tscharntke B. A. Hawkins (Eds) Multitrophic Level Interactions Cambridge University Press Cambridge 148–173
J. J. M. Alphen Particlevan M. E. Visser (1990) ArticleTitleSuperparasitism as an adaptive strategy for insect parasitoids Annu. Rev. Entomol. 35 59–79 Occurrence Handle10.1146/annurev.en.35.010190.000423 Occurrence Handle2405774
J. Baaren Particlevan J.-P. Nenon (1996) ArticleTitleHost location and discrimination mediated through olfactory stimuli in two species of Encyrtidae Entomol. Exp. Appl. 81 61–69 Occurrence Handle10.1007/BF00187839
W. A. Giessen Particlevan W. J. Lewis L. E. M. Vet F. L. Wäckers (1993) ArticleTitleThe influence of host site experience on subsequent flight behavior in Microplitis croceipes (Cresson) (Hymenoptera: Braconidae) Biol. Control 3 75–79 Occurrence Handle10.1006/bcon.1993.1012
J. C. Lenteren Particlevan (1976) ArticleTitleThe development of host discrimination and the prevention of superparasitism in the parasite Pseudeucoila bochei (Hym.: Cynipidae) Neth. J. Zool. 26 1–83
J. C. Lenteren Particlevan (1981) Host discrimination by parasitoids D. A. Nordlund R. L. Jones W. J. Lewis (Eds) Semiochemicals—Their Role in Pest Control Wiley New York 153–179
J. J. A. Loon Particlevan J. G. Boer Particlede M. Dicke (2000) ArticleTitleParasitoid–plant mutualism: parasitoid attack of herbivore increases plant reproduction Entomol. Exp. Appl. 97 219–227 Occurrence Handle10.1023/A:1004032225239
L. E. M. Vet M. Dicke (1992) ArticleTitleEcology of infochemical use by natural enemies in a tritrophic context Annu. Rev. Entomol. 37 141–172 Occurrence Handle10.1146/annurev.en.37.010192.001041
S. B. Vinson (1985) The behavior of parasitoids G. A. Kerkut L. I. Gilbert (Eds) Comprehensive Insect Physiology, Biochemistry and Pharmacology Pergamon Press New York 417–469
U. Wittstock N. Agerbirk E. J. Stauber C. E. Olsen M. Hippler T. Mitchell-Olds J. Gershenzon H. Vogel (2004) ArticleTitleSuccessful herbivore attack due to metabolic diversion of a plant chemical defense Proc. Natl. Acad. Sci. USA 101 4859–4864 Occurrence Handle10.1073/pnas.0308007101 Occurrence Handle15051878
Acknowledgments
The authors thank Joachim Ruther, Joop van Lenteren, and Monika Hilker for advice and comments on an earlier version of this manuscript; Leo Koopman, Frans van Aggelen, and André Gidding for culturing the insects. Funding by the Bresillac Foundation is gratefully acknowledged. All experiments complied with the current laws of The Netherlands.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fatouros, N.E., van Loon, J.J.A., Hordijk, K.A. et al. Herbivore-Induced Plant Volatiles Mediate In-Flight Host Discrimination by Parasitoids. J Chem Ecol 31, 2033–2047 (2005). https://doi.org/10.1007/s10886-005-6076-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-005-6076-5