Abstract
Fusarium wilt of tomato, a disease caused by the soilborne fungus Fusarium oxysporum f. sp. lycopersici, causes major losses to tomato production. Chemical fungicides and resistant cultivars are the main control strategies, but new races of the pathogen appear to be able to overcome resistance genes in currently grown cultivars. Therefore, there is a need to identify natural antifungal compounds and develop new agents to control F. oxysporum f. sp. lycopersici. The culture filtrate (F31D-CF) from isolate F31D, a bacterial isolate collected from paddy field soil in Shimane Prefecture in 2019, significantly inhibited in vitro conidial germination of F. oxysporum f. sp. lycopersici, indicating a fungicidal effect against this pathogen. The amount of the inhibitory compound in F31D-CF increased from 1 to 3 days of incubation. F31D-CF significantly suppressed disease development in tomato plants. Sequence analysis of the 16S rDNA region of the isolate revealed 99% similarity with the type strain of Streptomyces plumbeus. Thin layer chromatography–bioautography of F31D-CF showed that the compound inhibiting F. oxysporum f. sp. lycopersici growth had an Rf of 0.57. The effective compound in F31D-CF has a molecular weight of > 3000 and is heat stable below 100 °C. Our results suggest that secondary metabolites of isolate F31D have potential for developing a new fungicidal agent against F. oxysporum f. sp. lycopersici.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tomato is the second most important economic vegetable after potato. The worldwide production area devoted to tomato (Solanum lycopersicum L.) is increasing as consumption increases at an average rate of 3% annually (Leonardi et al. 2000; Serio et al. 2006). The plant is susceptible to many pathogens and pests that can reduce yield and quality in the field, while postharvest losses also occur due to improper handling. Fusarium wilt, a systemic disease caused by a soil-borne fungus, is one of the most important plant diseases as it can cause severe losses in more than 120 different crops, including banana, beans, capsicum, chickpeas, cotton, melons, peas, potato, and tomato (Gonzalez-Cendales et al. 2016; Schwarz and Grosch 2003). Fusarium wilt of tomato caused by Fusarium oxysporum f. sp. lycopersici is especially devastating in warm humid subtropical and temperate regions of the world, where it causes yield losses of 10–90% (Agrios 2005; Singh and Kamal 2012). The control of Fusarium wilt of tomato is difficult due to the ability of this pathogen to proliferate within the vascular tissues of the host. Several disease management strategies are available, such as cultural techniques (growing resistant cultivars, crop rotation), biological control, and chemical control (Abo-Elyousr and Mohamed 2009). Resistant cultivars are the most effective measure for controlling Fusarium wilt (Amini 2009; Beckman 1987), but new races of the pathogen appear to be able to overcome resistance genes in currently grown cultivars (Arie 2019). Soil fumigation with methyl bromide (Ben-Yephet et al. 1994; Li et al. 2014) and application of some chemical fungicides (azoxystrobin, benomyl, carbendazim, and prochloraz) can effectively control Fusarium wilt of tomato (Diehl and Fehrmann 1999; Minton 1986). However, excessive and continuous application of synthetic fungicides may induce resistance or reduce the susceptibility of the pathogenic fungus (Barak and Edgington 1984; Hahn 2014; Ishii 2006; Leadbeater 2014). Therefore, there is a need to identify natural substances that can be used to develop new antifungal agents that provide broad action and long-lasting control.
Microbial products have proven potential as biological and chemical control agents against fungal diseases in various crops (Joshi et al. 2008; Shimizu et al. 2000). Different microorganisms, even strains of the same species, differ physiologically and thus produce different compounds, so a wide range of taxa need to be screened for activity. Members of Streptomyces, a genus of Actinobacteria, can inhibit various plant pathogens, including Fusarium (Kim et al. 2011; Shimizu et al. 2000, 2009), and inhibitory compounds have been isolated from Streptomyces species (Lee et al. 2005; Park et al. 2006). In our previous studies, we found isolates of the genus Streptomyces that suppressed plant pathogens such as Magnaporthe oryzae and Colletotrichum orbiculare (Tamura et al. 2019; Ueno et al. 2016, 2019).
Herein, we report that a culture filtrate of a new Streptomyces plumbeus isolate, designated F31D, has fungicidal activity against F. oxysporum f. sp. lycopersici.
Materials and methods
Plants and pathogen
Tomato seeds of the cultivar Ponderosa (Tsurusin Co., Ltd., Nagano, Japan) were sown in plastic tubes (80 × 70 mm) containing commercial garden soil (Kumiai Nippi Engeibaido No. 1, Nihon Hiryo Co. Ltd., Tokyo, Japan). Seedlings were then grown according to standard procedures in an incubator (26 ± 2 °C) with 14 h light/10 h dark and used at the first true leaf stage for inoculation tests.
F. oxysporum f. sp. lycopersici (strain MAFF 305914, race 2; MAFF 744006, race 1) was grown on potato sucrose agar (PSA; 200 g/l potatoes, 2.0% w/v sucrose, 2.0% w/v agar) slants until use. Strain MAFF 305914 and MAFF 744006 were obtained from the Genetic Resources Center, National Agriculture and Food Research Organization. After sufficient growth on PSA plates, mycelial disks (8 mm diameter) of F. oxysporum f. sp. lycopersici were individually added to test tubes containing 20 ml of potato sucrose broth. The liquid cultures were incubated for 4–5 days at 26 ± 2 °C in the dark, with constant shaking on a rotary shaker (180 rpm) to induce synchronous conidiation. These conidia were used as inoculum.
Bacterial isolation and culture
We isolated bacteria from soil previously collected from four locations in Honjo District, Matsue City, Shimane Prefecture, Japan, using the methods of (Lemtukei et al. 2016). Bacteria were isolated from soil samples using humic acid–vitamin (HV) agar (Hayakawa and Nonomura 1987; Ueno et al. 2016). The soil samples were stored at 4 °C until use, then a small amount of soil was suspended in a microtube containing 1 ml distilled water. Each sample (10–100 μl) was spread on plates of HV agar containing 10 μg/ml cycloheximide and 10 μg/ml nalidixic acid. All plates were incubated for 4 days at 26 ± 2 °C. Then a single colony of each isolate was grown for 4 days at 26 ± 2 °C on nutrient glucose agar (NGA) (8 g nutrient broth, 10 g glucose, and 20 g agar in 1000 ml deionized water). Distinct colonies growing on NGA were added to individual test tubes containing 3 ml PC-1 medium (10 g each of starch, polypeptone, molasses, and beef extract in 1000 ml deionized water, pH 7.2) and incubated on a rotary shaker (130 rpm) for 7 days at 26 ± 2 °C. The cultures were used in dual culture assays.
Of 63 bacteria isolated from the soil samples, only isolate F31D inhibited the mycelial growth of F. oxysporum f. sp. lycopersici in dual culture assays (Fig. S1); therefore, it was selected for further study. The isolate was suspended in 15% v/v glycerol solution and stored at − 80 °C until use.
Preparation of culture filtrates
Isolate F31D was grown on Luria–Bertani (LB) agar (10 g bacto-tryptone, 10 g sodium chloride, 5 g yeast extract, 20 g agar in 1000 ml deionized water), and test tubes containing LB broth (20 ml) were inoculated with a colony of the isolate. For testing the inhibitory effects of filtrates from cultures of different ages, the liquid cultures were incubated at 26 ± 2 °C in the dark for 1–7 days with constant shaking on a rotary shaker (180 rpm). The culture was filtered through No. 101 filter paper (Advantec Toyo, Toyo Roshi Kaisha, Ltd., Tokyo, Japan). After the bacterial cells were removed by 0.22-μm micropore membrane filters (Syringe Filter Nylon 0.22 μm/φ32mm: AS ONE Corp., Osaka, Japan), the remaining solution was designated as F31D-CF, the culture filtrate from isolate F31D. Selected F31D-CF samples underwent cold or heat treatment at − 20, 37, 50, 100, or 121 °C for 20 min.
Agar well diffusion assay
A conidial suspension of F. oxysporum f. sp. lycopersici strain MAFF 305914 (106 conidia/ml, 100 μl) was subcultured on PSA. Subsequently, wells 8 mm in diameter were punched into the PSA and filled with 200 μl of F31D-CF samples that had been incubated for different durations (1, 2, 3, 4, 5, 6, or 7 days). The samples were then incubated at 26 ± 2 °C for 2 days. As a control, some wells were filled with LB broth. In addition, we set up a similar experiment using F31D-CF (3-day-old culture) samples to test the temperature stability of the CF to cold (− 20 °C for 60 min) or heat (37, 50, or 100 for 60 min, or 121 °C for 20 min). Any area (mm2) of inhibition of F. oxysporum f. sp. lycopersici inhibition area in the presence and absence of F31D-CF was measured using LIA32 software (https://www.agr.nagoya-u.ac.jp/~shinkan/LIA32/index-e.html). Experiments were repeated three times, with five dishes per experiment.
Effect of culture filtrate on in vitro germination of F. oxysporum f. sp. lycopersici
Three drops (150 µl) of F. oxysporum f. sp. lycopersici strain MAFF 305914 conidia (105 conidia/ml) in F31D-CF (3-day-old culture) were dropped onto glass slides and maintained in a moist chamber at 26 ± 2 °C. After 24 h, 300 conidia per experiment were examined with a light microscope to count germinated conidia and determine the percentage germination as (Number of conidia germinated/Total number of conidia) × 100. The experiments were repeated three times.
In vitro fungicidal activity of culture filtrate
F. oxysporum f. sp. lycopersici strain MAFF 305914 conidia (105 conidia/ml) were suspended in 1 ml of F31D-CF (3-day-old culture) or in LB as a control and incubated at 4 °C for 24 h. The suspension was then centrifuged at 20,000×g for 15 min, the supernatant removed, and 1 ml of distilled water added. The suspension was pipetted onto PSA plates containing 20 ppm chloramphenicol. After 4 days at 26 ± 2 °C, the mycelial area (mm2) of F. oxysporum f. sp. lycopersici was measured using LIA32 software (https://www.agr.nagoya-u.ac.jp/~shinkan/LIA32/index-e.html). Experiments were repeated three times, with 10 petri dishes per experiment.
Effect of culture filtrate on Fusarium wilt in tomato
Tomato seedlings at the first true leaf stage were inoculated with 106 conidia/ml F. oxysporum f. sp. lycopersici (strain MAFF 744006, race 1, and strain MAFF 305914, race 2) by the root dip method (30 min) in the presence of either 10 ml LB or F31D-CF (3-day-old culture), then transferred to plastic pots (7 cm in diameter) containing commercial garden soil. All seedlings were maintained in a growth chamber at 28 °C with 14 h light and 10 h dark. Three weeks after inoculation, symptoms on five tomato plants were scored on a scale of 0–3: 0, no symptoms; 1, yellowing; 2, wilting; 3, dead). The experiments were independently done three times.
Identification of bacterial isolate F31D
Genomic DNA, extracted from a bacterial colony of F31D as described by Suzuki et al. (2006), was used as a template for PCR amplification of the 16S rDNA region using previously described primers (Table S1) (Huong et al. 2007; Matsui et al. 2009) and a GeneAtlas G thermocyler (Astec Co, Ltd., Fukuoka, Japan). The thermocycling conditions were initial denaturation at 95 °C for 30 s; 30 cycles of denaturation at 95 °C for 30 s, annealing at 62 °C for 30 s, elongation at 72 °C for 90 s, and final extension at 72 °C for 10 min. Amplicons were purified using a NucleoSpin Gel and PCR Clean-up Kit (Macherey–Nagel GmbH & Co. KG, Düren, Germany) and sequenced using the Big Dye Terminator Cycle Sequencing Kit (Applied Biosystems, Carlsbad, CA, USA) on an ABI PRIZM 3130xl Genetic Analyzer (Applied Biosystems). The BLAST suite of programs was to search the DNA Data Bank of Japan for matching sequences. 16S rDNA sequences of reference strains were obtained from the NITE Biological Resource Center database (https://www.nite.go.jp/nbrc/index.html). Multiple sequence alignments were generated using CLUSTAL W (Thompson et al. 1994). Genetic distances between the aligned DNA sequences were calculated using Kimura’s two-parameter model (Kimura 1980) and the neighbor-joining method (Saitou and Nei 1987) in MEGA X (https://www.megasoftware.net/). Bootstrap percentages were calculated with 1000 replicates. Actinoalloteichus cyanogriseus (NBRC 13344) was used as the outgroup.
Ethyl acetate extraction of culture filtrate
For an initial characterization of the inhibitory compounds in F31D-CF, samples (20 ml) of the F31D-CF (3-day-old culture) were extracted twice with 40 ml of ethyl acetate. The ethyl-acetate-soluble fraction was added to distilled water and evaporated at 45 °C under reduced pressure until only the aqueous fraction remained, then the aqueous volume was adjusted to 20 ml. The ethyl-acetate-insoluble fraction was evaporated at 45 °C under reduced pressure until only the aqueous fraction remained, then the aqueous volume was adjusted to 20 ml. F31D-CF (3-day-old culture, 0.5 ml) was also separated by ultrafiltration using an Amicon Ultra 0.5-ml 3 K centrifugal filter (MilliporeSigma, Burlington, MA, USA) into two fractions (< 3000 and ≥ 3000 Da). The volume of each sample was adjusted to 0.5 ml with distilled water. Inhibitory activity was assayed using the agar well diffusion assay described above. Experiments were done three times, with five petri dishes per experiment.
Thin layer chromatography assay for inhibitory compounds
The culture filtrate of isolate F31D (tenfold concentration from 3-day-old culture, 100 µl) was spotted onto silica gel thin layer chromatography (TLC) plates (Silica Gel 60, Merck KGaA, Darmstadt, Germany) and separated using 4:1:1 (v/v/v) n-butanol–acetic acid–distilled water as the solvent. A conidial suspension of Fusarium oxysporum f. sp. lycopersici (> 1 × 107 conidia/ml) was then sprayed on the plates in the presence of molten PSA containing 20 ppm chloramphenicol. After 7 days in a moist chamber at 26 ± 2 °C, the plates were examined for zones of growth inhibition.
Statistical analyses
Significant differences (p < 0.05) in the means between treatment groups were determined using a t test, the Tukey–Kramer test or Kruskal–Wallis test in SPSS ver. 22.0 for Windows (IBM, Armonk, NY, USA).
Results
Inhibitory activity of F31D-CF on mycelial growth of F. oxysporum f. sp. lycopersici
Based on the extent of the inhibition of mycelial growth on plates, the amount of inhibitory compound in F31D-CF increased with incubation duration. As shown in Fig. 1, growth of F. oxysporum f. sp. lycopersici colonies in petri dishes was inhibited after treatment with F31D-CF from cultures incubated for 2–7 days compared with growth of the control on plates without F31D-CF (Fig. 1). Mean (± SD) mycelial inhibition areas (mm2, mean ± SD) in petri dishes treated with F31D-CF from cultures of different incubation durations were as follows: 0 days, 0; 1 day, 0; 2 days, 608.6 ± 203.0; 3 days, 838.5 ± 261.5; 4 days, 706.8 ± 332.4; 5 days, 455.3 ± 137.5; 6 days, 511.5 ± 78.8, 7 days, 278.6 ± 223.3 mm2 (Fig. 1).
Time course of inhibitory activity of culture filtrates of isolate F31D against mycelial growth of Fusarium oxysporum f. sp. lycopersici, determined by the agar well diffusion method on potato sucrose agar (PSA) plates. A conidial suspension of F. oxysporum f. sp. lycopersici (106 conidia/ml, 100 μl) was subcultured on PSA. Subsequently, wells of 8 mm in diameter were punched into the agar, filled with 200 μl of culture filtrate of isolate F31D growin Luria–Bertani broth (LB), and incubated at 26 ± 2 °C for 2 days. F. oxysporum f. sp. lycopersici plates showed inhibition zones a of different diameters b when exposed to culture filtrates of isolate F31D from colonies incubated for different durations; LB was used as a control. Data are means of the results from three separate experiments. Error bars represent the standard deviation of the mean. Means with different letters differed significantly in a Tukey–Kramer test (p < 0.05)
Inhibition of in vitro conidial germination of F. oxysporum f. sp. lycopersici by F31D-CF
After exposure to F31D-CF, 6.8 ± 3.6% of the conidia germinated, in contrast to 91.8 ± 5.3% of the control conidia, which were not exposed to the CF.
Inhibition of in vitro colony growth from conidia pretreated with F31D-CF
The mean (± SD) mycelial area of F. oxysporum f. sp. lycopersici that developed from conidia preteated with CF for 24 h was 5706.9 ± 43.7 mm2, significantly less compared with that from conidia pretreated with LB broth (418.8 ± 268.08 mm2, Fig. 2).
In vitro assay of fungicidal activity of culture filtrates of isolate F31D. Conidia of Fusarium oxysporum f. sp. lycopersici (105 conidia/ml) were treated for 24 h with the culture filtrate at 4 °C. Next, the filtrate was removed after centrifugation, and distilled water was added. Each conidial suspension was pipetted onto potato sucrose agar with chloramphenicol. Colonies were counted after 4 days at 26 ± 2 °C. Representative plates are shown. Control: Luria–Bertani broth. Experiments were done three times with 10 petri dishes per treatment per experiment. Error bars represent the standard deviation from the mean. An asterisk indicates a significant difference (t test, p < 0.05)
Suppression of Fusarium wilt in tomato by F31D-CF
After inoculation of tomato plants with Fusarium oxysporum f. sp. lycopersici (race 1 or race 2), plants that were pretreated with F31D-CF had a disease index approximately fivefold lower than that of plants without F31D-CF (Fig. 3). In race 2-inoculated plants, the mean (± SD) disease index for tomato plants with F31D-CF was 0.5 ± 1.0 and 2.6 ± 0.6 for those without (Fig. 3b); the disease index was 0 for control plants treated with LB broth but not inoculated with the fungus (Fig. 3b). In race 1-inoculated tomato plants, the mean (± SD) disease index for those treated with F31D-CF was 0.5 ± 0.5 and 2.6 ± 0.5 6 for those without (Fig. 3c); the disease index was 0 for control plants treated with LB broth but not inoculated with the fungus (Fig. 3d). LB broth had no toxic effect in the control plants (Fig. 3a, c), nor was F31D-CF phytotoxic to tomato plants (Fig. S2).
Suppression of Fusarium wilt in tomato plants by culture filtrate of isolate F31D. Tomato seedlings at the first true leaf stage were inoculated with Fusarium oxysporum f. sp. lycopersici (106 conidia/ml: race 1 and race 2) by the root dip method (30 min) in the absence or presence of the culture filtrate of isolate F31D (3-day-old culture, 10 ml) and then transferred into plastic pots (7 cm in diameter) containing commercial garden soil. Luria–Bertani broth was used as a control. All seedlings were maintained in a growth chamber at 26 ± 2 °C with 14 h light, 10 h dark. Three weeks after inoculation, symptoms were scored (0, healthy; 1, yellowing; 2, wilting; 3, dead). Data are means (± SD) from three separate experiments with five tomato plants per treatment per experiment. Means with different letters differed significantly in a Kruskal–Wallis test (p < 0.05). a, b: race 2, c, d: race 1
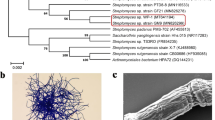
Phylogenetic analysis of 16S rDNA sequence from isolate F31D
The phylogenetic analysis showed that isolate F31D was most closely related to strain NBRC 13708T of Streptomyces plumbeus (Fig. 4). Isolate F31D (Accession LC599499) shared 99.0% similarity with S. plumbeus NBRC 13708T in the 16S rDNA region, which corresponds to a two-nucleotide difference in 1450 bp. Therefore, isolate F31D is considered to be a member of the genus Streptomyces.
Characterization of inhibitory compounds in F31D-CF
In the mycelial growth assays of the ethyl-acetate-soluble and ethyl-acetate-insoluble fractions of F31D-CF, the soluble fraction did not inhibit growth of F. oxysporum f. sp. lycopersici, but a mean (± SD) inhibition zone of 762.2 ± 132.9 mm2 formed in the presence of the insoluble fraction. When F. oxysporum f. sp. lycopersici was treated with the fraction consisting of compounds with a molecular weight < 3000 Da, the inhibition area was 0 mm2, compared with 595.3 ± 192.7 mm2 for compounds in the fraction > 3000 Da. In tests of cold- and heat-treated F31D-CF on mycelial growth, we found that the inhibitory compound in F31D-CF is heat stable at less than 100 °C. The mycelial inhibition areas (mm2) in petri dishes inoculated with F. oxysporum f. sp. lycopersici and exposed to cold- or heat-treated F31D-CF were as follows: 37 °C, 847.0 ± 95.7; 50 °C, 834.3 ± 88.0; 70 °C, 851.8 ± 70.3; 100 °C, 474.7 ± 166.5; 121 °C, 0; − 20 °C, 851.5 ± 92.5. In the control (F31D-CF not cold- or heat-treated), the inhibition area was 856.3 ± 101.5 mm2.
Characterization of inhibitory compounds by TLC bioautography of F31D-CF
To further determine the nature of the inhibitory compound(s) in F31D-CF, compounds were separated with TLC, and each was tested to determine whether it inhibited the growth of F. oxysporum f. sp. lycopersici colonies. A growth inhibition zone was detected at an Rf value of 0.57 (Fig. 5).
Thin layer chromatography (TLC) assay of culture filtrate of isolate F31D in relation to growth inhibition of Fusarium oxysporum f. sp. lycopersici. TLC plates with culture filtrate spots were developed in 4:1:1 (v/v/v) n-butanol–acetic acid–distilled water, then sprayed with molten potato sucrose agar inoculated with more than 107 conidia/ml and incubated at 26 ± 2 °C for 7 days
Discussion
In the present study, the amount of the inhibitory compound in culture filtrate of F31D, a bacterial isolate found in soil collected from a paddy field in Shimane Prefecture in 2019, significantly increased from 1 to 3 days of incubation, but decreased from 4 days of incubation onward (Fig. 1). In addition, F31D-CF exhibited fungicidal activity against F. oxysporum f. sp. lycopersici (Fig. 2). This result suggests that isolate F31D may produce inhibitory compounds against other microorganisms to protect itself from other microorganisms during early stages of growth or, alternatively, the inhibitory compound may be necessary for its own growth. In future experiments, perhaps screenings for inhibitory strains and secondary metabolites should focus on early stages of microbial growth.
Sequence analysis of the 16S rDNA region of isolate F31D revealed 99% similarity with Streptomyces plumbeus (Fig. 4). Among the few reports on pathogen control by secondary metabolites of S. plumbeus, Park et al. (1977) discovered new antimetabolites produced by S. plumbeus, which they named plumbemycin A (molecular weight 381.278) and B (380.294). Kim et al. (2020) reported that S. plumbeus strain CA5 produces a polyene macrolide, lucensomycin (molecular weight 708.3595), that is effective against gray mold of grapes. However, inhibitory activity of a culture filtrate of S. plumbeus against F. oxysporum f. sp. lycopersici has not been previously demonstrated. According to our tests, the inhibitory compound of isolate F31D-CF is heat-stable below 100 °C and has a molecular weight greater than 3000 kDa (Fig. 5); it did not exhibit chitinase or β-glucanase activity (data not shown). These results suggest that the fungicidal compound of isolate F31D-CF is not chitinase, β-glucanase, lucensomycin, plumbemycin A, or plumbemycin B. In the TLC bioautography of F31D-CF, one compound was detected, and we estimated that the inhibitory compound of F31D-CF is highly polar. Further research on the nature and identity of the fungicidal compounds in F31D-CF is needed.
Pretreatment with the culture filtrate from S. plumbeus from F31D-CF suppressed Fusarium wilt in tomato caused by F. oxysporum f. sp. lycopersici in the soil without being phytotoxic to tomato (Fig. 3, Fig. S2). Generally, soil-borne pathogens are controlled before planting by using biological, chemical, and physical methods. In our present study, F31D-CF indicated a fungicidal effect against F. oxysporum f. sp. lycopersici. These results suggest that it may be possible to sterilize the plant pathogen-contaminated soil causes of tomato diseases such as Fusarium wilt by treated the soil with an inhibitory compound of the isolate F31D-CF before planting. However, F31D-CF has not yet been tested against other pathogenic fungi and bacteria that infect tomato, so further studies are required to investigate the potential of F31D-CF against other tomato diseases. Our study suggests that the culture filtrate of S. plumbeus isolate F31D can contribute to the development of new fungicides against Fusarium wilt of tomato.
Data availability
The nucleotide sequence data reported are available in the DDBJ/EMBL/GenBank database under Accession No. LC599499.
References
Abo-Elyousr KAM, Mohamed HM (2009) Biological control of Fusarium wilt in tomato by plant growth-promoting yeasts and rhizobacteria. Plant Pathol J 25:199–204
Agrios GN (2005) Plant pathology, 5th edn. Elsevier Academic Press, New York, pp 523–526
Amini J (2009) Physiological race of Fusarium oxysporum f. sp. lycopersici in Kurdistan Province of Iran and reaction of some tomato cultivars to race 1 of pathogen. Plant Pathol J 8:68–73
Arie T (2019) Fusarium diseases of cultivated plants, control, diagnosis, and molecular and genetic studies. J Pestic Sci 44:275–281
Barak E, Edgington LV (1984) Cross-resistance of Botrytis cinerea to captan, thiram, chlorothalonil, and related fungicides. Cand J Plant Pathol 6:318–320
Beckman CH (1987) The nature of wilt diseases of plants. APS Press, St Paul, p 165
Ben-Yephet Y, Reuven M, Szmulewich Y, Mor Y (1994) Effect of methyl bromide on the control of Fusarium oxysporum f.sp. dianthi propagules in carnation greenhouse soil and on inoculum increase after one growth cycle of carnation. Crop Prot 13:357–361
Diehl T, Fehrmann H (1999) Wheat fusarioses: influence of infection date, tissue injury and aphids on leaf and ear attack. J Plant Dis Prot 96:393–407
Gonzalez-Cendales Y, Catanzariti AM, Baker B, Mcgrath DJ, Jones DA (2016) Identification of I-7 expands the repertoire of genes for resistance to Fusarium wilt in tomato to three resistance gene classes. Mol Plant Pathol 17:448–463
Hahn M (2014) The rising threat of fungicide resistance in plant pathogenic fungi: Botrytis as a case study. J Chem Biol 7:133–141
Hayakawa M, Nonomura H (1987) Humic acid-vitamin agar, a new medium for the selective isolation of soil actinomycetes. J Ferment Technol 65:501–509
Huong NL, Itoh K, Suyama K (2007) Diversity of 2,4-dichlorophenoxyacetic acid (2,4-d) and 2,4,5-trichlorophenoxyacetic acid (2,4,5-T)-degrading bacteria in Vietnamese soils. Microb Environ 22:243–256
Ishii H (2006) Impact of fungicide resistance in plant pathogens on crop disease control and agricultural environment. JARQ 40:205–211
Joshi S, Bharucha C, Desai AJ (2008) Production of biosurfactant and antifungal compound by fermented food isolate Bacillus subtilis 20B. Bioresour Technol 99:4603–4608
Kim JD, Han JW, Lee SC, Lee D, Hwang IC, Kim BS (2011) Disease control effect of strevertenes produced by Streptomyces psammoticus against tomato fusarium wilt. J Agric Food Chem 59:1893–1899
Kim JD, Kang JE, Kim BS (2020) Postharvest disease control efficacy of the polyene macrolide lucensomycin produced by Streptomyces plumbeus strain CA5 against gray mold on grapes. Postharvest Biol Tec 162:111115
Kimura M (1980) A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16:111–120
Leadbeater AJ (2014) Plant health management: fungicides and antibiotics. In: Van Alfen NK (ed) Encyclopedia of agriculture and food systems. Academic Press, London, pp 408–424
Lee JY, Lee JY, Moon SS, Hwang BK (2005) Isolation and antifungal activity of 4-phenyl-3-butenoic acid from Streptomyces koyangensis strain VK-A60. J Agric Food Chem 53:7696–7700
Lemtukei D, Tamura T, Nguyen QT, Kihara J, Ueno M (2016) Antagonistic potential of isolated microorganisms from soil in Shimane prefecture against rice blast disease cause by Maganporthe oryzae. Bull Fac Life Env Sci Shimane Univ 21:9–12
Leonardi C, Ambrosino P, Esposito F, Fogliano V (2000) Antioxidative activity and carotenoid and tomatine contents in different typologies of fresh consumption tomatoes. J Agric Food Chem 48:4723–4727
Li Y, Mao L, Yan D, Ma T, Shen J, Guo M, Wang Q, Ouyang C, Cao A (2014) Quantification of Fusarium oxysporum in fumigated soils by a newly developed real-time PCR assay to assess the efficacy of fumigants for Fusarium wilt disease in strawberry plants. Pest Manag Sci 70:1669–1675
Matsui T, Kato K, Namihira T, Shinzato N, Semba H (2009) Stereospecific degradation of phenylsuccinate by actinomycetes. Chemosphere 76:1278–1282
Minton EB (1986) Half a century dynamics and control of cotton disease. In: Proceedings of Beltwiae cotton conference, National Cotton Council of America, Memphis, TN, USA, pp 33–35
Park BK, Hirota A, Sakai H (1977) Structure of plumbemycin A and B, antagonists of l-threonine from Streptomyces plumbeus. Agric Biol Chem 41:573–579
Park HJ, Lee JY, Hwang IS, Yun BS, Kim BS, Hwang BK (2006) Isolation and antifungal and antioomycete activities of staurosporine from Streptomyces roseoflavus strain LS-A24. J Agric Food Chem 54:3041–3046
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Schwarz D, Grosch R (2003) Influence of nutrient solution concentration and a root pathogen (Pythium aphanidermatum) on tomato root growth and morphology. Sci Hortic 97:109–120
Serio F, Ayala O, Bonasia A, Santamaria P (2006) Antioxidant properties and health benefits of tomato. In: Govil JN, Singh VK, Arunchalam C (eds) Recent progress in medicinal plants vol 3: search for natural drugs, vol 13. Studium Press, Houston, pp 159–179
Shimizu M, Nakagawa Y, Sato Y, Furumai T, Igarashi Y, Onaka H, Yoshida R, Kunoh H (2000) Studies on endophytic actinomycetes (Ι) and Streptomyces sp. isolated from Rhododendron and its antifungal activity. J Gen Plant Pathol 66:360–366
Shimizu M, Yazawa S, Ushijima Y (2009) A promising strain of endophytic Streptomyces sp. for biological control of cucumber anthracnose. J Gen Plant Pathol 75:27–36
Singh AK, Kamal S (2012) Chemical control of wilt in tomato (Lycopersicon esculentum L.). Int J Hortic 2:5–6
Suzuki S, Taketani H, Kusumoto K, Kashiwagi Y (2006) High-throughput genotyping of filamentous fungus Aspergillus oryzae based on colony direct polymerase chain reaction. J Biosci Bioeng 102:572–574
Tamura T, Shinzato N, Ito M, Ueno M (2019) Microbial secondary metabolite induction of abnormal appressoria formation mediates control of rice blast disease caused by Magnaporthe oryzae. J Phytopathol 167:156–162
Thompson J, Higgins D, Gibson T (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Ueno M, Nguyen TQ, Shinzato N, Matsui T (2016) Antifungal activity of collected in subtropical region, Okinawa, against Magnaporthe oryzae. Trop Agric Dev 60:48–52
Ueno M, Tamura T, Yano Y, Ito M, Shinzato N (2019) Streptomyces strain 5–94, obtained from the subtropical region in Okinawa, has inhibitory activity against Colletotrichum orbiculare. Trop Agric Dev 63:192–197
Acknowledgements
The authors are thankful to the Faculty of Life and Environmental Science, Shimane University, for financial support to publish this report. This research study was supported and funded by the Project for the Promotion and Enhancement of the Afghan Capacity for Effective Development of the Japan International Cooperation Agency (JICA).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Abdullah, Z.K., Kihara, J., Gondo, Y. et al. Suppressive effect of secondary metabolites from Streptomyces plumbeus isolate F31D against Fusarium oxysporum f. sp. lycopersici, the causal agent of Fusarium wilt of tomato. J Gen Plant Pathol 87, 335–343 (2021). https://doi.org/10.1007/s10327-021-01020-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10327-021-01020-x