Abstract
Rice, a staple food crop worldwide, suffers devastating yield losses as a result of blast disease caused by Magnaporthe oryzae Cav. The adverse effects of chemicals on the environment are rising concerns for sustainable and eco-friendly approaches. The use of antagonistic microbes for the management of rice blast appears to be a sustainable solution to this challenge. Herein, we isolated 20 Streptomyces strains from rice rhizosphere, among which the isolate STR-2 exhibited maximum inhibition of mycelial growth of M. oryzae accounting for 50% reduction over control. The isolate STR-2 was identified as S. chrestomyceticus through 16S rRNA gene sequencing. In vitro tests demonstrated its ability to produce antifungal and bioactive compounds and also synthesize siderophore, IAA, and phosphate-solubilizing agents, thereby promoting plant growth upon inoculation on rice seeds. GC–MS analysis showed the presence of volatiles, antifungal, antimicrobial, and antioxidant compounds with different retention times. The crude antibiotic extract of 0.5% of S. chrestomyceticus STR-2 reduced the mycelial growth of M. oryzae over the control. Application of talc-based formulation of Streptomyces chrestomyceticus STR-2 resulted in the least disease incidence (15.89%) with the highest disease reduction of 65.26% over untreated control under field condition. These findings indicate the potential of S. chrestomyceticus as a potential bio-inoculant against rice blast disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rice (Oryza sativa) is the most valuable staple food crop for more than 50% of the global population. Various biotic and abiotic constraints occur during the cultivation of rice crop, such as insect pests, diseases, weeds and drought. Among the diseases, rice blast caused by Magnaporthe oryzae (anamorph Pyricularia oryzae Cav.) is considered as one of the most important phytopathogens which incurs huge economic loss to the farmers [1]. The annual yield losses due to rice blast ranges from 10 to 30% which can extend up to 50% during disease epidemics [2]. Rice blast disease outbreaks are a serious and recurrent problem in all rice growing regions of the world. The pathogen infects aerial parts of plant, including leaves, nodes, panicles and grains at all stages of development and is difficult to manage during the advanced stages [3]. When the pathogen attacks at grain developmental stage, it causes chaffiness, incomplete grain filling and poor grain quality which directly affects the yield. Various chemicals are used for the management of this disease, which leads to development of fungicide-resistant strains and creates environmental impacts [4].
As an alternative, biological control is a promising strategy, which can be exploited for the management of rice blast. Biological control agents protect the crop from harmful pathogens through production of antifungal metabolites or inducing systemic resistance in the host [5]. Of late, bacterial bioagents viz., Pseudomonas and Bacillus, are used for the management of various diseases of agricultural crops [6]. Among the alternate microbial candidates, actinobacteria are natural soil dwelling bacteria used for disease management program [7]. They can able to survive under extreme environmental conditions [8]. Among actinobacteria, the genus Streptomyces is a gram-positive, aerobic, non-motile, catalase positive and non-acid fast bacteria with filamentous nature that resembles fungi. Streptomyces spp. shows several modes of action by the production of antibiotics, cell wall-degrading enzymes, secondary metabolites and growth-promoting compounds. About 75% of commercially available antibiotics are derived from Streptomyces spp. [9]. Notably, some antibiotics viz, Blasticidin–S and Kasugamycin produced by Streptomyces have been used as fungicides to control rice blast, which were safely used with low mammalian toxicity and lack of phytotoxicity toward rice plants [10]. Spraying mixture of spore suspension of the pathogen M. oryzae and Streptomyces sindeneusis isolate 263 on rice seedlings resulted in strong inhibition of the pathogen and suppression of leaf symptoms [11]. An antibiotic, antifungalmycin 702 isolated from Streptomyces padanus JAU4234 inhibited the mycelial growth of M. grisea and reduced the formation of conidia and suppressed the conidial germination and appressorium formation [12]. The cell extract of Streptomyces erythrochromogenes 3–45 showed inhibitory activity against M. oryzae by abnormal formation of appressorium and suppressed blast lesions [13]. A novel bioactive compounds such as albofungin and chloroalbofungin was identified from Streptomyces chrestomyceticus BCC 24770 that showed strong antifungal activity against Curvularia lunata, A. brassicicola, Colletotrichum capsici, and Colletotrichum gloeosporioides [14]. Thus, Streptomyces strains have important application in agricultural field as biological control agent against various phytopathogenic fungi. This approach offers a better alternative to control rice blast disease which is exploited in this study.
Materials and Methods
Isolation and Characterization of Pathogen
The plant samples infected with rice blast were collected and isolated by tissue segment method using Potato Dextrose Agar (PDA) medium. The colony characters were observed for cultural identification. The obtained pure culture was inoculated in sterilized stem bits of collateral weed host (Digitaria sanguinalis) to induce sporulation of pathogen. The morphological characters such as mycelial type and structure of conidia were observed under light microscope. Pathogenicity test was conducted to prove the Koch’s postulate and to identify the virulent isolate. The total genomic DNA extraction was carried out by following standard protocol [15] and subjected to PCR amplification using universal primer pairs, ITS1 (TCGGTAGGTGAACCTGCGG) and ITS4 (TCCTCCGCTTATTGATATGC) [16]. The amplified PCR product was sequenced and the species was identified based on the percentage homogeneity and the molecular phylogeny was evaluated using MEGA 7 [17].
Isolation and Characterization of Actinobacteria
Random rhizospheric samples of healthy rice plants were collected from different geographical locations, which was air-dried, pre-heated, and serially diluted up to 10–5 dilution and plated on Ken Knight’s agar medium (1 g glucose, 0.1 g MgSO4, 0.1 g KCl, 0.1 g (NH4)2SO4, 0.1 g KH2PO4, 20 g Agar, 1000 ml water) by pour plate method and incubated at 28 °C [18]. After 5 days of incubation, the colonies showing powdery growth were selected and streaked on fresh medium to obtain pure culture. The actinobacteria was identified by cultural, morphological, and biochemical means as per the standard protocol of Bergey’s manual of systematic bacteriology [19].
In Vitro Screening of Actinobacterial Isolates Against M. oryzae
Dual-Culture Technique
The antagonistic effect of actinobacteria against the pathogen was tested by dual-culture technique [20]. A 9 mm diameter of actively growing culture disc of M. oryzae was cut and placed at one side and a gentle streak of actively growing actinobacteria was done on the other side of the sterile Petri plate containing PDA medium. Three replications were maintained in each treatment. The Petri plate containing the pathogen alone was used as control. The plates were incubated at room temperature and the radial growth of the pathogen in the treatment was measured when the control plate was fully grown. The percent growth inhibition is calculated using the following formula,
where C represents radial growth of the pathogen in control and T represents radial growth of the pathogen in treatment.
Bioassay of Crude Extract of Actinobacteria Against Mycelial Growth of Pathogen by Agar Well Method
The effective five actinobacterial isolates viz., STR-1, STR-2, STR-10, STR-17, and STR-18 were selected and tested for bioassay of crude extract by agar plate method. The molten PDA medium was poured in sterile Petri plate and allowed to solidify. Four wells were formed in the Petri plate containing PDA medium leaving 1 cm from the periphery using 9 mm sterilized cork borer. The actively growing M. oryzae was kept in the center of the Petri plate. The crude extract was poured into the wells at 100 µl (0.1%), 200 µl (0.2%), and 500 µl (0.5%) per well. A control well was maintained by pouring sterile water. The mycelial growth and inhibition zone were recorded after 8 days of incubation.
Molecular Characterization of Isolate STR-2
DNA Isolation
The pure culture of actinobacterial isolate STR-2 was grown on Ken Knight’s broth and incubated in an orbital shaker for 7 days. The bacterial suspension was then transferred to 1.5-ml Eppendorf tube and centrifuged at 8000 rpm for 10 min. After centrifugation, the supernatant was discarded and the pellet was resuspended in 500 µl of 1X TE buffer (pH-8.0) and 30 µl of 10% SDS, mixed thoroughly, and then incubated for 1 h at 37 °C. Then, 100 µl of 5 M NaCl and 80 µl of CTAB were added and incubated at 65 °C for 10 min. An equal volume of chloroform was added and centrifugation was carried out at 10,000 rpm for 15 min. The aqueous, viscous supernatant was transferred to a new centrifuge tube and equal volume of ice-cold isopropanol was added and incubated for 1 h at −20 °C. DNA pellet was obtained by centrifugation at 12,000 rpm for 10 min. The pellet was washed with 70% ethanol and air-dried. The supernatant was carefully removed and the pellet was dried briefly. The pellet was redissolved in 50 µl of Milli-Q water and stored at −20 °C.
Amplification of 16S rRNA Gene of Isolate STR-2
The isolated DNA was subjected to PCR amplification using the primers 27F (5′AGAGTTTGATCCTGGCTCAG 3′) and 1492R (5′GGTTACCTTGTTACGACTT 3′) [21]. The PCR conditions were set at initial denaturation at 95 °C for 5 min; 32 cycles of denaturation at 95 °C for 30 s, annealing temperature at 55 °C for 90 s, initial extension at 72 °C for 120 s and final extension at 72 °C for 5 min. The PCR products were resolved by electrophoresis in 1% agarose gel. The amplified PCR product was sequenced at Chromous Biotech Pvt. Ltd, Bangalore, India.
Phylogenetic and Sequence Analyses
The sequence was submitted to NCBI, GenBank and accession number was obtained. From the BLAST search, 6 closely related species of Streptomyces were selected. The nucleotide sequences obtained were aligned together to deduce the complete sequence and used for multiple sequence alignment using CLUSTALW program. The phylogenetic tree was constructed using 16S rRNA gene sequence from the GenBank, evaluated using the neighbor-joining method with 1000 bootstrap replicates using MEGA 7 [17].
Effect of Cell-Free Culture Filtrate of S. chrestomyceticus STR-2 Against Mycelial Dry Weight of M. oryzae
The effect of culture filtrate of S. chrestomyceticus STR-2 on mycelial growth of pathogen was tested [22]. The culture was inoculated in Ken Knight’s broth and incubated for 5 days in shaker at 28 ± 2 °C for 150 rpm. It was then subjected to centrifugation at 10,000 rpm for 10 mins and the supernatant was collected to obtain cell-free culture (CFC). The CFC was filtered through bacterial proof filter (0.2 µm pore size) and 5 ml of the filtrate was poured to 95 ml sterilized Potato dextrose broth (PDB) in 250 ml conical flask. A 9 mm of actively growing mycelial disc of M.oryzae was aseptically inoculated into it, which served as treatment. The PDB with mycelial disc of M. oryzae alone without any culture filtrate is used as control and incubated at 28 ± 2 °C for 7 days. After incubation period, the mycelia were taken and dried in oven at 60 °C for 8 h and the results were expressed on dry weight basis and compared with control using the formula,
where W1 is the mycelial dry weight of the pathogen in control and W2 is mycelial dry weight of the pathogen in treatment.
Effect of Volatile Compounds of S. chrestomyceticus STR-2 Against M. oryzae
The effect of volatile organic compounds (VOCs) produced by S. chrestomyceticus STR-2 against M. oryzae was tested [23]. The isolate was allowed to grow on yeast malt extract medium until full growth was observed. The lids of the plate were then replaced with PDA medium containing 9 mm actively growing mycelial disc of M. oryzae. The two plates were covered together by face to face by covering with parafilm ensuring no physical contact between pathogen and antagonistic actinobacteria. The control plate was maintained with pathogen alone without any actinobacteria-seeded plates. The experiment was repeated thrice. The mycelial growth reduction of the pathogen was measured after the control plate achieved full growth, using the following formula,
where C represents radial growth of the pathogen in control and T represents radial growth of the pathogen in treatment.
GC–MS Analysis of Crude Extract of S. chrestomyceticus STR-2
The most effective antagonistic S. chrestomyceticus STR-2 was cultured at 28 ± 2 °C for 5 days in yeast malt extract broth. After incubation period, the broth was centrifuged at 5000 rpm and the supernatant was adjusted to pH 2.0 using concentrated HCl. An equal volume of ethyl acetate was added with the collected supernatant and kept overnight in orbital shaker at 150 rpm. Then, the organic solvent layer was separated using separating funnel. The separated solvent was filtered using filter paper with a pinch of sodium sulfate and evaporated in rotary evaporator up to the volume of 5 ml. The residue was resuspended with ethyl acetate (HPLC graded), filtered using filter paper with a pinch of sodium sulfate. Using bacterial membrane filter (0.2 µm), the precipitate was filtered. The antibiotics, volatiles, and secondary metabolites present in the sample were detected by injecting 1 µl of sample in Capillary Standard Non-Polar Column (0.25 mm × 0.25 µm × 30 m length) of GC—MS (Perkin Elmer Ltd.) in which Helium was used as carrier gas (1.0 ml/min). The m/z peaks representing mass-to-charge ratio, characteristic of the antimicrobial fractions was compared with those of the corresponding organic compounds in the NIST library [24].
Evaluation of S. chrestomyceticus STR-2 for Its Growth Promotion Activity
Production of IAA
IAA production test was carried out for the isolate S. chrestomyceticus (STR-2) by preparing 20 ml volume of Ken Knight’s broth amended with 0.1% tryptophan which served as treatment. The broth without 0.1% tryptophan was maintained as control and sterilized. The broths were inoculated with loop full of STR-2 culture and incubated at 28 ± 2 °C for 5 days. After incubation period, the cell-free culture was prepared by centrifugation at 10,000 rpm for 10 min. The supernatant (1 ml) was added with 2 ml of Salkowski reagent (1 ml of 0.5 M FeCl2 in 50 ml of 35% perchloric acid) and kept in dark for 30 min. Development of pink color indicates the production of IAA which was quantified by spectrophotometer at 530 nm wavelength and expressed as µg/ml using a standard curve [25].
Siderophore Production
Siderophore production by S. chrestomyceticus STR-2 was tested on Chrome Azurol S blue agar (CAS) as described [26]. The full-grown STR-2 culture was streaked on CAS plates and incubated at 30 °C for 5 days in dark condition. Yellow to orange-colored zone around the colony indicates the production of siderophore.
Phosphate Solubilization
The actinobacterial isolate S. chrestomyceticus STR-2 was tested for phosphate solubilization using Pikovskaya’s agar medium [27]. A loop full of fully grown actinobacterial culture was streaked on sterile Petri plate containing Pikovskaya’s agar medium and incubated at 30 °C for 4 days. A clear halo zone surrounding the colony indicates solubilization of phosphate.
Assessing Growth-Promoting Activity by Roll Towel Method
The plant growth-promoting activity was assessed based on seedling vigor index using the standard roll towel method [28]. Fifty paddy seeds were surface sterilized with 1% Sodium hypochlorite solution for 2 min, subsequently washed with sterile water thrice, and then dried. The seeds were then treated with the S. chrestomyceticus STR-2 suspension at 106 cfu/ml containing 100 mg of Carboxy Methyl Cellulose (CMC). The seeds were soaked for 12 h and kept over pre-soaked germination paper and rolled along with the seeds, which served as the treatment. The seeds soaked in sterile water served as control. They were incubated in growth chamber for 10 days. The germination percentage of seeds, root length, and shoot length of individual seedlings were measured and the vigor index is calculated using the formula,
Efficacy of S. chrestomyceticus STR-2 Against Rice Blast Disease in Pot Culture
Based on the results obtained from in vitro studies, pot culture experiment was conducted for the management of rice blast disease using talc formulation of S. chrestomyceticus STR-2 [29]. For the preparation of formulation, 400 ml of 48 h old culture broth of S. chrestomyceticus STR-2 was mixed with 1 kg of sterile talc and 10 g of Carboxy Methyl Cellulose (CMC) and then stored. The pot culture experiment was conducted on susceptible cv. ASD 16 for two seasons (Kharif and Rabi season) in the year 2020. The experiment was carried out in a randomized block design with 10 treatments replicated thrice. For each replication, three plants/pot were maintained. The seeds were treated with talc formulation @ 10 g/kg of seed at the time of sowing. Seedling root dip of S.chrestomyceticus STR-2 was done @ 10 g/l at the time of transplanting. Soil application was carried out at 30 days after transplanting @ 10 g/pot by thoroughly mixing with well-decomposed FYM. Foliar application was given @ 0.5% on 50 days after transplanting. Tricyclazole @ 1 g/l was used as a chemical check. The plants were sprayed with spore suspension of M. oryzae (106 cfu/ml) at active tillering stage. The plants without any treatment but inoculated with M. oryzae alone served as control. The disease incidence was recorded on 30 DAT and the percent reduction over control was calculated [11].
Efficacy of S. chrestomyceticus STR-2 on Rice Blast Disease Under Field Condition
An experimental field trial was carried out in a farmer’s field at Muppathuvetti village in Ranipet district in a blast susceptible rice cv. ADT 47 to evaluate the efficacy of S. chrestomyceticus STR-2 against rice blast disease. Ten treatments were replicated thrice by following randomized block design. Talc-based formulation of S. chrestomyceticus STR-2 was applied through seed treatment, seedling root dip, soil application, and foliar spray. Seed treatment was done by treating paddy seeds with talc-based formulation of S. chrestomyceticus @ 10 g/kg of seeds. Seedling root dip was done by dipping paddy seedlings while transplanting in talc-based formulation of S. chrestomyceticus @ 10 g/l of water. Soil application was carried out at 30 days after transplanting @ 2.5 kg/ha mixed with well-decomposed FYM. Foliar spray @ 0.5% was given at 50 DAT. Tricyclazole @ 1 g/l was used as a chemical check. A control plot was also maintained. The percent disease index and yield obtained in each experiment were calculated.
Statistical Analysis
The data were statistically analyzed using the AGRES version 92. The percentage values of the disease incidence are arcsine-transformed. Data were subjected to analysis of variance (ANOVA) at two significant levels (P < 0.05 and P < 0.01) and means were compared by Least significant difference (LSD).
Results
Isolation and Characterization of Pathogen
A total of fifteen pathogenic isolates were characterized based on colony color, melanin production, colony morphology, and conidial characters. The conidia were small and pyriform in shape with 2 septate, 3 celled in which the middle cell was broader than the other two cells. By performing pathogenicity test, the isolate IS (Kar)-6 was found to be more virulent, which was further characterized through PCR amplification of genomic DNA using ITS1 and ITS4 universal primer pairs. The results revealed an amplicon of about 560 bp in agarose gel electrophoresis which was sequenced. The sequence obtained was deposited in GenBank with the accession number MN960043. Similar sequences were retrieved from NCBI, GenBank database and dendrogram was constructed using UPGMA method (Fig. S1).
Isolation and Characterization of Actinobacteria
A total of twenty isolates of actinobacteria were isolated from rhizosphere region. The growth rate of isolated actinobacteria varied from moderate to good and the colony color varied from white to brown color. It took 5 to 6 days to sporulate and chain of spores was observed in all the isolates under 40X magnification of light microscope. The isolated actinobacteria gave positive reaction to gram staining test by retaining crystal violet color.
In Vitro Screening of Actinobacterial Isolates Against M. oryzae
Dual-Culture Technique
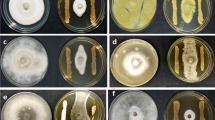
Twenty isolates of actinobacteria were screened in vitro against the mycelial growth of M. oryzae by dual-culture technique. Among the twenty isolates, the isolate STR-2 exerted maximum mycelial growth inhibition (50%) depicting 45 mm of colony diameter of the pathogen compared to control (90 mm). This was followed by STR-1 and STR-18 with 47.78% inhibition. The lowest inhibition was recorded in the isolate STR-14 with 59 mm mycelial growth recording 34.44% inhibition over control (Fig. 1a and b).
a and b In vitro efficacy of actinobacteria against the mycelial growth of M. oryzae by dual-culture assay. Plates were incubated at room temperature at 28 ± 2 °C for 7 days. Each value is the mean of three replications. Error bars indicate standard deviation obtained from three replications per treatment
Bioassay of Crude Extract of Actinobacteria Against Mycelial Growth of Pathogen by Agar Well Method
The five effective isolates were tested against the mycelial growth of M. oryzae by agar well method and incubated until the control plate was completely grown. The crude antibiotic extract from STR-2 showed reduced mycelial growth (1.3 cm) @ 0.5% over the control. The least effect was noticed in isolate STR-17 (2.6 cm) (Fig. 2a and b).
Molecular Characterization of Isolate STR-2
The effective actinobacterial isolate STR-2 was subjected to molecular characterization to confirm the isolate at species level. PCR amplification was done using 27F and 1492R primer pairs, the amplified fragment of 1500 bp which was sequenced further. The sequence was BLAST searched in NCBI database, which showed 100% nucleotide sequence identity to S. chrestomyceticus and was deposited in GenBank with accession number MW505980. Similar sequences were retrieved from the NCBI, GenBank database and dendrogram tree was constructed using UPGMA method by comparing the study isolate with the existing isolates from the database (Fig. 3).
Phylogenetic tree of S. chrestomyceticus STR-2 small subunit ribosomal RNA gene with other nucleotide sequences from the GenBank. rDNA homology searches were performed using the BLAST program and the sequences were submitted to GenBank. Clustering was determined by UPGMA analysis and the nucleotide sequences were aligned using CLUSTAL X 1.81. Superscript T denotes the type strains
Effect of Cell-Free Culture Filtrates of S. chrestomyceticus STR-2 on Mycelial Dry Weight of M. oryzae
The effect of culture filtrate of S. chrestomyceticus STR-2 on mycelial weight of the pathogen was performed. It was found that the reduction in mycelial dry weight (0.034 g) was observed in STR-2 compared to control (0.208 g). The percent dry weight was found to be 83.65% (Fig. 4).
Effect of Volatile Compounds Produced by S. chrestomyceticus STR-2 Against M. oryzae
The effective actinobacterial isolate S. chrestomyceticus STR-2 was tested for the effect of volatile compounds against the pathogen. The results revealed that S.chrestomyceticus STR–2 inhibited the mycelial growth (2.1 cm) of M. oryzae (76.66% reduction over the control) (Fig. 4).
GC–MS Analysis of Crude Extract of S. chrestomyceticus STR-2
The GC–MS analysis of actinobacterial isolate S. chrestomyceticus STR-2 confirmed the presence of antimicrobial compounds and volatiles with different retention times. The results revealed the presence of antimicrobial compounds such as Undecane, n-Hexadecanoic acid, Benzamide, Pentadecane, Eicosane, Tetradecanoic acid, 1, 2-Benzene dicarboxylic acid, Heneicosane, Nonadecane 1-chloro, and 1-heptacosanol (Table 1).
Evaluation of S. chrestomyceticus STR-2 for Its Growth Promotion Activity
Production of IAA
The antagonistic isolate S. chrestomyceticus STR-2 was tested for the production of IAA. Color change from pale yellow to deep yellow was observed, which indicates positive response for the production of IAA (Fig. 5a). Quantification of the IAA production was performed in S. chrestomyceticus STR-2 (11.59 µg/ml) against the control (1.83 µg/ml) (Fig. 5b).
Evaluation of S. chrestomyceticus STR-2 for its growth-promoting activity. The effective actinobacteria isolates were tested for their growth promotion attributes viz., IAA production, phosphate solubilization, siderophore production, germination percentage, root length, shoot length, and vigor index by roll towel method. Error bars indicate standard deviation obtained from three replications per treatment
Production of Siderophore
The siderophore production by antagonistic actinobacterial isolate S. chrestomyceticus STR-2 was tested using CAS medium. The S. chrestomyceticus STR-2 changed blue color of the medium to light yellowish fluorescent color indicating the positive reaction for the production of siderophore (Fig. 5a).
Phosphate Solubilization
The antagonistic isolate S. chrestomyceticus STR-2 was tested for phosphate solubilization by Pikovskaya’s agar medium which showed positive reaction by the formation of halo clear zone around the colony (Fig. 5a).
Assessing Plant Growth-Promoting Activity by Roll Towel Method
The roll towel method was followed to evaluate the growth-promoting efficiency of S. chrestomyceticus STR-2. The seeds pre-treated with the bacterial suspension of S. chrestomyceticus STR-2 showed improvement in plant growth-promoting parameters viz., germination %, root length, shoot length, and vigor index over untreated seeds (Fig. 5c).
Efficacy of Antagonistic S. chrestomyceticus STR-2 on Blast Disease of Rice Under Pot Culture
The effective antagonistic S. chrestomyceticus STR-2 was used in different combination to combat rice blast disease in pot culture experiment. The experimental results stated that the treatment T8 consisting of seed treatment of S. chrestomyceticus STR-2 @ 10 g/kg of seed + seedling root dip @ 10 g/l of water + soil application at 30 days after transplanting @ 10 g/kg of soil + foliar spray at 50 days after transplanting @ 0.5% recorded the least disease incidence of 14.62 (79.72% reduction over control). This was followed by treatment T8 which consists of seedling root dip @ 10 g/l of water + soil application at 30 days after transplanting @ 10 g/kg of soil + foliar spray at 50 days after transplanting @ 0.5% which recorded 17.89 PDI (75.19% disease reduction over control). Foliar spray of tricyclazole @ 1 g/l of water recorded 12.35 PDI resulting in 82.87% disease reduction over control. The treatment T8 showed significant mean difference, thus outperforming the disease reduction among all the other treatments in this experiment (Fig. 6).
Effect of talc-based actinobacterial formulation of S. chrestomyceticus STR-2 on blast disease in rice cv. ASD 16 under pot culture. Data are presented as percent disease index and represents the mean value for two season trials. Error bars indicate standard deviation obtained from 20 replicates per treatment
Effect of Antagonistic S. chrestomyceticus STR-2 on the Management of Blast Disease of Rice Under Field Condition
Among all the treatments, the treatment T8 consisting of seed treatment with S. chrestomyceticus STR-2 @ 10 g/kg of seed + seedling root dip @ 10 g/l of water + soil application at 30 days after transplanting @ 2.5 kg/ha + foliar spray at 50 days after transplanting @ 0.5% recorded least disease index of 15.89% with highest disease reduction of 65.26 over control. This was followed by the treatment T7, consisting of seedling root dip @ 10 g/l of water + soil application at 30 days after transplanting @ 2.5 kg/ha of soil + foliar spray at 50 days after transplanting @ 0.5% which recorded 19.55 PDI with 57.25% disease reduction over control. The comparative check with foliar spray of tricyclazole @ 1 g/l of water recorded 71.09% reduction of disease over control with PDI of 13.22. The treatment T8 was significantly different from all the other treatments tested. The treatment T8 also registered a maximum yield of 5800 kg/ha over untreated control (3900 kg/ha). The treatment T7 and T6 succeeded the treatment T8 with the yield of 5500 and 5350 kg/ha, respectively (Table 2).
Discussion
Rice (O. sativa L) is known for “global grains” grown over an area of 149 million hectares under diverse cultural conditions which constitute 689 million tons consumed by more than half of global population [30]. Among diseases, rice blast caused by M. oryzae is one of the most destructive diseases that cause huge economic loss to the farmers. Streptomyces spp. has the potential to control phytopathogens by producing of various secondary metabolites [31]. Hence, this study focuses on systematic screening and analysis of Streptomyces isolates to investigate their potential to control rice blast fungus.
In the present research, 15 rice blast pathogen isolates were cultured from different geographical locations which were further confirmed by cultural and morphological characters [32]. Molecular identification was done for the most virulent pathogen using ITS1 and ITS4. About 20 actinobacterial strains were isolated from rhizosphere samples. Diverse cultural characters were observed and chain of spores was observed under light microscope which is the characteristic feature of Streptomyces [33]. These isolates were screened against rice blast pathogen.
Among the twenty actinobacterial isolates tested, all the isolates showed a wide spectrum of action against rice blast pathogen in vitro by dual-culture technique. Among which, five effective actinobacterial isolates were screened further to identify the potential one. Members of the genus Streptomyces have been reported to produce volatile organic compounds as a tool for inhibition of pathogens. By the paired plate technique, the most effective actinobacterial isolate STR-2 showed 76.66% reduction over control by the production of volatiles [34]. The effect of culture filtrate of efficient isolate STR-2 against the mycelial dry weight of M. oryzae revealed that the reduction in the mycelial weight by 83.65% [35]. The isolate which showed potent antimicrobial activity was characterized by 16S rRNA sequence analysis to assign a taxonomic unit [36]. Accordingly, the culture STR-2 represents a novel species of the genus Streptomyces, S. chrestomyceticus.
The application of GC–MS was employed in identifying novel bioactive secondary metabolites present in S. chrestomyceticus STR-2 using ethyl acetate as solvent and the presence of novel antimicrobial compounds were detected. The compound, Undecane having antifungal activity and Hexadecanoic acid having antioxidant and antimicrobial property against rice blast were reported [37]. The effect of crude extract of actinobacteria was tested by agar well method. The result revealed that the crude extract from the isolate S. chrestomyceticus STR-2 exhibited 85.55% reduction over control at 0.5% concentration. Our results are in accordance with the bioactive compounds produced by Streptomyces sp. UPMRS4 which showed 55.3% to 98.33% mycelial inhibition of P. oryzae compared to control [38].
The plant growth-promoting attributes of actinobacteria such as the production of siderophores, solubilization of inorganic phosphate, and phytohormone production such as IAA were tested in vitro [39]. Growth promotion was assessed by pre-inoculation of rice seeds with S. chrestomyceticus STR-2 which resulted in increased germination %, root length, and shoot length compared to control [40]. The results of pot culture experiment revealed that the combination of treatment viz., seed treatment, seedling root dip, soil application, and foliar spray with the isolate STR-2 S. chrestomyceticus exhibited high antifungal activity, thereby reducing the rice blast. A study reported that Streptomyces strain PM5 produce antifungal compound, SPM5C-1 which completely inhibited the mycelial growth of P. oryzae at 25 µg/ml concentration in vitro and 76.1% disease reduction at 500 µg/ml under greenhouse condition [41]. The rice seedlings treated with actinobacterial isolates JSN1.9, SKB2.14 and SKB2.3 resulted in disease reduction of 88.11%, 88.02%, and 87.6%, respectively, upon 21-day post-inoculation of pathogen [11]. Upon screening of four different Streptomyces strains (W1, PC 12, D 4.1, and D 4.3) against rice blast under greenhouse condition, the strain PC 12 showed the lowest disease severity (31.4%) compared to control (87.5%) [42].
In the present study, the effect of S. chrestomyceticus STR-2 was evaluated under field condition which revealed that the combined treatment viz., seed treatment, seedling root dip, soil application, and foliar spray recorded the highest disease reduction (65.26%) compared to control. A similar result was reported in Streptomyces strain UPMR54 that suppressed blast disease (67.9%) and promoted rice growth and yield [43]. The foliar treatment of Streptomyces endus OsiSh-2 at 107 spores/ml reduced the blast severity by production of mycolytic enzymes, IAA, and antibiotics [44]. The foliar treatment of rice seedlings with culture filtrate of Streptomyces hygroscopicus OsiSh-2 showed disease reduction of 28.3% compared to control [45]. Similarly, the isolate Streptomyces albidoflavus OsiLf-2 showed least disease severity of rice blast under field condition [46]. Seven actinobacterial isolates having chitinolytic activity were evaluated, which resulted in inhibition of rice blast disease ranging from 17.7 to 72.5% [47]. A novel strain called Streptomyces xantholiticus inhibited the pathogen in vitro (92%) and in vivo. Moreover, the treated plants showed enhancement of resistance by the production of defense enzymes, such as polyphenol and peroxidase [48]. In a study, it was reported that the strain Streptomyces hygroscopicus OsiSh-2 exhibited strong antagonistic activity toward M. oryzae by hydrolase activity, induction of ROS, and biofilm formation in response to toxin produced by the pathogen (Mo-toxin) [49]. In a recent study, a crude extract of lipopeptides (CEL) was extracted from fermentation liquid of Streptomyces bikiniensis HD-087 which inhibited spore germination and appressorial formation of M. oryzae by destroying membrane integrity and through leakage of cellular components in vitro and reduced the disease index of blast by 76.9% in vivo [50]. Thus, Streptomyces spp. with antimicrobial, chitinolytic, and growth-promoting activity can be exploited for the management of rice blast.
Conclusion
It is evident that rhizospheric actinomycete, S. chrestomyceticus STR-2 suppressed rice blast pathogen, M. oryzae both under in vitro and in vivo conditions by several mechanisms, such as volatiles, production of secondary metabolites, and plant growth-promoting activity. The ability of biocontrol agents to promote plant growth is considered as an added advantage. Hence, S. chrestomyceticus STR-2 could be used as potential biocontrol agent of rice blast.
References
Dean R, Van Kan JA, Pretorius ZA, Hammond-Kosack KE, Di Pietro A, Spanu PD, Rudd JJ, Dickman M, Kahmann R, Ellis J (2012) The top 10 fungal pathogens in molecular plant pathology. Mol Plant Pathol 13:414–430. https://doi.org/10.1111/j.1364-3703.2011.00783.x
Ashkani S, Rafii MY, Shabanimofrad M, Miah G, Sahebi M, Azizi P, Tanweer FA, Akhtar MS, Nasehi A (2015) Molecular breeding strategy and challenges towards improvement of blast disease resistance in rice crop. Front Plant Sci 6:886. https://doi.org/10.3389/fpls.2015.00886
Wilson RA, Talbot NJ (2009) Under pressure: investigating the biology of plant infection by Magnaporthe oryzae. Nat Rev Microbiol 7:185–195. https://doi.org/10.1038/nrmicro2032
Kato H (2001) Rice blast disease. Pestic Outlook 12(1):23–25. https://doi.org/10.1039/B100803J
Harish S, Parthasarathy S, Durgadevi D, Anandhi K, Raguchander T (2019) Plant growth-promoting rhizobacteria: harnessing its potential for sustainable plant disease management. In: Kumar A, Meena VS (eds) Plant growth promoting rhizobacteria for agricultural sustainability. Springer, Singapore, pp 151–187. https://doi.org/10.1007/978-981-13-7553-8_8
Harish S, Kavino M, Kumar N, Saravanakumar D, Soorianathasundaram K, Samiyappan R (2008) Biohardening with plant growth promoting rhizosphere and endophytic bacteria induces systemic resistance against banana bunchy top virus. Appl Soil Ecol 39(2):187–200. https://doi.org/10.1016/j.apsoil.2007.12.006
Reddy KRK, Jyothi G, Sowjanya C, Kusumanjali K, Malathi N, Reddy KRN (2016) Plant growth-promoting actinomycetes: Mass production, delivery systems, and commercialization. In: Plant growth promoting actinobacteria. Springer, Singapore, pp 287–298. https://doi.org/10.1007/978-981-10-0707-1_19
Vurukonda SSKP, Giovanardi D, Stefani E (2018) Plant growth promoting and biocontrol activity of Streptomyces spp. as endophytes. Int J Mol Sci 19:952. https://doi.org/10.3390/ijms19040952
Law JW, Ser HL, Khan TM, Chuah LH, Pusparajah P, Chan KG, Goh BH, Lee LH (2017) The potential of Streptomyces as biocontrol agents against the rice blast fungus, Magnaporthe oryzae (Pyricularia oryzae). Front Microbiol 8:3. https://doi.org/10.3389/fmicb.2017.00003
Copping LG, Duke SO (2007) Natural products that have been used commercially as crop protection agents. Pest Manage Sci 63:524–554. https://doi.org/10.1002/ps.1378
Harsonowati W, Astuti RI, Wahyudi AT (2017) Leaf blast disease reduction by rice-phyllosphere actinomycetes producing bioactive compounds. Gen J Plant Pathol 83:98–108. https://doi.org/10.1007/s10327-017-0700-4
Xiong ZQ, Tu XR, Wei SJ, Huang L, Li XH, Lu H, Tu GQ (2013) In vitro antifungal activity of antifungalmycin 702, a new polyene macrolide antibiotic, against the rice blast fungus Magnaporthe grisea. Biotechnol Lett 35(9):1475–1479. https://doi.org/10.1007/s10529-013-1229-z
Tamura T, Shinzato N, Ito M, Ueno M (2019) Microbial secondary metabolite induction of abnormal appressoria formation mediates control of rice blast disease caused by Magnaporthe oryzae. J Phytopathol 167(3):156–162. https://doi.org/10.1111/jph.12782
Taridaporn B, Lapanun S, Supothina S, Rachtawee P, Chunhametha S, Suriyachadkun C, Boonruangprapa T, Auncharoen P, Chutrakul C, Vichai V (2016) Polycyclic tetrahydroxanthones from Streptomyces chrestomyceticus BCC 24770. Tetrahedron 72(5):775–778. https://doi.org/10.1016/j.tet.2015.12.045
Mior ZA, Tong PE, Mohammadpourlima M, Yun WM (2017) Morphological and molecular characterizations of rice blast fungus, Magnaporthe oryzae. Pak J Agric Sci 54:765–772. https://doi.org/10.21162/PAKJAS/17.3786
Srivastava D, Shamim MD, Kumar D, Pandey P, Khan NA, Singh KN (2014) Morphological and molecular characterization of Pyricularia oryzae causing blast disease in rice (Oryza sativa) from North India. Int J Sci Res Publ 4:1–9
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0. for bigger datasets. Mol Biol Evol 33:1870–1874
Garcha S, Katyal P, Sharma V (2016) Microbial diversity in soil under different land use systems in sub-mountainous zone of Punjab. J Indian Soc Soil Sci 64(3):271–275. https://doi.org/10.5958/0974-0228.2016.00038.4
Parte A, Whitman WB, Goodfellow M, Kämpfer P, Busse HJ, Trujillo ME, Suzuki KI (2012) Bergey’s manual of systematic bacteriology: volume 5: the actinobacteria. Springer, New York
Boukaew S, Prasertsan P (2014) Suppression of rice sheath blight disease using a heat stable culture filtrate from Streptomyces philanthi RM-1-138. Crop Prot 61:1–10. https://doi.org/10.1016/j.cropro.2014.02.012
Masand M, Sivakala KK, Menghani E, Thinesh T, Anandham R, Sharma G, Sivakumar N, Jebakumar SRD, Jose PA (2018) Biosynthetic potential of bioactive Streptomycetes isolated from arid region of the Thar desert, Rajasthan (India). Front Microbiol 9:687. https://doi.org/10.3389/fmicb.2018.00687
Li Q, Jiang Y, Ning P, Zheng L, Huang J, Li G, Jiang D, Hsiang T (2011) Suppression of Magnaporthe oryzae by culture filtrates of Streptomyces globisporus JK-1. Biol Control 58(2):139–148. https://doi.org/10.1016/j.biocontrol.2011.04.013
Tamreihao K, Ningthoujam DS, Nimaichand S, Singh ES, Reena P, Singh SH, Nongthomba U (2016) Biocontrol and plant growth promoting activities of a Streptomyces corchorusii strain UCR3-16 and preparation of powder formulation for application as biofertilizer agents for rice plant. Microbiol Res 192:260–270. https://doi.org/10.1016/j.micres.2016.08.005
Manigundan K, Joseph J, Ayswarya S, Vignesh A, Vijayalakshmi G, Soytong K, Gopikrishnan V, Radhakrishnan M (2020) Identification of biostimulant and microbicide compounds from Streptomyces sp. UC1A-3 for plant growth promotion and disease control. Int J Agric Technol 16:1125–1144
Tang YW, Bonner J (1948) The enzymatic inactivation of indole acetic acid; the physiology of the enzyme. Am J Bot 35:570–578. https://doi.org/10.2307/2438053
Schwyn B, Neilands JB (1987) Universal chemical assay for the detection and determination of siderophores. Anal Biochem 160:47–56. https://doi.org/10.1016/0003-2697(87)90612-9
Nautiyal CS (1999) An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol Lett 170:265–270. https://doi.org/10.1111/j.1574-6968.1999.tb13383.x
Gopalakrishnan S, Humayu P, Vadlamudi S, Vijayabharathi R, Bhimineni RK, Rupela O (2012) Plant growth-promoting traits of Streptomyces with biocontrol potential isolated from herbal vermicompost. Biocontrol Sci Technol 22(10):1199–1210. https://doi.org/10.1080/09583157.2012.719151
Adhilakshmi M, Latha P, Paranidharan V, Balachandar D, Ganesamurthy K, Velazhahan R (2014) Biological control of stem rot of groundnut (Arachis hypogaea L.) caused by Sclerotium rolfsii Sacc. with actinomycetes. Arch Phytopathol Plant Prot 47(3):298–311. https://doi.org/10.1080/03235408.2013.809224
Hasan NA, Rafii MY, Rahim HA, Ali NS, Mazlan N, Abdullah S (2016) Morphological and molecular characterization of fungal pathogen, Magnaphorthe oryzae. AIP Conf Proc. https://doi.org/10.1063/1.4940263
Zarandi ME, Bonjar GHS, Dehkaei FP, Moosavi SAA, Farokhi PR, Aghighi S (2009) Biological control of rice blast (Magnaporthe oryzae) by use of Streptomyces sindeneusis isolate 263 in greenhouse. Am J Appl Sci 6(1):194–199. https://doi.org/10.3844/ajas.2009.194.199
Aruna J, Kumar SV, Rambabu R, Ramesh S, Yashaswini C, Bhaskar B, Madhavi KR, Balachndran SM, Ravindrababu V, Prasad MS (2016) Morphological characterization of five different isolates of Pyricularia oryzae causing rice blast disease. Progress Res 11:3377–3380
Salim FM, Sharmili SA, Anbumalarmathi J, Umamaheswari K (2017) Isolation, molecular characterization and identification of antibiotic producing actinomycetes from soil samples. J Appl Pharm Sci 7:69–75. https://doi.org/10.7324/JAPS.2017.70909
Boukaew SA, Plubrukam PP (2013) Effect of volatile substances from Streptomyces philanthi RM-1-138 on growth of Rhizoctonia solani on rice leaf. Biol Control 58:471–482. https://doi.org/10.1007/s10526-013-9510-6
Aldesuquy HS, Mansour FA, Abo-Hamed SA (1998) Effect of the culture filtrates of Streptomyces on growth and productivity of wheat plants. Folia Microbiol 43(5):465–470. https://doi.org/10.1007/BF02820792
Chen YD, Zhou QD, Gao Z, Xie J, Luo Y (2018) Growth promotion and disease suppression ability of a Streptomyces sp. CB-75 from banana rhizosphere soil. Front Microbiol 8:2704. https://doi.org/10.3389/fmicb.2017.02704
Saleh M (2018) Evaluation of endophytes isolated from rice leaves for their antifungal activities against Pyricularia oryzae causative blast disease. Egypt J Phytopathol 46:193–214. https://doi.org/10.21608/ejp.2018.87788
Awla HK, Kadir J, Othman R, Rashid TS, Wong MY (2016) Bioactive compounds produced by Streptomyces sp. isolate UPMRS4 and antifungal activity against Pyricularia oryzae. Am J Plant Sci 7:1077. https://doi.org/10.4236/ajps.2016.77103
Newitt JT, Prudence SMM, Hutchings MI, Worsley SF (2019) Biocontrol of cereal crop diseases using Streptomycetes. Pathogens 8:78. https://doi.org/10.3390/pathogens8020078
Mun BG, Lee WH, Kang SM, Lee SU, Lee SM, Lee DY, Shahid M, Yun BW, Lee IJ (2020) Streptomyces sp. LH 4 promotes plant growth and resistance against Sclerotinia sclerotiorum in cucumber via modulation of enzymatic and defense pathways. Plant Soil. https://doi.org/10.1007/s11104-019-04411-4
Prabavathy VR, Mathivanan N, Murugesan K (2006) Control of blast and sheath blight diseases of rice using antifungal metabolites produced by Streptomyces sp. PM5. Biol Control 39:313–319. https://doi.org/10.1016/j.biocontrol.2006.07.011
Chaiharn M, Theantana T, Pathom-Aree W (2020) Evaluation of biocontrol activities of Streptomyces spp. against rice blast disease fungi. Pathogens 9:126. https://doi.org/10.3390/pathogens9020126
Awla HK, Kadir J, Othman R, Rashid TS, Hamid S, Wong MY (2017) Plant growth-promoting abilities and biocontrol efficacy of Streptomyces sp. UPMRS4 against Pyricularia oryzae. Biol Control 112:55–63. https://doi.org/10.1016/j.biocontrol.2017.05.011
Xu T, Li Y, Zeng X, Yang X, Yang Y, Yuan S, Hu X, Zeng J, Wang Z, Liu Q (2017) Isolation and evaluation of endophytic Streptomyces endus OsiSh-2 with potential application for biocontrol of rice blast disease. J Sci Food Agric 97:1149–1157. https://doi.org/10.1002/jsfa.7841
Xu T, Cao L, Zeng J, Franco CMM, Yang Y, Hu X, Liu Y, Wang X, Gao Y, Bu Z (2019) The antifungal action mode of the rice endophyte Streptomyces hygroscopicus OsiSh-2 as a potential biocontrol agent against the rice blast pathogen. Pest Biochem Physiol 160:58–69. https://doi.org/10.1016/j.pestbp.2019.06.015
Gao Y, Zeng XD, Ren B, Zeng JR, Xu T, Yang YZ, Hu XC, Zhu ZY, Shi LM, Zhou GY (2020) Antagonistic activity against rice blast disease and elicitation of host-defence response capability of an endophytic Streptomyces albidoflavus OsiLf-2. Plant Pathol 69:259. https://doi.org/10.1111/ppa.13118
Ilsan NA (2017) Antifungal activity of phyllosphere actinobacteria against Pyricularia oryzae. In: 2nd international seminar on global health (ISGH), pp 308–315
Awla HK (2021) Effect of Streptomyces xantholiticus on rice blast disease reduction and enzyme activity. Polytechnic J 11(1):112–117. https://doi.org/10.25156/ptj.v11n1y2021.pp112-117
Liu Y, Chen N, Gao Y, Bu Z, Niu S, Wang Y, Liu X, Zhu Y (2021) Physiological, biochemical and proteomic insight into the response system of Streptomyces hygroscopicus OsiSh-2 to rice blast fungus toxins. Appl Soil Ecol 167:104058. https://doi.org/10.1016/j.apsoil.2021.104058
Liu W, Wang J, Li S, Zhang H, Meng L, Liu L, Ping W, Du C (2022) Genomic and biocontrol potential of the crude lipopeptide by Streptomyces bikiniensis HD-087 against Magnaporthe oryzae. Front Microbiol. https://doi.org/10.3389/fmicb.2022.888645
Acknowledgements
The authors are thankful to the Department of Plant Pathology, Agricultural College & Research Institute, Madurai, Tamil Nadu Agricultural University, Tamil Nadu, India for extending necessary infrastructural facilities to carry out the above research work. The author acknowledges the help rendered by M. Nivedha during the course of the experiments.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or non-profit sectors.
Author information
Authors and Affiliations
Contributions
RR : investigation, experimentation, and analysis, SH : conceptualization, supervision, validation, editing, and project administration, KK and GA : methodology and resources, MA and RK : data interpretation and analysis.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
No human and/or animal participants were involved in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
284_2023_3205_MOESM1_ESM.jpg
Supplemental Figure S1: Phylogenetic tree of Magnaporthe oryzae large subunit ribosomal RNA gene with other nucleotide sequences from the GenBank. rDNA homology searches were performed using the BLAST program and the sequences were submitted to GenBank. Clustering was determined by UPGMA analysis and the nucleotide sequences were aligned using CLUSTAL X 1.81. (TIF 31304 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rahila, R., Harish, S., Kalpana, K. et al. Antifungal Metabolites of Streptomyces chrestomyceticus STR-2 Inhibits Magnaporthe oryzae, the Incitant of Rice Blast. Curr Microbiol 80, 107 (2023). https://doi.org/10.1007/s00284-023-03205-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00284-023-03205-3