Abstract
Chinese medicinal plants and their surrounding rhizospheric soil serve as promising sources of actinobacteria. A total of 180 actinobacteria strains were isolated from the rhizosphere soil, leaves, stems, and roots of nine selected plants and have been identified as potential biocontrol agents against Fusarium oxysporum f. sp. cucumerinum. An endophytic strain CNS-42 isolated from Alisma orientale showed the largest zone of inhibition demonstrating a potent effect against F. oxysporum f. sp. cucumerinum and a broad antimicrobial activity against bacteria, yeasts, and other pathogenic fungi. The in vivo biocontrol assays showed that the disease severity index was significantly reduced (P < 0.05), and plant shoot fresh weight and height increased greatly (P < 0.05) in plantlets treated with strain CNS-42 compared to the negative control. This isolate was identified as Streptomyces sp. based on cultural, physiological, morphological characteristics, and 16S rRNA gene analysis. Further bioassay-guided isolation and purification revealed that staurosporine was responsible for its antifungal and plant growth promoting activities and the latter property of staurosporine is reported for the first time. The in vivo assay was further performed and indicated that staurosporine showed good growth promoting effect on the plant shoot biomass of cucumber. This is the first critical evidence identifying CNS-42 as a biocontrol agent for the soil borne pathogen, F. oxysporum f. sp. cucumerinum.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fusarium wilt of cucumber (Cucumis sativus L.) caused by Fusarium oxysporum f. sp. cucumerinum is one of the major limitations for the stable production of cucumber, especially in greenhouse vegetable production (Vakalounakis et al. 2004). A few traditional chemical methods have been used for the control of this disease. However, environmental problems could arise due to the chemical dispersion. Thus, previous studies focusing on environmentally friendly biocontrol agents have been conducted and several potential candidates have been reported (Pavlou and Vakalounakis 2005; Postma et al. 2008; Wei et al. 2008; Xian and Peng 2008; Xue et al. 2008).
Actinobacteria play a significant role in the control of plant diseases and their biocontrol mechanisms have been clearly demonstrated. Diversified secondary metabolites have been identified from actinobacteria including various antibiotics, antitumor and immunosuppressive agents, and plant growth hormones (Fiedler et al. 2008; Schulz et al. 2009; Strobel et al. 1999). Several studies have reported the antimicrobial activities of actinobacteria in the protection of host plants against pathogens thus promoting the growth of such plants (de Araújo et al. 2000; Schippers et al. 1987; Taechowisan et al. 2005; Tahvonen 1982). Nishimura et al. (2002) isolated an endophytic Streptomyces strain AOK-30, which showed antagonistic effect against pathogenic fungi from the cuckoo families of flowering plants. Shimizu et al. (2006) identified that disease resistance could be induced by some non-antagonistic endophytic Streptomyces sp. on tissue-cultured seedlings of rhododendron. Cao et al. (2004) indicated that endophytic actinobacteria could reduce the impact of ‘take-all’ on wheat.
Actinobacteria are widespread in the environment and most of the species are chemo-organotrophic, aerobic, mesophilic, and grow optimally at a pH near neutral (Goodfellow and Williams 1983; Williams and Wellington 1982). They are affected directly by the presence of available carbon sources and their numbers are especially high in land rich in organic matters (Alexander 1977). A large number of actinobacteria have been isolated and described from traditional sources and many researchers have started to select and isolate untapped microbes from special environments. Kitouni et al. (2005) identified some strains with antimicrobial activities from water, soil, and tree barks and an endophytic Streptomyces from the medicinal plant Hibbertia scandens capable of producing antibiotics with a broad antimicrobial spectrum was isolated in Australia by Castillo et al. (2003). Two novel strains were recently isolated from the rhizosphere soil of TCMs (Wei et al. 2009). One of them showed strong inhibitory activity against the growth of Mycobacterium smegmatis and the other isolate could inhibit the growth of Pseudomonas aeruginosa. Yuan et al. (2009) isolated a total of 959 strains from 62 rhizosphere soil samples of medicinal plants from Yunnan province and Chongqing area, China, among which 31 % of these isolates showed positive bioactivities.
In this study, we attempted to investigate endophytic or rhizospheric actinobacteria from Chinese medicinal plants as potential biocontrol agents against F. oxysporum f. sp. cucumerinum and a plant growth promoter of cucumber. A total of 180 actinobacteria were isolated from the rhizosphere soils, leaves, stems, and roots of selected plants. An endophytic strain CNS-42, identified as Streptomyces sp., isolated from Alisma orientale showed the strongest antagonistic effect against F. oxysporum f. sp. cucumerinum in vivo. Further bioassay-guided purification and in vivo evaluation revealed that staurosporine was responsible for its antifungal and plant growth promotion activities.
Materials and methods
General experimental procedures
UV spectra were obtained on a Cary 50 spectrophotometer. NMR spectra were obtained on a Bruker Avance DRX600 spectrometer. HRESIMS measurements were obtained on a Brukermicro TOF mass spectrometer. Resin HP-20 (Diaion, Japan), and ODS-A (YMC, Japan) were used for purification. RP-HPLC was carried out using an Eclipse XDB-C18 column (5 μm) semi-preparative column (9.4 × 250 mm) on a Shimadzu 20A series HPLC.
Isolation of the actinobacteria from Chinese medicinal plants and rhizosphere soils
Nine Chinese medicinal plants including A. orientale, Latycodon grandiflorum, Curcuma longa, Platycodon grandiflorum, Polygonatum cyrtonema, Rhizoma paridis, Rhodiola crenulata, Notopterygium incisum, and Astragalus membranaceus, together with their rhizosphere soils were collected in the Sichuan province, southwest of China. The method and media used for isolating endophytic actinobacteria of plant samples were as described by Zhao et al. (2011). Isolation from the rhizosphere soils was done by serial dilution method, and 10−3–10−5 dilutions were used.
In order to confirm the success of the sterilization process, aliquots of the sterile distilled water from the final rinse were inoculated on International Streptomyces Project-2 (ISP2) medium plates, incubated at 28 °C, and observed for microbial growth. If no microbial growth occurred on the surface of the medium, the sterilization was considered complete (Qin et al. 2009).
Incubation of endophytic strains was performed at 28 °C until single colonies could be detected. The aerial mycelium of colonies were isolated and purified on ISP2 medium. The stocks were prepared on ISP2 and kept at −70 °C (under 30 % of glycerol) for long-term storage and at 4 °C as source cultures.
Preliminary screening of the antimicrobial activities of the isolates
To select antagonistic strains, the antimicrobial activity of each isolate was tested against the pathogenic fungi (F. oxysporum f. sp. cucumerinum, this strain has been preserved at the Microbial Natural Product Library, Institute of Microbiology, Chinese Academy of Sciences, with the accession number SCPF151). The spores of actinomycetes were spread on ISP-2 and cultivated for 7 days (three repetitions). The pathogenic fungus (F. oxysporum f. sp. cucumerinum) was inoculated on potato dextrose agar (PDA) medium for 1 week (Shomura et al. 1979). A mycelia disk (6-mm diameter) of each pathogen was transferred to the center of freshly prepared PDA medium on different plates, and three disks of actinomycetes (6-mm diameter) were inoculated onto the margin areas 3 cm from the central pathogen colony, which was followed by incubation at 28 °C for 5–7 days.
Antimicrobial spectrum of CNS-42
In order to investigate the antimicrobial spectrum of the strain CNS-42, which demonstrated the strongest inhibition of F. oxysporum on agar medium, six pathogenic fungi (including F. oxysporum, Colletotrichum orbiculare, Rhizoctonia solani, Alternaria solani, F. graminearum, and Curvularia lunata), Saccharomyces cerevisiae, and three bacteria (including Escherichia coli ATCC35218, Bacillus subtilis ATCC31785, and Staphylococcus aureus ATCC25923) were used in this study. The pathogenic fungi and yeast have been preserved at the Microbial Natural Product Library, Institute of Microbiology, Chinese Academy of Sciences, with the accession number SCPF151, SCPF152, SCPF153, SCPF154, SCPF155, SCPF156, and SCYF151, respectively. Mycelia disk of strain CNS-42 (6 mm diameter) was cut from the plates and inoculated in a 250 mL conical flask containing 50 mL liquid medium. The flask was then placed in a rotary shaker at 200 rpm and 28 °C for 7 days. After incubation, the culture medium was separated from the mycelium by centrifugation at 5,000 rpm for 10 min. The supernatant was used for the antimicrobial activity test using the method described previously (Thakur et al. 2007). The zones of inhibition were determined after 24 h of incubation at 37 °C for bacteria. Antifungal activity of CNS-42 was tested by the methods described in the previous section. Each experiment was replicated three times and the average zone of inhibition was calculated.
In vivo biocontrol assay using CNS-42
Four treatments (A1, B1, C1, and D1) were carried out to evaluate the ability of strain CNS-42 to control the severity of disease and promote growth of cucumber. The soil used in the experiment was steam-sterilized for 2 h at 121 °C. In the treatment A1, the spore suspension of F. oxysporum f. sp. cucumerinum (2 mL, 3 × 104–4 × 104 cfu/mL) was spread in the soil when cucumber was planted. In the treatment B1, both the mycelia suspension of strain CNS-42 (2 mL, 3 × 106–4 × 106 cfu/mL) and the spore suspension of F. oxysporum f. sp. cucumerinum (2 mL, 3 × 104–4 × 104 cfu/mL) were spread in the soil at the same time when cucumber was planted. In the treatment C1, no microorganism solution was added to the soil. For the treatment D1, the mycelia suspension of strain CNS-42 (2 mL, 3 × 106–4 × 106 cfu/mL) was spread in the soil as cucumber was planted. For each treatment, five replicates of cucumber plantlets (2-week old) were cultivated in each 15 cm diameter plastic pots. The experiment was carried out in a greenhouse at an average temperature of 25 °C, with relative humidity at about 60 % and 12 h of illumination per day at 11.8 W/m2. The plantlets were watered once every 2 days from the second day onwards and no fertilizers were added to the soil. After 6 weeks of cultivation, five plants of each treatment were removed from the pot randomly and their shoot fresh weights and heights were recorded. Disease evaluation was graded according to the wilt index (WI) scale and internal examination for the extent of rhizome discoloration graded according to the vascular discoloration index (VDI), as reported by Saravanan et al. (2003).
Preliminary identification of CNS-42
The morphological and cultural characteristics of the CNS-42 were determined by Gram staining and light microscopy of 14-day-old cultures grown on various International Streptomyces Project (ISP) media (Shirling and Gottlieb 1966). Colors of aerial and substrate mycelia were determined with the ISCC-NBS centroid color charts. The main physiological characteristics of CNS-42 were performed as described by Nishimura et al. (2002). Morphological features of spores and mycelia were observed by scanning electron microscopy (Quanta 200, USA) after desiccation and coating the cells with gold (Brunk et al. 1981). This strain has been preserved at the China General Microbiological Culture Collection Center (accession no. 4.7127).
DNA extraction, sequencing and analysis
Genomic DNA extraction of strain CNS-42 was performed as described by the TINAamp Bacteria DNA Kit (TIANGENG BIOTECH, Beijing, China), and PCR amplification of the 16S rRNA gene was carried out with universal primers (27f: 5′-AGAGTTTGATCCTGGCTCAG-3′; 1492r: 5′-TACGGCTACCTTGTTACGACTT-3′). The conditions used for thermal cycler (TaKaRa) was according to the following amplification profile: 95 °C for 5 min followed by 30 cycles consisting of denaturation at 95 °C for 30 s, primer annealing at 55 °C for 30 s, and primer extension at 72 °C for 1 min 45 s. At the end of the cycles, the reaction mixture was kept at 72 °C for 7 min and then cooled to 4 °C. The PCR products were purified by electrophoresis in 1.0 % agarose gels with ethidium bromide staining, and were isolated from the agarose gel by using a E.Z.N.A.™ Gel Extraction Kit (OMEGA bio-tek, China). The extracted PCR product was connected with pMD 18-T Vector (TaKaRa) and cloned. The recombined plasmids were sequenced by TSINGKE Biological Technology Ltd. (Beijing, China).
The 16S rRNA gene sequence determined was compared with the GenBank databases by using the BLAST search program. Phylogenetic and molecular evolutionary analyses were conducted using the software included in MEGA 4.0 package. The 16S rRNA gene sequence of the strain CNS-42 and 13 type strains of actinobacteria were aligned using the Clustal X 1.83 program (Thompson et al. 1994). The evolutionary tree was inferred by using the neighbor-joining method (Saitou and Nei 1987) from the evolutionary distance data corrected by Kimura’s two-parameter model (Kimura 1980) and the topology of the phylogenetic tree was evaluated by bootstrap resampling method with 1,000 replicates (Felsenstein 1985). The 16S rRNA gene sequence reported in this article was assigned by the GenBank with the accession number KJ596488.
Fermentation, isolation, and purification of staurosporine
Strain CNS-42 was cultivated on an ISP2 agar (Nishimura et al. 2002) plate at 28 °C for 5 days. Ten 250 mL Erlenmeyer flasks containing 40 mL of AM2 medium (starch 0.5 %, glucose 2 %, soybean meal 1 %, peptone 0.2 %, yeast extract 0.2 %, NaCl 0.4 %, K2HPO4 0.05 %, MgSO4·7H2O 0.05 %, CaCO3 0.2 %, sea salt 3.4 %, pH 7.8) was inoculated with pieces of well-grown agar cultures of the strain CNS-42 and incubated at 28 °C, 220 rpm for 4 days, which were used as seed cultures for the large-scale fermentation. Aliquots (15 mL) of the seed cultures were aseptically transferred to 135 × 1 L Erlenmeyer flasks, each containing 300 mL of AM2 medium, and the flasks were incubated at 28 °C, 220 rpm for 7 days. The broth was combined and centrifuged (8,000 rpm, 15 min, 4 °C) to yield a supernatant and a mycelial cake. The supernatant was extracted with EtOAc (v/v: 1:1) for three times, while the mycelial cake was extracted with acetone (3 × 1 L). The organic layers were combined and dried to afford a crude extract (Figure S1a). The crude extract (14 g) was fractionated by ODS-MPLC using gradient elution from 20 to 80 % aqueous MeOH for 80 min to provide 11 fractions (Frs. 1–11). The active faction (Fr. 5, 810 mg) was fractionated by ODS-MPLC (35 × 2.2 cm) using an elution of 32 % acetonitrile (ACN) in water to give four sub-fractions (Frs. 5–1 to 5–4). The active sub-fraction (Fr. 5–2, 450 mg) was further purified by ODS-MPLC (35 × 2.2 cm) using a gradient elution from 35 to 70 % ACN/H2O in 120 min to provide an antifungal compound 3 (90 mg) with an MIC value of 6.25 μg/mL (Figure S1b). Fr. 6 (65 mg) showed antifungal activity and were subjected to ODS-MPLC (35 × 2.2 cm, 20 % ACN/H2O), followed by reversed phase HPLC preparation (Zorbax SB-C18 250 × 9.4, 5 μm column, 2 mL/min, gradient elution from 35 to 70 % in 60 min) to afford 2 (21 mg) showed antifungal activity with an MIC value of 3.125 μg/mL (Figure S1b). Fraction 9 (2.4 g) was purified first by recrystallization to obtain staurosporine (1, 200 mg), and this compound showed the best anti-fungal activity (MIC = 0.1 μg/mL).
Evaluation of the growth promoting potential of staurosporine on cucumber
The in vivo growth promoting activity of staurosporine on cucumber was evaluated. Four treatments (A2, B2, C2 and D2) were carried out with four concentrations of 0, 50, 150 and 300 μg/L of staurosporine, respectively. The soil used in the experiment was steam-sterilized for 2 h at 121 °C. These experiments were carried out in a greenhouse at an average temperature of 25 °C, with relative humidity of about 60 % and 12 h of illumination per day at 11.8 W/m2. The plantlets were watered once every 2 days from the second day onwards and no fertilizers were added to the soil. After 6 weeks of cultivation, all plants of each treatment were removed from the pot randomly and the weights of fresh and dry biomass were recorded. The dry biomass was measured after the deactivation of enzymes at 105 °C for 40–60 min and drying to constant weight at 75 °C.
General antimicrobial assays
Antimicrobial assays were performed according to the Antimicrobial Susceptibility Testing Standards outlined by the Clinical and Laboratory Standards Institute (CLSI) using the bacteria S. aureus (ATCC 6538), P. aeruginosa (PAO1) and B. subtilis (ATCC 6633). For each organism, a loopful of glycerol stock was streaked on an LB-agar plate, which was incubated overnight at 37 °C. A single bacterial colony was picked and suspended in Mueller–Hinton Broth to approximately 1 × 104 cfu/mL. A two-fold serial dilution of each compound to be tested (4,000–31.3 μg/mL in DMSO) was prepared and an aliquot of each dilution (2 μL) was added to a 96-well flat-bottom microtiter plate (Greiner). Vancomycin and ciprofloxacin were used as positive controls and DMSO as the negative control. An aliquot (78 μL) of bacterial suspension was then added to each well (to give final compound concentrations of 100–0.78 μg/mL in 2.5 % DMSO) and the plate was incubated at 37 °C aerobically for 16 h. Finally, the optical density of each well at 600 nm was measured with an EnVision 2103 Multi-label Plate Reader (Perkin-Elmer Life Sciences). MIC values were defined as the minimum concentration of compound that inhibited visible bacterial growth. All the experiments were performed in triplicates.
Antifungal assays
Antifungal and bioassays were performed according to a previously described (Zhang et al. 2007) protocol modified from the Clinical and Laboratory Standards Institute M-27A methods (National Committee for Clinical Laboratory Standards 2002) using the fungus Candida albicans SC5314. A colony of C. albicans was picked from a YPD agar plate and suspended in RPMI 1640 to a concentration of 1 × 104 cfu/mL. A two-fold serial dilution of each compound to be tested (4,000–31.3 μg/mL in DMSO) was prepared and an aliquot of each dilution (2 μL) was added to a flat bottom, 96-well microtiter plate (Greiner). Ketoconazole was used as the positive control and DMSO as the negative control. An aliquot (78 μL) of the fungal suspension was added to each well (to give final compound concentrations of 100–0.78 μg/mL in 2.5 % DMSO) and the plate was incubated at 35 °C for 16 h. Finally, the optical density of each well at 600 nm was measured with an EnVision 2103 Multilabel Plate Reader (Perkin-Elmer Life Sciences) and the antifungal MICs were defined as the minimum concentration of compound that inhibited visible fungal growth.
Statistical analysis
Analysis of variance (ANOVA) was conducted on collected data, and the mean values of plant shoot fresh weight, root fresh weight, height and disease index were statistically analyzed using the LSD test. Differences were considered to be significant when the probability was <0.05.
Results
In vitro antagonistic activities
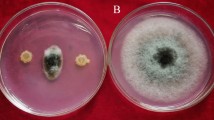
Among the 180 isolates, only 21 strains showed antimicrobial activity to the pathogenic fungi (Table S1). The endophytic strain CNS-42 showed the strongest inhibitory activity against F. oxysporum f. sp. cucumerinum with inhibition zone larger than 20 mm (Fig. 1a). In addition, CNS-42 showed antimicrobial activity to F. oxysporum, C. lunata, C. orbiculare, S. cerevisiae, R. solani, E. coli, A. solani, B. subtilis and S. aureus indicating a good antimicrobial spectrum.
Overview of assessment of strain CNS-42 as a potential biocontrol agent of Fusarium oxysporum f. sp. cucumerinum and a plant growth promoter. a Screening antifungal activity of microbes from TCMs and their rhizosphere soil (the host medicinal plant A. orientale; strain CNS-42; inhibition zone of strain CNS-42 against F. oxysporum f. sp. cucumerinum, grown on PDA medium for 7 days). b Growth promotion of cucumber inoculated with strain CNS-42 and/or F. oxysporum f. sp. cucumerinum. Cucumber plants grown in soil inoculated with (A1) F. oxysporum f. sp. Cucumerinum alone, (B1) F. oxysporum f. sp. Cucumerinum and CNS-42, (C1) neither F. oxysporum f. sp. cucumerinum nor CNS-42 as a control, (D1) CNS-42. c Structure of active compound staurosporine (1) isolated from CNS-42. d Growth promotion of cucumber treated with staurosporine (1). Cucumber plants grown in soil containing 0 (A2), 50 (B2), 150 (C2), and 300 (D2) μg/L.)
In vivo biocontrol assay of strain CNS-42
In the greenhouse experiment, the average shoot fresh weight and height of cucumber plants were measured a month after planting and the results are shown in Table 1 and Fig. 1b. With the application of the endophytic actinomycete strain CNS-42, the shoot fresh weight and height of cucumber were increased (P < 0.05). The average shoot fresh weight and height of cucumber (49.40 g, 78.88 cm) with treatment D1 were higher than those of treatment C1 (45.22 g, 74.94 cm), which clearly demonstrated the promotion effect of CNS-42 on cucumber growth.
The application of endophytic actinomycete CNS-42 could also decrease the impact of F. oxysporum f. sp. cucumerinum on cucumber shoot fresh weight and height significantly (P < 0.05). In treatment A1, the average shoot fresh weight and height were 31.10 g and 52.76 cm, respectively, with the introduction of the spore suspension of F. oxysporum f. sp. cucumerinum. In treatment B1, when CNS-42 was introduced to inhibit the spore suspension of F. oxysporum f. sp. cucumerinum, the shoot fresh weight and height were 41.66 g and 71.90 cm, respectively.
Even though some visual external wilt symptoms (yellowing of leaves) of plantlets in both A1 and B1 were observed and leaf chlorosis emerged firstly in older leaves and then spread to younger leaves, after 3 weeks of inoculation, treatment B1 which had endophytic actinomycete CNS-42 applied to the plant could effectively decrease the impact of F. oxysporum f. sp. cucumerinum on cucumber growth as seen from the observation of the severity of the disease a month after their planting. For A1 experiment, a total of 16 (80 %) plantlets showed wilt symptoms and the disease index reached 50. Otherwise, for B1 experiment only 9 plantlets (45 %) showed wilt symptoms and the disease index was reduced to 25 due to the introduction of CNS-42. In the case of the controls, the plantlets for D1 and C1 experiments remained healthy, without any signs of leaf chlorosis.
Characterization and identification of the isolate CNS-42
Colonies of CNS-42 grown on GT (soluble starch 2.0 %, l-asparagine 0.05 %, KNO3 0.1 %, K2HPO4·H2O 0.05 %, NaCl 0.05 %, MgSO4·7H2O 0.05 %, pH 7.5) media at 35 °C for 4 days were observed to be 2–6 mm in diameter. The colonial and morphological properties (Fig. 2) and physiological characteristics (Table S3) suggest that CNS-42 is a member of the genus Streptomyces. Substrate mycelium is light yellow in color. Aerial mycelium produced on GT medium was gray. No diffusible pigments are obviously produced. Aerial hyphae differentiate into short, curly spores chains with smooth surface.
Through 16S rRNA gene sequence analysis, an amplified fragment of about 1,500 bp was obtained and compared with sequences of the reference species of actinobacteria contained in GenBank. The results indicated that strain CNS-42 clustered within the genus Streptomyces and exhibited the closest phylogenetic affinity (Figure S2) and highest sequence similarity to Streptomyces fradiae NBRC 12773.
Structure elucidation and antibacterial activity of staurosporine
The structure of staurosporine was elucidated based on UV, MS analysis, 1H-NMR, 13C-NMR, and comparison with those of reported literature (Li et al. 2011). Staurosporine showed typical UV–Vis spectra (Figure S3) similar to those of known indolecarbazoles. A molecular formula C28H26N4O3 of staurosporine was deduced from its HRESIMS ([M + H]+ m/z 467.2099, cald. 467.2078) (Figure S4). The structure of staurosporine for the identification of specific staurosporine type could be determined by further comparing its 1H-NMR (Figure S5) and 13C-NMR (Figure S6) with those reported previously (Li et al. 2011) (Table S2).
The pure compound staurosporine showed activities with MIC values of 0.1 µg/mL against C. albicans and 12.5 µg/mL against B. subtilis respectively.
Growth promoting activity of staurosporine
Three doses of staurosporine, 50 (B2), 150 (C2), and 300 (D2) µg/L, together with a negative control (A2), were applied to the growth promotion experiments. The results demonstrated that the height, size, and number of leaves were increased in B2, C2, and D2 treatments (Fig. 1d). The height, fresh and dry biomass in the treatments of B2 and D2, i.e. 50 and 300 µg/L of staurosporine, respectively, were significantly higher than those of the control treatment (P < 0.05, Table 2). However, the results indicated that there was no growth promoting effect of staurosporine on the root biomass of plants.
Discussion
As compared to actinomycetes in the rhizosphere, endophytic actinomycetes are not subjected to competition with soil bacteria and can colonize plants well. Thus the endophytic actinomycetes can be considered as potential biocontrol agents (Cao et al. 2004). The endophytic Streptomyces plays important roles in the development and health of plants by enhancing nutrient assimilation or producing secondary metabolites (Kizuka et al. 2002; Matsukuma et al. 1994). Endophytic Streptomyces have been investigated as potential biological control agents of soil-borne fungal plant pathogens in previous studies and been revealed as a promising resource for the development of agricultural supplies (Cao et al. 2004; Nishimura et al. 2002; Shimizu et al. 2006; Taechowisan et al. 2005). Previous studies on endophytic actinobacteria discovered several Streptomyces sp. with a variety of activities against Micrococcus luteus or F. oxysporum (Sardi et al. 1992), bacteria, yeast, and filamentous fungi (Cidaria et al. 1993; Shimizu et al. 2001). Actinomycin X2 and fungichromin produced by endophytic S. galbus R-5 conferred some level of protection from diseases on seedlings of rhododendron (Shimizu et al. 2001).
However, our study revealed that the substances produced within tissues might not be the primary cause of acquired resistance of seedlings treated with strain CNS-42. Resistance in cucumber seedlings induced by the spread of CNS-42 in the soil may not be as a result of the production of antibiotics within tissues. As Yuan and Crawford (1995) indicated, there is no general relationship between in vitro antagonism of biocontrol candidate and in vivo disease suppression. Further field tests on the biocontrol effects of CNS-42 are underway to confirm the usefulness of the isolate under actual biocontrol situations.
Strain CNS-42 had a broad spectrum of activities against bacteria, yeasts and pathogenic fungi, and could protect cucumber seedlings from F. oxysporum f. sp. cucumerinum disease to a desirable degree when spread on the soil surface in pots. Based on cultural, physiological, morphological characteristics and the 16S rRNA gene analysis, CNS-42 was determined as one strain of Streptomyces sp. Further bioassay-guided isolation and purification revealed that staurosporine was responsible for its antifungal and plant growth promoting activities, which was undiscovered in previous reports. The in vivo assay was further evaluated and indicated that staurosporine showed good growth promoting effect on the aboveground biomass of cucumber growth. In the previous studies, staurosporine had been confirmed as a potent inhibitor of protein kinase C (McGlynn et al. 1992) and can induce cell apoptosis (Couldwell et al. 1994).
Our study is the first to demonstrate and report antifungal and plant growth promoting activities of staurosporine from endophytic actinobacteria. In addition, this is the first report of screening and identification of endophytic actinobacteria associated with the Chinese medicinal plant A. orientale. The present study indicates the great potentials of strain CNS-42 as a biological control agent against F. oxysporum f. sp. cucumerinum, which causes the root and stem rot disease of cucumber. The mechanism involved in plant growth promotion and the range of the plant species that respond to staurosporine are still under investigation in our lab.
References
Alexander M (1977) Introduction to soil microbiolgy. Wiley, New York
Brunk U, Collins VP, Arro E (1981) The fixation, dehydration, drying and coating of cultured cells of SEM. J Microsc 123:121–131
Cao L, Qiu Z, Dai X, Tan H, Lin Y, Zhou S (2004) Isolation of endophytic actinomycetes from roots and leaves of banana (Musa Acuminata) plants and their activities against Fusarium oxysporum f. sp. cubense. World J Microbiol Biotechnol 20:501
Castillo U, Harper JK, Strobel GA, Sears J, Alesi K, Ford E, Lin J, Hunter M, Maranta M, Ge H, Yaver D, Jensen JB, Porter H, Robison R, Millar D, Hess WM, Condron M, Teplow D (2003) Kakadumycins, novel antibiotics from Streptomyces sp. NRRL 30566, an endophyte of Grevillea pteridifolia. FEMS Microbiol Lett 224:183–190
Cidaria D, Borgonovi G, Pirali G (1993) AB023, novel polyene antibiotics. I. Taxonomy of the producing organism, fermentation and antifungal activity. J Antibiot 46:251–254
Couldwell WT, Hinton DR, He S, Chen TC, Sebat I, Weiss MH, Law RE (1994) Protein kinase C inhibitors induce apoptosis in human malignant glioma cell lines. FEBS Lett 345:43–46
de Araújo JM, Da Silva AC, Azevedo JL (2000) Isolation of endophytic actinomycetes from roots and leaves of maize (Zea mays L.). Braz Arch Biol Technol 43:447–451
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791
Fiedler HP, Bruntner C, Riedlinger J, Bull AT, Knutsen G, Goodfellow M, Jones A, Maldonado L, Pathom-aree W, Beil W, Schneider K, Keller S, Sussmuth RD (2008) Proximicin A, B and C, novel aminofuran antibiotic and anticancer compounds isolated from marine strains of the actinomycete Verrucosispora. J Antibiot 61:158–163
Goodfellow M, Williams ST (1983) Ecology of actinomycetes. Annu Rev Microbiol 37:189–216
Kimura M (1980) A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16:111–120
Kitouni M, Boudemagh A, Oulmi L, Reghioua S, Boughachiche F, Zerizer H, Hamdiken H, Couble A, Mouniee D, Boulahrouf A, Boiron P (2005) Isolation of actinomycetes producing bioactive substances from water, soil and tree bark samples of the north–east of Algeria. J Med Mycol 15:45–51
Kizuka M, Enokita R, Takahashi K, Okamoto Y, Otsuka T, Shigematsu Y, Inoue Y, Okazaki T (2002) Studies on actinomycetes isolated from plant leaves. New plant growth inhibitors A-79197-2 and -3 from Dacthylosporangium aurantiacum SANK 61299. Actinomycetologica 16:14–16
Li XB, Tang JS, Gao H, Ding R, Li J, Hong K, Yao XS (2011) A new staurosporine analog from Actinomycetes Streptomyces sp. (172614). J Asian Nat Prod Res 13:765–769
Matsukuma S, Okuda T, Watanabe J (1994) Isolation of actinomycetes from pine litter layers. Actinomycetology 8:57–65
McGlynn E, Liebetanz J, Reutener S, Wood J, Lydon NB, Hofstetter H, Vanek M, Meyer T, Fabbro D (1992) Expression and partial characterization of rat protein kinase C-delta and protein kinase C-zeta in insect cells using recombinant baculovirus. J Cell Biochem 49:239–250
Nishimura T, Meguro A, Hasegawa S, Nakagawa Y, Shimizu M, Kunoh H (2002) An endophytic actinomycete, Streptomyces sp. AOK-30, isolated from mountain laurel and its antifungal activity. J Gen Plant Pathol 68:390–397
Pavlou GC, Vakalounakis DJ (2005) Biological control of root and stem rot of greenhouse cucumber caused by Fusarium oxysporum f. sp. radicis-cucumerinum, by lettuce soil amendment. Crop Prot 24:135–140
Postma J, Stevens LH, Wiegers GL, Davelaar E, Nijihuis EH (2008) Biological control of Pythium aphanidermatum in cucumber with a combined application of Lysobacter enzymogenes strain 3.1T8 and chitosan. Biol Control 48:301–309
Qin S, Li J, Chen HH, Zhao GZ, Zhu WY, Jiang CL, Xu LH, Li WJ (2009) Isolation, diversity, and antimicrobial activity of rare actinobacteria from medicinal plants of tropical rain forests in Xishuangbanna, China. Appl Environ Microbiol 75:6176–6186
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Saravanan T, Muthusamy M, Marimuthu T (2003) Development of integrated approach to manage the fusarial wilt of banana. Crop Prot 22:1117–1123
Sardi P, Saracchi M, Quaroni S, Petrolini B, Borgonovi GE, Merli S (1992) Isolation of endophytic Streptomyces strains from surface-sterilized roots. Appl Environ Microbiol 58:2691–2693
Schippers B, Bakker AW, Bakker PAHM (1987) Interactions of deleterious and beneficial rhizosphere microorganisms and the effect of cropping practices. Annu Rev Microbiol 25:339–358
Schulz D, Nachtigall J, Riedlinger J, Schneider K, Poralla K, Imhoff JF, Beil W, Nicholson G, Fiedler HP, Sussmuth RD (2009) Piceamycin and its N-acetylcysteine adduct is produced by Streptomyces sp. GB 4-2. J Antibiot 62:513–518
Shimizu M, Furumai T, Igarashi Y, Onaka H, Nishimura T, Yoshida R, Kunoh H (2001) Association of induced disease resistance of rhododendron seedlings with inoculation of Streptomyces sp. R-5 and treatment with actinomycin D and amphotericin B to the tissue-culture medium. J Antibiot 54:501–505
Shimizu M, Meguro A, Hasegawa S, Nishimura T, Kunoh H (2006) Disease resistance induced by nonantagonistic endophytic Streptomyces ssp. on tissue-cultured seedlings of rhododendron. J Gen Plant Pathol 72:351–354
Shirling EB, Gottlieb D (1966) Methods for characterization of Streptomyces species. Int J Syst Evol Microbiol 16:313–340
Shomura T, Yoshida J, Amano S, Kojima M, Inouye S, Niida T (1979) Studies on Actinomycetales producing antibiotics only on agar culture. I. Screening, taxonomy and morphology–productivity relationship of Streptomyces halstedii, strain SF-1993. J Antibiot 32:427–435
Strobel GA, Miller RV, Martinez-Miller C, Condron MM, Teplow DB, Hess WM (1999) Cryptocandin, a potent antimycotic from the endophytic fungus Cryptosporiopsis cf. quercina. Microbiology 145:1919–1926
Taechowisan T, Lu C, Shen Y, Lumyong S (2005) Secondary metabolites from endophytic Streptomyces aureofaciens CMUAc130 and their antifungal activity. Microbiology 151:1691–1695
Tahvonen R (1982) Preliminary experiments into the use of Streptomyces spp. isolated from peat in the biological control of soil and seed-borne diseases in peat culture. J Sci Agric Soc Finl 54:357–369
Thakur D, Yadav A, Gogoi BK, Bora TC (2007) Isolation and screening of Streptomyces in soil of protected forest areas from the states of Assam and Tripura, India, for antimicrobial metabolites. J Med Mycol 17:242–249
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Vakalounakis DJ, Wang Z, Fragkiadakis GA, Skaracis N, Li DB (2004) Characterization of Fusarium oxysporum isolates obtained from cucumber in China by pathogenicity, VCG, and RAPD. Plant Dis 88:645–649
Wei L, Jiang CH, Shu JW (2008) Growth-promotion and biocontrol of cucumber fusarium wilt by marine Bacillus subtilis 3512A. J Shenyang Agric Univ 39:182–185
Wei YZ, Zhang YQ, Li QP, Yu LY, ZY Q (2009) Screening and identification of two rare actinomycetes exhibiting antimicrobial activity. J Biol 26:7–9
Williams ST, Wellington EMH (1982) Actinomycetes. In: Page AL, Miller RH, Keency OR (eds) Methods of soil analysis, part 2, chemical and microbiological properties, 2nd edn. American Society of Agronomy/Soil Science Society of America, Madison, pp 969–987
Xian CW, Peng HZ (2008) Effects of arbuscular mycorrhizal fungi on fusarium wilt of cucumber seedlings. Mycosystema 27:395–404
Xue LC, Guang HJ, Jian W, Bao LL (2008) Biocontrol effect of Paenibacillus polymyxa BRF21 and Bacillus subtilis BRF22 on fusarium wilt disease of cucumber and tomato. Chin J Eco-Agric 16:446–450
Yuan WM, Crawford DL (1995) Characterization of Streptomyces lydicus WYEC108 as a potential biocontrol agent against fungal root and seed rots. Appl Environ Microbiol 61:3119–3128
Yuan LJ, Zhang GL, Zhang YQ, Yu LY, Zhang YQ (2009) Preliminary research about population diversity and bioactivity of rhizosphere actinomycetes from medicinal plant. Chin J Antibiot 34:463–504
Zhang L, Yan K, Zhang Y, Huang R, Bian J, Zheng C, Sun H, Chen Z, Sun N, An R, Min F, Zhao W, Zhuo Y, You J, Song Y, Yu Z, Liu Z, Yang K, Gao H, Dai H, Zhang X, Wang J, Fu C, Pei G, Liu J, Zhang S, Goodfellow M, Jiang Y, Kuai J, Zhou G, Chen X (2007) High-throughput synergy screening identifies microbial metabolites as combination agents for the treatment of fungal infections. Proc Natl Acad Sci USA 104:4606–4611
Zhao K, Penttinen P, Guan T, Xiao J, Chen Q, Xu J, Lindstrom K, Zhang L, Zhang X, Strobel GA (2011) The diversity and anti-microbial activity of endophytic actinomycetes isolated from medicinal plants in Panxi plateau, China. Curr Microbiol 62:182–190
Acknowledgments
The authors are grateful to Dr. Qi Li of Chinese Academy of Sciences and Prof. Isoamro Yamaguchi of The University of Tokyo, for kindly correcting this manuscript. This work was supported in parts by the National Program on Key Basic Research Project (2013AA102802, 2013ZX10005004 and 2011ZX11102-011-11), the Chinese National Natural Science Fund (31070004, 81102369, 30911120483, 81102356, 30901849, 30973665, 30911120484) and the specialized research fund for the doctoral program of higher education 20060626006. LZ is an Awardee for National Distinguished Young Scholar Program in China. Krishna Bolla is thankful to CAS and TWAS for the financial support.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Xiaolin Li and Pei Huang have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, X., Huang, P., Wang, Q. et al. Staurosporine from the endophytic Streptomyces sp. strain CNS-42 acts as a potential biocontrol agent and growth elicitor in cucumber. Antonie van Leeuwenhoek 106, 515–525 (2014). https://doi.org/10.1007/s10482-014-0220-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10482-014-0220-6