Abstract
Wastewater from the livestock industries contains high concentration of nutrients, organic pollutants, suspended solids, and pathogenic microorganisms. Discharge of livestock wastewater without proper treatment can therefore cause serious pollution, calling for advanced treatment methods. For instance, electrochemical treatments are gaining attention because they are clean and flexible. Compared to conventional biological treatment methods, electrochemical processes exhibit higher pollutant removal efficiencies within shorter time periods. Here, we review the electrochemical treatment of livestock wastewater with focus on electrocoagulation, electrooxidation, and electro-disinfection. We present factors controlling effective process design and operation. Pollutants removal from livestock wastewater, economic analysis, and scaling-up considerations are also discussed. The oxidation of electrodes during electrocoagulation induces periodical replacement and thus influences the operational cost. Dimensionally stable metal oxide-layered titanium electrodes such as Ti/PbO2, Ti/RuO2, Ti/IrO2, and Ti/IrO2–Ta2O5–Pt are becoming popular due to their stability. Reactor design and operational parameters govern the effectiveness of the treatment process. Further pilot-scale studies are required to scale up and demonstrate the potential of electrochemical livestock wastewater treatment techniques.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The indisputable world population growth is causing an augmentation in the consumption of meat and dairy products (Huber et al. 2010). Consequently, the livestock farming practices have become more intensive to fulfill the dietary requirement. Intensive livestock farming systems inevitably lead to increased generation of wastewater. Livestock wastewater contains high amount of nutrients, organic pollutants, suspended solids, xenobiotics, and pathogenic microorganisms and has negative environmental consequences along with human health concerns if discharged untreated (Burkholder et al. 2007; Greger and Koneswaran 2010; Wang et al. 2011; Hu et al. 2017; Shim et al. 2021). Treatment of high pollutant-laden livestock wastewater is therefore of utmost importance considering human and environmental health and to meet up the elevating demand for safe and clean water globally.
Up till now, various biological, physical, and chemical treatment methods have been applied for high-strength livestock wastewater remediation; however, still there is a need of processes that can degrade the organic matters in wastewater effectively. Highly polluted waste streams like livestock wastewater generally use biological treatment methods to stabilize the organic matter either aerobically or anaerobically. Among the biological treatment methods, anaerobic digestion is the most commonly employed to comply with the wastewater treatment goals (Kranert et al. 2012). Although anaerobic digestion has the advantage of producing green energy in the form of biogas (Afazeli et al. 2014), nutrients (nitrogen and phosphorus) cannot be removed effectively (Shi et al. 2018). Moreover, anaerobic digestion requires extortionate retention time and large spaces for the effective stabilization of wastewater (Adekunle and Okolie 2015). Further, a reduction in process performance may be observed due to the presence of non-biodegradable or slowly degradable compounds like antibiotics in livestock wastewater (Álvarez et al. 2010). Considering the above, electrochemical treatment technologies can be an effective alternative to overcome the drawbacks of conventional biological treatment processes (Bejan et al. 2005).
Electrochemical treatment of waste streams is not a new invention of modern science. It has been practiced since before the last century. Michael Faraday is called the pioneer of electrolytic treatment technology as he first mentioned the principle of electrolysis in 1820 (Chen et al. 2005). The process occurs in an electrolytic or a salt solution that helps to transfer the ions between two electrodes. After applying electric current, the positive ions shift toward the cathode, whereas the negative ions advance toward the anode. At the initial developmental stages, the primary focus of the process was to deodorize and disinfect the sewage wastewater (Vik et al. 1984). The application of electrochemical processes in sewage wastewater treatment was first mentioned in England in 1889 (Comninellis and Chen 2010). As time passes, electrolytic treatment has become more popular due to several benefits in treating highly polluted and complex municipal, industrial, and agro-industrial wastewaters. The benefits of electrolytic treatment of wastewater include (a) electrons, the sole facilitators of the treatment process, are inherently clean reagents and perform as heterogeneous catalysts, (b) cost-effectiveness, (c) simple in design, (d) could be energy efficient based on application, (e) versatility and flexibility, and (f) ability to treat a diverse array of pollutants (organic, inorganic, and biochemical) (Rajeshwar et al. 1994; Ibanez et al. 2014).
Electrochemical removal of pollutants from livestock waste streams including swine, dairy, and poultry is well illustrated in the literature. The major electrochemical technologies employed for the treatment of highly polluted livestock wastewater are electrocoagulation, electrooxidation, and electro-disinfection. The electrolytic treatment of livestock wastewater has recently drawn the interest of researchers due to the growing importance of the post-treatment of the anaerobically digested effluents. Considering the significance of the context, this review is focused on the electrochemical treatment of high-strength effluents from livestock farms. To be more specific, this review discusses the status of contemporary electrolytic treatment technologies for the remediation of livestock wastewater and provides fundamental information on treatment approaches, process design and operational parameters, economic feasibility, scaling-up consideration, and a direction for future studies in this field.
Technologies for electrochemical treatment of livestock wastewater
Electrocoagulation
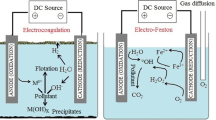
Electrocoagulation enables in situ generation of coagulants during the treatment process when the electric current is passed through sacrificial metal anode materials such as aluminum (Al), iron (Fe), mild steel, and stainless steel (Mook et al. 2014; Lourinho and Brito 2021). Through the introduction of electric current, electro-dissolution of sacrificial anodes takes place and highly charged metal cations are produced which ultimately play the role of actual coagulation agents and remove pollutants from waste streams. Electricity from a direct current source is therefore considered as the main driving force for the solicited chemical reactions in electrocoagulation. The generalized and electrode-specific reactions that occur during electrocoagulation are shown in Table 1 (Eqs. 1–11) (Mollah et al. 2004; Liu et al. 2010; Kabdaşlı et al. 2012; Sahu et al. 2014; Moussa et al. 2017).
Metal cations (M+) are generated due to the oxidation of metal (M) anode. Depending on the total cation concentration and pH, the highly charged synthesized M+ react further and generate metal polymeric hydroxides near the anode. The overall mechanism of the electrocoagulation process was elucidated by Mollah et al. (2004) (Fig. 1). The ionic strength of the solution increases with treatment time, and the colloidal particles having negative charges showed strong affinity toward the anode due to electrophoresis. The particles are aggregated as the electrostatic interparticle repulsion is minimized due to the electrical double-layer compression (Moussa et al. 2017) and mutual collision of electrogenerated hydroxides neutralizes the charges of the colloidal particle (Barrera-Díaz et al. 2011). The mechanism of vanquishing the electrostatic repulsive barrier and authorizing aggregation of particles is denoted as coagulation. The flocs formed due to coagulation generally have high absorption capacity and hence can entrap the pollutants (sweep coagulation) or make a bond with them before removal either through flotation or sedimentation (Ghernaout et al. 2009). On the other hand, simultaneous evolution of hydrogen takes place at the cathode and produces bubbles (Linares-Hernández et al. 2009), which may enable the formed flocs to float on the surface of the treated solution. The main steps of electrocoagulation are shown in Fig. 2. Studies reported that iron anode generally performs better in alkaline conditions, while aluminum anode shows better results in neutral or weakly acidic conditions (Inan et al. 2004; Can et al. 2006). However, depending on wastewater pH, operating conditions, pollutant types and concentration, and electrode materials, the main mechanism of the electrocoagulation may change during the treatment process, and sometimes other electrochemical and chemical phenomena may also occur.
General mechanism of electrocoagulation processes. With the introduction of electric current from an external direct power source, the anode material oxidizes, while the cathode undergoes reduction and reductive deposition of the material takes place. Reprinted from Journal of Hazardous Materials, Vol. 114 (1-3), Mollah et al., Fundamentals, present and future perspectives of electrocoagulation, pp. 199-210, Copyright (2004), with permission from Elsevier
Major steps involved in the electrocoagulation of wastewater. The overall electrocoagulation process is an association of electrochemistry, coagulation, and floatation. Anode dissolution, OH– ions and H2 gas production, and coagulant formation belong to electrochemistry. Target pollutants are removed from the waste stream during coagulation and flotation via destabilization of repulsive forces.
There are several advantages associated with the electrocoagulation treatment, which include simple reactor designs, faster reactions without the addition of chemical coagulants and oxidants, no need of microorganisms, and reduced sludge production. However, the requirement of a minimum level of conductivity and wastewater pretreatment steps like equalization, pH adjustment, etc., to ensure efficient process operation are some of the drawbacks mentioned in previous studies (Mollah et al. 2004; Kabdaşlı et al. 2012). In addition, the rapid consumption of electrodes due to oxidation, electrode passivation, periodical replacement of electrodes, and electricity consumption may lead to high operational costs. Most electrocoagulation studies were performed in small-scale batch reactors for a shorter duration of time; therefore, the performance of full-scale electrocoagulation processes in long runs requires further studies.
Electrooxidation
In electrooxidation, organic pollutants from waste streams which are difficult to treat using conventional biological and chemical methods can be easily removed (Drogui et al. 2007). Anodic oxidation during the treatment is performed either by electrochemical combustion or by electrochemical conversion (Comninellis and Pulgarin 1993). The electrooxidation process is generally classified as (a) direct anodic oxidation and (b) indirect anodic oxidation. Deng and Englehardt (2007) demonstrated the pollutant removal pathways in direct and indirect anodic oxidation (Fig. 3).
Pollutant removal pathways in electrochemical indirect and direct oxidation. Electrochemically generated mediators perform the oxidation process during indirect oxidation, while pollutants are oxidized on the electrode surface during direct oxidation. Reprinted from Waste Management, Vol. 27 (3), Deng Y. and Englehardt J. D., Electrochemical oxidation for landfill leachate treatment, pp. 380-388, Copyright (2007), with permission from Elsevier
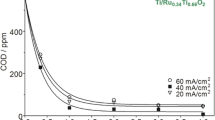
In the case of direct anodic oxidation, direct oxidation of the pollutants takes place at the anode surface via physical and chemical adsorption of active oxygen as hydroxyl radicals (°OH) and oxygen in the oxide lattice (MOx+1), respectively (Comninellis 1994). The overall mechanism of direct anodic oxidation is presented in Table 2 through Eqs. 12–17. Organic pollutants in the wastewater are oxidized completely when reacted with °OH (Eq. 13) (Mook et al. 2012). Hydroxyl radicals are electrophilic in nature and can react promptly and non-selectively with organic compounds rich in electrons (Stasinakis 2008). The chemical absorption occurs at the dimensionally stable electrodes, viz. iridium (IV) oxide (IrO2), lead (IV) oxide (PbO2), ruthenium (IV) oxide (RuO2), tin (IV) oxide (SnO2), etc., where the MOx+1 reacts with organic materials (Mook et al. 2014) (Eq. 15). In addition, molecular oxygen (O2) can be formed due to electrolysis of H2O (parasitic reaction) during direct anodic oxidation (Drogui et al. 2007) which is an influencing factor governing the treatment efficiency of the process. The production of O2 results in reduced process performance. To tackle this, anodes such as boron-doped diamond film on titanium (Ti) electrodes and valve metal (DiaChem) electrodes are found to be helpful (Troster et al. 2002; Chen et al. 2003; Chen 2004). Both the Ti/boron-doped diamond and DiaChem electrodes possess higher oxygen evolution overpotential and prioritize direct anodic oxidation over oxygen evolution at increased current density. Martinez-Huitle and Ferro (2006) denoted °OH and MOx+1 generation, anode material characteristics, and applied electric current as the main factors determining the technical performance of the direct anodic oxidation process. Moreover, it is also visible from Eqs. 16–17 that physically and chemically adsorbed °OH increases O2 formation and thus highlights the significance of adding oxidizing agents to improve the overall oxidation efficiency.
On the other hand, oxidizing agents including chlorine (Cl2), hypochlorite ion (OCl–), hydrogen peroxide (H2O2), ozone (O3), and peroxodisulfate (O8S22–) are commonly used as mediators in indirect anodic oxidation (Canizares et al. 2002, 2005). Chlorine and hypochlorite are usually employed to remove oxidizable pollutants as oxide electrodes are quite active in terms of Cl2 formation (Feng et al. 2003; Bersier et al. 2008). However, the application of oxidizing agents during indirect anodic oxidation can produce mutagenic and carcinogenic by-products [organic chlorinated intermediates (RCl)] (Bergmann and Rollin 2007; Maljaei et al. 2009). The reactions below depict the organic pollutants (R) degradation mechanism during indirect anodic oxidation with chlorine (Table 3; Eqs. 18–22) (Mook et al. 2012):
Apart from removing organic pollutants, direct and indirect anodic oxidation are also found to be effective in removing both free ammonia (NH3) and ionized ammonium (NH4+) from wastewater (Table 4). Ammonia removal through direct anodic oxidation may occur at the electrode–liquid interface (Kapałka et al. 2010), while ammonia can also be removed via indirect oxidation (Anglada et al. 2009). However, the mechanism of ammonia removal through electrooxidation has not been fully explored. At alkaline conditions (pH > 9), anodic adsorption of NH3 takes place in the reaction zone nearby the electrode surface and is oxidized directly to nitrogen gas (N2) through a three-electron exchange, whereas at acidic conditions (pH < 7), although the dominant nitrogen species exist as NH4+ in liquid, it is difficult to mineralize the ammonia ions. Considering this, Gerischer and Mauerer (1970) suggested an ammonia removal mechanism which is now widely accepted by researchers. According to their suggested mechanism, deprotonation of NH3 is done by hydroxide (OH–) in three steps: generation of H2O molecule and release of electron → absorption of M(°NHa) and M(°NHb) → formation of N–N (dimeric) species (Eqs. 23–26). The dimeric nitrogen species finally deprotonated to N2, desorbed, and then released (Eq. 27). Studies reported that the deprotonated M(°N) formed during this process is poisonous and can cause a blocking effect at the active sites in electrode surface (Bonnin et al. 2008).
Furthermore, the ammonia from waste streams may also be removed by indirect oxidation via generation of hypochlorite (HOCl) during anodic oxidation of Cl– (Cabeza et al. 2007; Zhang et al. 2010; Panizza and Martinez-Huitle 2013). Li and Liu (2009) mentioned that, due to slow indirect oxidation of °OH, ammonia removal during the treatment is mainly attributed to the indirect oxidation of HOCl. The overall ammonia removal mechanism during indirect oxidation is depicted in Eqs. 28–32. The oxidation of chloride ions (Cl−) forms HClO which reacts further with free NH3 or NH4+ through breakpoint chlorination to generate chlorinated by-products of ammonia. The derivatives are hydrolyzed further and produce completely oxidized products such as N2, H2O, Cl−, and H+. However, nitrogen trichloride (NCl3) and nitrate (NO3−) as intermediates and products, respectively, may be formed depending on the pH and ionic properties of the wastewater (Jafvert and Valentine 1992; Chiang et al. 1995; Li and Liu 2009). In general, the electrochemical reactions in an electrooxidation process mainly rely on the experimental conditions, and more precisely, the characteristics of the electrodes used during the treatment. Electrochemical oxidation can further be advantageously coupled with conventional biological livestock wastewater treatment processes. Hence, further studies can be directed toward evaluating the performance of these combined processes.
Electro-disinfection
Disinfection is one of the most commonly used wastewater treatment processes for the efficient removal of various pathogenic microorganisms. Among different water disinfectants, the application of chlorine or chlorine compounds has been considered a common approach (Hossain et al. 2014). However, excessive addition of chlorine in the presence of natural organic matter leads to residual carcinogenic chloroform generation in the water (Ghernaout et al. 2011). Although other disinfection techniques, viz. ozonation, advanced oxidation, ultraviolet radiation, and solar photo-fenton, are effective in terms of instant disinfection (Hijnen et al. 2006; Gilboa and Friedler 2008; Lee et al. 2011; Ferro et al. 2015; Al-Qodah et al. 2018; Maniakova et al. 2021), these treatment methods are generally costly and often show reduced treatment efficiency after completion of the process. Rutala and Weber (2008) stated a set of criteria of a perfect disinfectant process which includes removal of a wide range of pathogens within a short time, safe for human health, should not produce harmful by-products during or after treatment, easily applicable, cost-effective, high water solubility, non-corrosive for the equipment, and safe to dispose. Considering the above-mentioned aspect, electrochemical disinfection has gained more attention among researchers in recent years (Simas et al. 2019). The process has advantages including abatement of an extended range of microorganisms, nominal chemical usage, no issues with storage, space-saving, easy operation, and low maintenance cost (Schmalz et al. 2009).
During electrochemical disinfection of wastewater, influent flows through a disinfection chamber equipped with electric current-charged electrodes (Diao et al. 2004). The most influencing parameters of the electro-disinfection process are reactor configuration, electrodes, electrolyte composition, microorganisms, current density, and pH (Chen 2004; Kerwick et al. 2005; Jeong et al. 2007, 2009; Schmalz et al. 2009). According to Mook et al. (2014), the setups of the electrochemical disinfection treatment process can be divided into two groups: (a) direct electrolyzer and (b) mixed oxidants generator. In a direct electrolyzer, oxidants are produced directly from the wastewater using the electrolyzer, while strong oxidizing species such as active chlorine (Cl2, HOCl, and OCl−), chlorine dioxide (ClO2), O3, H2O2, and other short-live radicals are fabricated from the supplied concentrated brine solution in the mixed oxidants generator (Diao et al. 2004; Kerwick et al. 2005; Jeong et al. 2007). The reactions that take place in different electro-disinfection processes are exhibited in Table 5 (Eqs. 33–41). In the field of electrochemical disinfection of waste streams, electro-chlorination is regarded as one of the most popular methods. Details of electro-chlorination can be obtained from the study conducted by Cotillas et al. (2013). However, Jeong et al. (2009) reported that active chlorine and reactive oxygen species produced during wastewater oxidation possessed higher disinfection ability than that of electro-chlorination. For water where low or zero Cl– concentration is needed, electrochemical production of O3 (electro-ozonation) may serve as an alternative disinfection process. Ozone can be produced during electrolysis applying high O2 overvoltage and current density in a low temperature (Ghernaout and Ghernaout 2010). Kraft (2008) mentioned that a simple ‘sandwich’ electrode assembly configuration comprised of diamond anode/screen-printed electrode/cathode may improve the electrochemical O3 production during the treatment. On the other hand, H2O2, unlike other electro-disinfectants, can be generated at the cathode using the O2 present in wastewater as the reactant (Ghernaout and Ghernaout 2010) (Eq. 41), whereas the application of electrolytically produced H2O2 is still limited due to its lower oxidation potential.
For the past few years, wastewater treatment system including livestock has been reinvested from the circular economy perspective, where sustainable use and reuse of water has been considered along with other aspects like biogas production and by-products transformation. Due to the abundance of zoonotic pathogens in wastewater, it is of utmost importance to disinfect the wastewater before reuse and thus highlight the salience of electrochemical disinfection as an eco-friendly and sustainable process.
Factors affecting electrochemical treatment of livestock waste streams
The performance of any electrochemical treatment process depends on the design and operational parameters (Moussa et al. 2017). Design parameters include electrode material, interelectrode gap, electrode arrangement, and mode of operation of the reactor, while operational parameters such as current density or voltage, electrolyte concentration, pH, conductivity, temperature, operation time, and mixing speed play key roles in electrochemical reactions and regulate the performance of the treatment process (Hakizimana et al. 2017; Al-Qodah and Al-Shannag 2017). The detail of the design and operation parameters will be discussed below.
Reactor design parameters
Electrode material
The efficiency of electrochemical treatment is governed by the electrode material. Electrodes used in the treatment process can be classified into two types, including sacrificial electrodes and insoluble electrodes. Sacrificial electrodes are generally fabricated using polyvalent metallic materials. Metals such as Al and Fe are most widely used as sacrificial electrodes in the electrochemical treatment of wastewater to avail the benefits of multivalent ions coagulating properties. Some other metals such as silver (Ag), arsenic (As), barium (Ba), calcium (Ca), cadmium (Cd), cesium (Cs), magnesium (Mg), sodium (Na), silicon (Si), strontium (Sr), and zinc (Zn) can also be used as sacrificial electrodes. In addition, mild steel and stainless steel electrodes are utilized by the researchers due to their ability to perform electrocoagulation and because they are anodically soluble (Sahu et al., 2014). On the other hand, dimensionally stable anodes including SnO2, PbO2, graphite, nickel (Ni), etc., and boron-doped diamond electrodes are insoluble, easily available, and cost-effective (Al-Qodah and Al-Shannag 2017). Insoluble electrodes possess greater chemical resistance and show higher treatment efficiency. Furthermore, Comninellis (1994) proposed a simple classification considering the role of insoluble electrodes on oxygen evolution reaction where carbon, graphite, platinum (Pt), IrO2, and RuO2 were denoted as active anodes and antimony doped tin oxide (Sb:SnO2), PbO2, and boron-doped diamond were posited as inactive anode.
In general, the application of both dimensionally stable and sacrificial electrodes is evident from previous studies in terms of electrochemical treatment of swine wastewater (Table 6), whereas sacrificial electrodes are mostly used in dairy wastewater treatment (Table 7). The majority of the electrolysis of poultry manure studies employed insoluble electrodes such as Ti/Pt-IrO2 and Pt/Ti-IrO2 (Table 8). The physical and chemical properties of the sacrificial and insoluble electrodes are different in nature. While selecting electrodes, characteristics such as ion size and charge, oxidation potential, migration speed in the solution along with cost-effectiveness, regeneration competence, and toxicity or environmental impact are needed to be considered (Gürses et al. 2002; Moreno-Casillas et al. 2007; Al-Shannag et al. 2015). Moreover, as metal hydroxide plays a crucial role in electrochemical treatment, the polarity of the hydroxide bond and its structure and size have a significant effect on the adsorption capability of a specific hydroxide (Ghazouani et al. 2019; Chezeau et al. 2020). It is therefore highly important to consider the above-mentioned properties in the selection of electrode material for designing an efficient electrochemical treatment process. Recently, the application of dimensionally stable electrodes having a layer of precious metal oxide on titanium (Ti/PbO2, Ti/RuO2, Ti/IrO2, and Ti/IrO2–Ta2O5–Pt) in treating livestock waste streams has increased in comparison with other electrodes because of their high resistance against corrosion and conductivity. In addition, although not evident the performance of 3D electrodes fabricated from nanomaterials can also be tested in the electrolytic treatment of livestock waste streams. Most of the studies until now were conducted in small-scale reactors for comparatively shorter durations, so the performance of different electrode materials in treating livestock waste streams in long runs requires further studies.
Interelectrode gap
The interelectrode gap is an important design parameter that affects the performance of the electrochemical treatment process. In livestock wastewater treatment, most of the studies maintained interelectrode gaps of 1–2.5 cm (Tables 6 and 7). However, a higher interelectrode gap of 6 cm was reported in studies focused on the electrochemical treatment of poultry manure (Table 8) (Yetilmezsoy et al. 2009; Wang et al. 2015b, 2020). During treatment, it regulates the mobility of the ions through electrostatic attraction, influent residence time, and swirling velocity and ultimately influences removal efficiency of the treatment process (Asselin et al. 2008; Nasrullah et al. 2012; Khandegar and Saroha 2013b). The reactions that occurred in the electrolytic reactor are generally inversely proportional to the interelectrode gap (Cho et al. 2010). A narrow interelectrode gap results in the solids coagulation and colloidal materials adsorption on the electrodes, thus affecting the water flow in the reactor (Daneshvar et al. 2004), whereas a large distance between the electrodes will definitely hinder the electrolytic reaction due to a decrease in electrostatic attraction and an increase in ion mobility. The interelectrode gap also determines the amount of energy needed in the electrochemical treatment. A trivial spacing between electrodes results in low power consumption, while an increase in energy consumption is observed with the increase in the interelectrode gap (Bayramoglu et al. 2007). Several studies reported that a distance approximately 1 cm or in the range of 0.5–2 cm can minimize the solution agitation between the electrode and remove pollutants efficiently from wastewater (Chen et al. 2000; Mollah et al. 2001; Kim et al. 2003; Kobya et al. 2003, 2006; Daneshvar et al. 2006; İrdemez et al. 2006; Costa and Olivi 2009; Liu et al. 2009; Wang et al. 2009; Ghahremani et al. 2012; Benazzi et al. 2016; Melchiors et al. 2016; Zakeri et al. 2021).
Another important aspect of having an appropriate interelectrode gap is its influence on the ohmic potential drop or IR drop. The ohmic potential drop indicates the difference in potential needed for transferring the ions through the solution. This drop-in IR takes place due to solution resistance (Vasudevan et al. 2011) and has a significant influence on the reactor performance. IR drop can be expressed as
where I indicates the electrical current (A), D denotes the interelectrode gap (m), A represents the active anode surface area (m2); and k signifies the specific conductivity (mS/m). From Eq. (42), it can be said that an increase in the interelectrode gap increases the IR drop. Hence, the applied voltage should be augmented to uphold a constant current input to the reactor. Therefore, an optimum interelectrode gap should be maintained to prevent the rise in IR drop and thus to achieve maximum removal efficiency of the pollutants during electrolysis. Further studies are still needed to determine the electrode-specific optimal interelectrode gap.
Electrode arrangements
Electrochemical treatment processes are generally comprised of plate electrodes, and the solution (influent) circulates through interelectrode space (Chen 2004). Studies reported that the electrodes in an electrochemical system can be arranged in either monopolar or bipolar systems (Fig. 4) and the influent inside the reactor can circulate in either vertical or horizontal direction. In the monopolar-series arrangements (Fig. 4a), all the anodes are connected with each other, and likewise, all cathodes are also coupled with each other, resulting in identical electric current and stabilizing the voltage. This arrangement shows a relatively low difference in potential as the current is distributed between the electrodes (Moussa et al. 2017). In this configuration, each pair of anode and cathode epitomizes a small electrolytic of equal voltage and additive current. In terms of bipolar-series systems (Fig. 4b), the two outermost anode and cathode (monopolar) are connected to an external electric power supply and internal electrodes are bipolar. The internal bipolar electrodes possess antithetical charges on its two sides. The positive side is for metal oxidation, and the cathodic reaction takes place on the negative side (Mollah et al. 2001; Demirci et al. 2015).
Electrode arrangements (a) monopolar and (b) bipolar. In general, the monopolar arrangement of electrodes has lower removal efficiency and operational cost than that of the bipolar arrangement. Reprinted from Separation and Purification Technology, Vol. 61 (3), Drogui et al., Electrochemical removal of pollutants from agro-industry wastewaters, pp. 301-310, Copyright (2008), with permission from Elsevier
It is evident from the earlier studies on livestock wastewater that the difference in electrode arrangement will lead to contrasting results (Tables 6, 7, and 8). Contrary to bipolar electrode systems, monopolar electrode system requires a lower voltage and a higher current. Hence, while arranging the electrodes response parameters such as yield and efficiency are needed to be considered along with the cost of the electrochemical treatment process. In general, the monopolar electrode arrangement is more beneficial for the studies focused on treatment efficiency and cost management due to its low energy requirement (Naje and Abbas 2013). On the other hand, bipolar arrangement is more suitable if the treatment objective is to obtain the maximum yield (Bayramoglu et al. 2007). However, Laridi et al. (2005) reported a marginally higher treatment cost during electrolysis of liquid swine manure in monopolar electrode arrangement (0.29 USD/1000 L) than the bipolar arrangement (0.24 USD/1000 L). Although the treatment cost in the monopolar electrode system was a bit higher, the cost-effectiveness ratio for both the electrode systems was almost similar. In addition, the pH values of the discharged effluent were close to neutral in monopolar arrangement of electrodes. Therefore, it can be said that energy consumption and treatment objective together with maintenance cost should be given due consideration at the time of electrode arrangement.
Electrode shape and orientation
Very few works have been conducted to evaluate the role of electrode shape and orientation on the performance of the electrochemical treatment process. The rectangular-shaped electrodes are generally used in the electrochemical treatment of wastewater. Some studies used circular and cylindrical geometries and punched-holes-type electrodes (Logan 2005; Tran et al. 2009; Daghrir et al. 2014; Nassef 2014; Guitaya et al. 2015; Khandegar and Saroha 2016; Hakizimana et al. 2017; Al-Rubaiey and Al-Barazanjy 2017; Khandegar et al. 2018; Avancini Dias et al. 2019; Mohammadi et al. 2019). Circular-shaped electrodes embedded in insulation material are commonly used in laboratory-scale studies for kinetic measurements, while in technical applications disk-shaped electrodes are more preferable (Muddemann et al. 2019). On the other hand, punched-holes electrodes possess discharge current and subsequently higher collection efficiency is observed in comparison with the plane electrodes. Such phenomenon is subjected to the higher electric field density at the border of punched-holes electrodes, which is 1.2 times higher than the plane electrodes (Kuroda et al. 2003). Regarding electrode orientation, electrodes in the electrochemical system are generally installed vertically with a few cases of horizontal orientation (Harif et al. 2012). Muddemann et al. (2019) reported that electrodes having expanded metal shape are generally fixed horizontally adjacent to the bottom of the electrochemical reactor. Moreover, Fouad et al. (2009) observed a higher mixing efficiency when electrodes are settled in the horizontal direction in a batch reactor. In the electrochemical treatment of livestock wastewater, rectangular electrodes are mostly used in the vertical orientation; however, the shape and orientation of the electrode should be taken into account at the time of designing the treatment process.
Mode of operation
The electrochemical treatment process is often designed considering a specific purpose. Along with the form of the reactants and products, the operational mode of the reactor is also considered an important design parameter (Walsh and Mills 1994). Holt et al. (2005) classified the operational modes of an electrochemical reactor considering three major aspects, viz. batch versus continuous operation, feed flow direction, and method of aggregated pollutants separation.
Batch system reactors are convenient to study the time-dependent behavior of the treatment process with a constant feed working volume per treatment without any feed and product stream (Fig. 5a) and thus serve as a basis of continuous operation of electrochemical reactors through optimization of the process parameters, whereas continuous systems are known for inherent dynamic operations and stability (Fig. 5b). Hence, it is easy to study the thermodynamic and kinetic considerations using mathematical modeling in the continuous operation mode of electrochemical reactors. Moreover, the effect of influent load on reactor performance can be studied at different flow regimes. The electrode response concerning floc formation and its dissolution in the liquid can also be observed in continuous mode along with electrode passivation and corrosion over time (Mahesh et al. 2006a, b; Petsriprasit et al. 2010). Levenspiel (1998) mentioned that continuous process operation is more beneficial and cost-effective to treat large volumes of wastewater than batch systems. The second aspect considers the direction of feed flow (horizontal or vertical) (Jiang et al. 2002). In the electrolytic treatment of livestock wastewater, generally feed flow is horizontal, while the third aspect deliberates to the process related to the separation process of accumulated flocs. The accumulated pollutants during the electrochemical treatment can be separated using either in situ flotation or precipitation or downstream separation process such as centrifugation (Al-Qodah and Al-Shannag 2017). Not too many studies related to the electrolytic treatment of livestock wastewater have been carried out using continuous operation (Aitbara et al. 2014, 2021; Benazzi et al. 2016; Bassala et al. 2017; Chakchouk et al. 2017; Eulmi et al. 2019; Shim et al. 2020), while most of the studies have been conducted in batches. A few electrochemical studies on continuous operation have also been performed in conjunction with other treatment processes, but mostly on swine wastewater (Liu et al. 2011a; Diaz and Botte 2012; Lahav et al. 2013; Han et al. 2015; Wang et al. 2019b; Shim et al. 2020). Although there is a lack of understanding in the mechanistic perspective of the electrochemical treatment process due to the absence of an appropriate empirical or systematic approach, the selection of operational mode of the reactor during electrolysis of livestock wastewater solely depends on the primary objective of the treatment process.
Reactors used in electrochemical livestock wastewater treatment. (a) Batch reactor used for pollutants removal from dairy wastewater during electrolysis. Reprinted from Journal of Electroanalytical Chemistry, Vol. 775, Davarnejad R. and Nikseresht M., Dairy wastewater treatment using an electrochemical method: Experimental and statistical study, pp. 364-373, Copyright (2016), with permission from Elsevier. (b) Electrochemical removal of pollutants from swine wastewater using continuous flow reactor. Reprinted from Journal of Hazardous Materials, Vol. 180 (1-3), Cho et al., Effects of electric voltage and sodium chloride level on electrolysis of swine wastewater, pp. 535-541, Copyright (2010), with permission from Elsevier
Reactor operational factors
Current density
Current density is a key operational factor that strongly influences the performance of electrochemical treatment of waste streams (Hakizimana et al. 2017). Current density explains the ratio between electric current (I) and effective surface area of the electrode (A) and can be directly manipulated. This operational parameter regulates both the amount of coagulant input and gas bubbles’ generation rates and thus significantly affects the solution mixing and mass transfer at the electrode, simultaneously (Sahu et al. 2014). Hakizimana et al. (2017) reported the amount of electrical energy consumed (P) during electrochemical treatment as a function of operating time (t), potential (U), and electric current (I) (Eq. 43):
In general, the electrochemical treatment of wastewater can be carried out in two different ways. In the first mode (for electrocoagulation) where sacrificial electrodes are used, the treatment process is conducted by controlling and/or varying the applied current through the electrodes. Current density can be varied as a function of coagulant dosage during electrocoagulation which is dependent on the amount of electricity that passed through the treatment solution. However, in the second mode (for electrooxidation and electro-reduction processes) where insoluble electrodes are employed, mainly cell voltage is controlled (Ruotolo and Gubulin 2011). It is worth mentioning that applying a very high level of current could initiate secondary reactions and ultimately decreases the treatment efficiency by reversing the colloidal charges and redispersing them (Harif et al. 2012). This phenomenon can also reduce the lifetime of electrodes. Harif and Adin (2011) developed a conceptual model to predict the floc evaluation rate and structure under different applied currents (Fig. 6). Moreover, Liu et al. (2010) mentioned that the influent pH, temperature, and flow rate can influence the optimal current density of a treatment process and consistently encompasses a trade-off between operational costs and effective use of sacrificial electrodes. The current supply during electrochemical treatment limits the release amount of Al3+ or Fe2+ ions from the respective electrodes. Sahu et al. (2014) suggested that the current density should be maintained between 2 and 2.5 mA/cm2 with some periodical cleaning of electrode surface for the efficient and longer operation of an electrochemical treatment process without any major maintenance. However, in terms of electrochemical treatment of livestock wastewater a wide range of current density has been reported (Tables 6, 7, and 8). For sacrificial electrodes, the current density varied between 0.5 and 56 mA/cm2, while a current density of as low as 0.004 mA/cm2 was reported for insoluble electrodes (Won et al. 2016). Further studies can be done to determine electrode-specific optimal current density for a particular livestock waste stream.
Conceptual model for floc evaluation rate and structure prediction under high and low applied current situations. Along with affecting the surface charge of the generated stable sweep flocs, the amount of applied current governs the formation and size of flocs in a colloidal suspension via maintaining a subtle balance between the precipitates of metal hydroxide precursors at the early stages of the treatment process and generation of bubbles in the solution. The surface charge of the flocs also plays an important role in determining the precursor particles bonding strength, collision efficiency, and floc structure. Reprinted from Water Research, Vol. 45 (18), Harif T. and Adin A., Size and structure evolution of kaolin–Al(OH)3 flocs in the electroflocculation process: A study using static light scattering, pp. 6195-6206, Copyright (2011), with permission from Elsevier
Electrical conductivity
The higher ionic strength results in an increment in current density in the same cell voltage, or an increase in influent electrical conductivity at a constant current density decreases the cell voltage (Song et al. 2002; Kobya et al. 2003). Therefore, it is essential to examine the effect of influent electrical conductivity on pollutants removal in the electrochemical treatment of waste streams. The type and concentration of electrolytes are the main determinants of influent conductivity in a treatment process. Different types of salts such as sodium chloride (NaCl), barium chloride (BaCl2), potassium chloride (KCl), sodium sulfate (Na2SO4), and potassium iodide (KI) have been added as electrolytes during the treatment process. NaCl is the most commonly used electrolyte to increase electrical conductivity. The addition of NaCl in a treatment process provides an abundant number of Cl− that produce Cl at the anode and instantly form HOCl by reacting with water. The formed HOCl then reacts with NH3 present in the solution and produces N2 gas. Moreover, NaCl significantly minimizes the antagonistic effect of other anions like bicarbonate (HCO3−) and sulfate (SO42−) which can decrease the current efficiency by promoting calcium ion (Ca2+) and magnesium ion (Mg2+) deposition and oxide layer formation (Chen 2004). Holt et al. (2005) recommended that for effective and efficient electrochemical treatment of wastewater, Cl− concentration should be maintained well over 200 mg/L (0.02%). Furthermore, the production of Cl− during electrochemical treatment can also contribute to water disinfection. Future studies need to ascertain an optimum dose of electrolytes for efficient operation of electrochemical livestock wastewater treatment processes.
Temperature
Temperature is another process parameter that regulates the pollutant removal efficiency in the electrochemical treatment of waste streams (Sahu et al. 2014). However, none of the studies related to electrolysis of livestock wastewater have studied the effect of temperature on pollutant removal. Electrochemical treatment of livestock wastewater is generally performed at room temperatures (from 18 to 27 °C) (Ghahremani et al. 2012; Valente et al. 2012; Aitbara et al. 2014; Rahman and Borhan 2014; Han et al. 2015; Benaissa et al. 2016; Huang et al. 2018b). Han et al. (2015) found that minor fluctuations in temperatures (20.8 ± 3.4 °C) did not have any significant effect on turbidity removal from anaerobically digested swine wastewater. Vasudevan et al. (2009) studied the phosphate removal from high-strength synthetic wastewater through electrochemical treatment at different temperatures ranging from 20 to 60 °C and reported a decrease in removal efficiency at a low temperature (20 °C). They also mentioned that a decrease in the dissolution rate of the sacrificial anode at low temperatures might lead to a reduction in phosphate removal efficiency. Although it is well known that an increase in temperatures augments mass transfer and collision kinetics of the particles, Katal and Pahlavanzadeh (2011) observed a decrease in chemical oxygen demand (COD) and phenol removal efficiencies in electrochemical treatment of paper mill wastewater. They stated that such phenomena occurred due to the constriction of the large pores of aluminum hydroxide (Al(OH)3) gel at a high temperature. This constriction results in dense flocs formation, which are deposited on the electrode surface rather than floating in the system (Chen 2004). Such contradictory results necessitate the significance of studying the role of temperature in the electrochemical removal of pollutants from livestock wastewater. Further studies should be focused on the effect of temperature on pollutants removal during electrolysis of livestock wastewater, specifically increasing the temperature to enhance chemical reaction.
pH
The initial pH of the influent is an important operational parameter that affects the performance of the electrochemical treatment of wastewater. The pH regulates the ionic conductivity of the wastewater along with influencing the electrode dissolution and OH− speciation and controls the zeta potential of colloidal particles during electrolysis (Sahu et al. 2014). In fact, the type and amount of coagulant released to the influent are governed by the OH− speciation which ultimately determines the coagulation pathway. The coagulation mechanism explains the interaction between coagulant and pollutant particles in an electrochemical treatment. Therefore, it is important to maintain an optimum pH to achieve maximum removal efficiency of the pollutants during the treatment process. Although a theoretical perspective explains the relationship between pH and treatment efficiency of the electrochemical process, at acidic pH metal cations and ion-complexes in wastewater neutralize the superficial charges of colloidal particles, and a reduction in solubility can be observed. On the other hand, cations and ion-complexes are fully hydrolyzed to produce precipitates (amorphous hydroxides) and lead to adsorption/interparticle bridging and sweep coagulation at neutral or slightly alkaline pH (5–9) (Lourinho and Brito 2021). During electrolysis, livestock wastewater using sacrificial electrodes, particulate, and dissolved organic matter removal process can be explicated by the latter mechanism (Jiménez et al. 2012; Marriaga-Cabrales and Machuca-Martínez 2014). Hence, the efficacious electrochemical livestock wastewater treatment can be performed at a pH range of 6–8 (Tak et al. 2015). Depending on the type of wastewater and target pollutant, however, this range should be experimentally optimized.
Treatment time
Another significant operating factor in the electrolysis of wastewater is treatment time. According to Faraday’s law, theoretically, the duration of the experiment influences the amount of electrode dissolution and cation production from the electrodes during the electrochemical treatment process (Şengil and Özacar 2006; Drouiche et al. 2009). Destabilization and accumulation are the two major processes involved in electrolysis (Bazrafshan et al. 2012). The first step is generally short, while the second one requires a relatively long time. As the treatment time increases, both the electrode and energy consumption increases along with the treatment and maintenance cost which indicates the significance of the treatment duration (Tir and Moulai-Mostefa 2008). Moreover, electrolysis time also governs the treatment efficiency of the process by regulating the current density and pH of the influent (Abdel-Gawad et al. 2012). The mixing of influents in the reactor is also affected by the electrolysis time (Tak et al. 2015). With the increase in reaction time, hydrogen bubble production at the cathode intensifies. A rise in bubble production improves the influent mixing capacity and enhances the cell flotation ability, floc formation rate, and growth of aggregates and, consequently, escalates the removal efficiencies of the pollutants (Mouedhen et al. 2008; Uğurlu et al. 2008; Zaroual et al. 2009; Kabdaşlı et al. 2012). In the case of electrochemical treatment of swine and dairy wastewater, treatment durations between 30–90 and 30–120 min, respectively, were found to be sufficient when sacrificial electrodes were used, while a much higher treatment time was reported by the studies conducted with dimensionally stable electrodes. Studies also reported the phenomenon of time-dependent pollutant removal equilibrium (Laridi et al. 2005; Han et al. 2015). Therefore, longer reaction times may improve removal efficiency during electrochemical treatment until achieving equilibrium.
Mixing speed
Moderate mixing speed is generally employed to maintain steady conditions, restrict concentration gradient formation, and increase the mobility of the ions generated from anodic oxidation inside the electrochemical reactor during electrolysis (Naje et al. 2017). Proper mixing of influent also increases the mobility of the ions generated from anodic oxidation and attribute to early floc formation; consequently, the recovery efficiency of the pollutants improves. However, an optimal mixing speed should be maintained during the treatment process as flocs can collide and degrade if higher mixing speed is applied and ultimately hinders the process performance (Bayar et al. 2011; Khandegar and Saroha 2012, 2013a, 2014; Lekhlif et al. 2014; Khaled et al. 2019). In addition, a lower mixing speed can decrease the removal efficiency of the pollutants at a specific time due to reduced ion mobility. It is worth mentioning that mixing speed in electrochemical treatment of livestock wastewater has been rarely optimized. Bhushan and Kulkarni (2016) performed electrochemical treatment of dairy wastewater in batches using mixing speeds of 50, 100, 150, and 200 rpm. They reported the mixing speed of 150 rpm as an optimal for efficient removal of COD and biological oxygen demand (BOD). During electrochemical treatment of livestock wastewater, mixing speeds were commonly varied from 50 to 300 rpm, whereas higher mixing speeds of 500 and 800 rpm were also found in the existing literature (Tchamango et al. 2010; Borbón et al. 2011; Benaissa et al. 2016; Majlessi-Nasra et al. 2020). The information presented above thus highlights the significance of optimizing mixing speeds for efficient removal of pollutant from livestock waste streams.
Pollutant removal from livestock waste streams by electrochemical treatment
Reactor design and operational conditions are the determining factors governing the pollutants removal efficiency from livestock waste streams, which is apparent from past studies. Despite using the same electrode material, differences in design and operational parameters lead to significant variations in the process performance. Summary of the studies on the electrochemical treatment of livestock wastewater for the past two decades is presented in Tables 6, 7, and 8.
In the case of swine wastewater, the majority of the studies used laboratory-scale batch reactors, while continuous operations were generally performed in association with other treatment technologies (Liu et al. 2011b; Diaz and Botte 2012; Lahav et al. 2013; Mores et al. 2016b; Wang et al. 2019b). Han et al. (2015) used nanofiltration to reclaim nutrients from swine farm-originated biogas digestion slurry where electrolysis was employed as a pretreatment method. Another study reported the effectiveness of electrochemical treatment as a final polishing treatment of swine wastewater (Shim et al. 2020). The deodorization potential of electrochemical treatment has also been evident from earlier studies (Bejan et al. 2005; Ding et al. 2021). Electrocoagulation and electrooxidation are mainly used to treat swine wastewater electrochemically (Table 6). During electrocoagulation of swine wastewater, based on operational conditions COD, BOD, total organic carbon (TOC), soluble total organic carbon (STOC), ammonia nitrogen (NH3-N), and total phosphorus removal efficiencies generally varied in the range of 65.2–80, 50–87.3, 69–85, 94–96.1, 56.3–100, and 78.2–100%, respectively. Regarding NH4+, total nitrogen, orthophosphate, and color, the removal efficiencies were found to be 87, 61, 96, and 97%, respectively. However, lower removal efficiencies as low as 10–11.5% have also been observed for BOD, total nitrogen, and NH4+ (Mores et al. 2016a; Chen et al. 2021a, b). On the other hand, electrooxidation demonstrated much effective (around 90–100%) removal of pollutants from swine wastewater. Furthermore, effective removal of microorganisms from swine wastewater via electro-disinfection has been described in studies carried out by Bejan et al. (2007), Simas et al. (2019), and Shim et al. (2020). Considering the removal efficiencies of the pollutants, electrooxidation of swine wastewater appeared as an efficient treatment technology.
In the electrochemical treatment of dairy wastewater, most of the studies employed electrocoagulation to remove pollutants from the waste streams. Studies showed that COD, BOD, TOC, total nitrogen, total phosphorus, turbidity, and color removal efficiencies usually lie in the range of 70–98, 78–97.9, 60–81, 81–100, 81–98, 90–100, and 87.5–94.9%, respectively, depending on the experimental conditions (Table 7), whereas Smoczynski et al. (2013) and Chezeau et al. (2020) found lower turbidity (40%) and total nitrogen (10%) removal efficiencies, respectively, in their studies. Although limited studies applied electrooxidation technology for treating dairy wastewater, due to higher effectiveness and ease of application, control electrooxidation processes seem to gain more attention recently (Markou et al. 2017). Electro-oxidative removal of COD, BOD, total phosphorus, turbidity, and color commonly ranged between 75 and 97, 81 and 98, 81 and 98, 84 and 100, and 90 and 95%, respectively (Table 7). Nonetheless, lesser removal of COD (32%), total nitrogen (19%), and turbidity (70%) was also reported in previous studies (Ihara et al. 2006; Stylianou et al. 2020; Turan 2021). None of the studies reported microorganism removal potential of electrochemical treatment in dairy wastewater.
On the other hand, electrochemical oxidation is mainly employed in treating poultry manure (Table 8). All the studies used electrooxidation in combination with microalgae cultivation for removing pollutants from poultry manure except Yetilmezsoy et al. (2009). The study conducted by Yetilmezsoy et al. (2009) employed electrocoagulation for COD removal and decolorization of pretreatment poultry manure. They observed around 90 and 92% of COD and color removal, respectively, from poultry manure using Al electrodes. Although a similar COD removal (86%) was found when using Fe electrodes, the color removal efficiency was reduced to half (46%). On the other hand, Wang et al. (2015a) combined electrooxidation with microalgae cultivation for efficient removal of NH4+, turbidity, total phosphorus, and inorganic carbon as a pretreatment of anaerobically digested poultry manure using Ti/Pt-IrO2 electrodes, and the removal efficiencies ranged from 96.6 to 100%, except TOC (81.5%). Moreover, Ti/Pt-IrO2 electrodes were also found to be effective in removing all the bacteria from poultry manure (Wang et al. 2015a, 2017). On the contrary, Pt/Ti-IrO2 electrodes showed reduced removal efficiency of pollutants during electro-oxidative treatment of poultry manure (Wang et al. 2015b, 2020). Compared to studies on the electrolytic treatment of swine and dairy wastewater, very few studies were found in the available literature that applied electrochemical technologies to treat poultry manure. Therefore, electrochemical treatment of poultry manure still requires further investigation.
Economic perspective
The operating cost of any treatment process is the most important factor that must be taken into account as it determines the potentiality of the process upscaling (Vasudevan et al. 2012; Bazrafshan et al. 2015; Hashim et al. 2017). Electrochemical treatment processes therefore need to be cost-effective to ensure its large-scale applicability as there are many alternatives available for the treatment of high-strength livestock wastewater (Butler et al. 2011). In fact, the fluctuating energy prices worldwide and the utilization of electric current as the main operating parameter may epitomize a serious drawback that can discourage the full-scale application of electrochemical treatment processes (Moussa et al. 2017). Considering this, optimization of the electrochemical treatment process is a crucial step to demonstrate its cost-effectiveness before any large-scale application. Despite numerous publications on the electrolysis of livestock wastewater, systematic studies pertaining to process optimization are generally scarce.
Laridi et al. (2005) compared electrochemical and chemical treatment processes of liquid swine manure. They reported that the cost of electrochemical treatment was less than 0.30 USD/1000 L of swine manure, while a much higher cost (1.64 USD/1000 L) was involved in chemical treatment of swine manure. Based on their analysis and comparison, the electrochemical treatment appeared to be a more cost-efficient and eco-friendly process. Ikematsu et al. (2007) showed a comparison among the operating costs of different treatment methods for treating 1,000 L of swine wastewater. They found that electrolytic treatment of swine wastewater is the most cost-effective one in terms of treatment time and area required for treatment. Subsequently, Geraldino et al. (2015) employed a response surface methodology (RSM) using full experimental planning with three variables (pH, electric current intensity, and electrolytic reaction time) and three levels (+ 1, 0, − 1) to optimize and ascertain the interactions of important process parameters on the turbidity, color, and COD removal efficiency of dairy wastewater. They delineated that RSM was a suitable method for optimizing the operational parameters to maximize the pollutants removal efficiencies along with minimum operating costs. Furthermore, Kuokkanen et al. (2015) reported an operating cost of 0.36 USD/1000L of electrolytic treatment of dairy wastewater. The following equations can be used to calculate the energy consumption and operating cost of electrochemical treatment of wastewater using sacrificial electrodes (Eqs. 44–45) (Geraldino et al. 2015):
where Cen is the electric energy consumption (kWh/m3), U is the voltage (V), i is the DC current (A), t is the treatment time (h), and V is the volume of treatment sample (L).
where Cop is the operating cost, a is the energy cost, Cen is the energy consumption, b is the electrode cost, and Cel is the electrode consumption.
Rodriguez et al. (2007) denoted the electrochemical treatment process as a more economically as well as technologically convenient process than that of conventional processes like chemical precipitation. Liu et al. (2011a) conducted an experiment to enhance the recovery of nutrients from swine wastewater through recycling of electrochemically dissolved struvite. They mentioned that the combined total cost of chemical and energy consumption was 11.34 USD/1000 L when electrolytically dissolved struvite was used for crystallization, whereas the cost was 32.40 USD/1000 L during chemical precipitation of struvite. Wang et al. (2019a) also stated that orthophosphate recovery from swine wastewater using struvite precipitation electrolyzer would be more cost-effective and technically superior.
A detailed economic analysis of the electrochemical treatment process is necessary to determine the feasibility of the treatment process and can provide a realistic evaluation in comparison with other available treatment methods. The cost of electrolysis is mainly dependent on energy consumption and electricity price. With the increase in treatment time, the current density and energy consumption increase. The application of a sustainable renewable energy source such as photovoltaic solar cells could significantly minimize the treatment cost (Mook et al. 2014). Further studies on the prospective utilization of renewable energy sources in the electrolysis of livestock wastewater along with optimized operation conditions are therefore needed to develop an economically viable treatment process.
Scaling up
There are factors such as essential, systematic, and successiveness that are needed to be considered to scale up the primary laboratory-scale electrochemical processes to full industrial scale (Al-Qodah and Al-Shannag 2017). Essential factors include the final product, process optimization, transferring the process from the laboratory-scale to the pilot-scale, and finally to the full-scale (Den and Huang 2006). Holt et al. (2005) reported that there is a lack of a systematic approach to scale up the electrochemical treatment process from the laboratory to the industrial scale, whereas recently, a few pilot-scale studies had been conducted on electrolytic livestock wastewater treatment (Shim et al. 2020; Ding et al. 2021). Den and Huang (2006) suggested a framework comprising experimental assessment and model simulation for the electrochemical process scale-up (Fig. 7). In their framework, they mentioned that while scaling up an electrochemical treatment process, the reactor capacity and components in the pilot-scale plant should be geometrically similar to the laboratory-scale process to avoid possible hydrodynamic variation.
Framework for scaling up of electrochemical process based on the experimental observations and model simulation. The framework outlined in the figure can be utilized as a functional tool for the priority assessment of electrochemical treatment process scale-up. Republished with permission of American Society of Civil Engineers (ASCE), from Journal of Environmental Engineering, Den W. and Huang C., volume 132, edition 12 and year 2006; permission conveyed through
Shim et al. (2020) designed, constructed, and operated a pilot-scale novel integrated treatment process for pollutants removal and nutrient recovery from swine wastewater simultaneously. In that integrated process, the electrochemical treatment was performed as a final polishing treatment prior to the discharge of effluent to the natural environment (Fig. 8). The electrochemical reactor used in that study consisted of five electrolytic cells with a total treatment capacity of 833.3 L. Ding et al. (2021) evaluated the applicability of electrochemical H2S mitigation in a pilot-scale field test using laboratory-scale results and observed reduction efficiencies of 84% and 63.5% in hydrogen sulfide (H2S) concentration employing low carbon and stainless steel electrodes, respectively. In both studies, the pilot-scale processes were developed based on the laboratory-scale studies conducted earlier. A recent study on electrochemical treatment of cattle wastewater also highlights the importance of pilot-scale studies in verifying the laboratory-scale data (Stylianou et al. 2020). Results obtained from pilot-scale plants can provide important insights and help develop a better understanding of the treatment process in in situ conditions and thus encourage further full industrial-scale setups.
Pilot-scale swine wastewater treatment process comprised of biological treatment unit, struvite crystallization process, and electrochemical treatment system. Note that the integrated swine wastewater treatment unit applies electrochemical oxidation as a final polishing process. The electrochemical treatment unit decolorizes and disinfects the waste stream prior to dispense into the aquatic ecosystems. Reprinted from Shim et al. (2020) with permission from the multidisciplinary Digital Publishing Institute (MDPI) under an open access Creative Commons CC BY 4.0 License
Conclusion
High polluted livestock waste streams are known to have a deleterious effect on the environment. For the past couple of decades, treatment of livestock wastewater using electrochemical processes has attracted researchers around the globe. Electrocoagulation and electrooxidation are the commonly employed electrochemical techniques in the field of livestock wastewater treatment, while electro-disinfection is applied as a polishing treatment. Pollutants removal efficiency is mainly controlled by the reactor design and operational factors. Up till now, most of the studies on the electrolytic treatment of livestock waste streams have been conducted in batches at room temperatures (from 18 to 27 °C), while a few studies have been carried out using continuous operation. This review signifies the need of extensive research considering this aspect to make the electrochemical processes efficient and reliable for treating highly polluted livestock waste streams. Moreover, although a few pilot-scale studies are found in the literature, there are no studies on the full-scale electrochemical treatment of livestock wastewater. Multi-parameter optimization of the operational factors based on response surface methodology and artificial neural network, and simultaneous production of hydrogen can help scale up the process and thus prove the effectiveness of electrochemical technologies on the industrial scale. In addition, integration of electrochemical process with biological and/or other treatment technologies either as a pretreatment method or polishing step may further improve its performance and reveal the applicability of the electrochemical treatment processes in the long run. To sum up, based on the evidence presented and discussed in this review, it can be said that electrochemical treatment of livestock waste streams is a promising remedial approach to solve some of the environmental issues faced by the livestock industries.
Conflicts of interest
The authors declare no conflict of interest.
References
Abdel-Gawad SA, Baraka AM, Omran KA, Mokhtar MM (2012) Removal of some pesticides from the simulated waste water by electrocoagulation method using iron electrodes. Int J Electrochem Sci 7:6654–6665
Abdelhay A, Jum’h I, Albsoul A, Al Tarazi D, (2019) Dairy wastewater remediation using electrochemical oxidation on boron doped diamond anode (BDD). Desalin Water Treat 171:177–182. https://doi.org/10.5004/dwt.2019.24753
Adekunle KF, Okolie JA (2015) A review of biochemical process of anaerobic digestion. Adv Biosci Biotechnol 06:205. https://doi.org/10.4236/abb.2015.63020
Afazeli H, Jafari A, Rafiee S, Nosrati M (2014) An investigation of biogas production potential from livestock and slaughterhouse wastes. Renew Sustain Energy Rev 34:380–386. https://doi.org/10.1016/j.rser.2014.03.016
Aitbara A, Cherifi M, Hazourli S, Leclerc J-P (2014) Continuous treatment of industrial dairy effluent by electrocoagulation using aluminum electrodes. Desalin Water Treat 57:3395–3404. https://doi.org/10.1080/19443994.2014.989411
Aitbara A, Khelalfa A, Bendaia M et al (2021) Treatment of dairy wastewater by electrocoagulation using A-U4G (2017-Al) alloy and pure aluminum as electrode material. Euro-Mediterr J Environ Integr 6:19. https://doi.org/10.1007/s41207-020-00227-2
Akansha J, Nidheesh PV, Gopinath A et al (2020) Treatment of dairy industry wastewater by combined aerated electrocoagulation and phytoremediation process. Chemosphere 253:126652. https://doi.org/10.1016/j.chemosphere.2020.126652
Al-Qodah Z, Al-Shannag M (2017) Heavy metal ions removal from wastewater using electrocoagulation processes: a comprehensive review. Sep Sci Technol 52:2649–2676. https://doi.org/10.1080/01496395.2017.1373677
Al-Qodah Z, Al-Shannag M, Bani-Melhem K et al (2018) Free radical-assisted electrocoagulation processes for wastewater treatment. Environ Chem Lett 16:695–714. https://doi.org/10.1007/s10311-018-0711-1
Al-Rubaiey NA, Al-Barazanjy MG (2017) Study the efficiency of electrocoagulation system using conductivity measurements for the removal of zinc heavy metal. In: 2017 international conference on environmental impacts of the oil and gas industries: Kurdistan Region of Iraq as a Case Study (EIOGI). pp 42–47.
Al-Shannag M, Al-Qodah Z, Bani-Melhem K et al (2015) Heavy metal ions removal from metal plating wastewater using electrocoagulation: kinetic study and process performance. Chem Eng J 260:749–756. https://doi.org/10.1016/j.cej.2014.09.035
Álvarez JA, Otero L, Lema JM, Omil F (2010) The effect and fate of antibiotics during the anaerobic digestion of pig manure. Bioresour Technol 101:8581–8586. https://doi.org/10.1016/j.biortech.2010.06.075
Anglada A, Urtiaga A, Ortiz I (2009) Pilot Scale performance of the electro-oxidation of landfill leachate at boron-doped diamond anodes. Environ Sci Technol 43:2035–2040. https://doi.org/10.1021/es802748c
Asselin M, Drogui P, Benmoussa H, Blais J-F (2008) Effectiveness of electrocoagulation process in removing organic compounds from slaughterhouse wastewater using monopolar and bipolar electrolytic cells. Chemosphere 72:1727–1733. https://doi.org/10.1016/j.chemosphere.2008.04.067
Avancini Dias O, Perini Muniz E, da Silva Porto PS (2019) Electrocoagulation using perforated electrodes: an increase in metalworking fluid removal from wastewater. Chem Eng Process—Process Intensif 139:113–120. https://doi.org/10.1016/j.cep.2019.03.021
Barrera-Díaz C, Bilyeu B, Roa G, Bernal-Martinez L (2011) Physicochemical aspects of electrocoagulation. Sep Purif Rev 40:1–24. https://doi.org/10.1080/15422119.2011.542737
Bassala HD, Dedzo GK, Bememba CBN et al (2017) Investigation of the efficiency of a designed electrocoagulation reactor: application for dairy effluent treatment. Process Saf Environ Prot 111:122–127. https://doi.org/10.1016/j.psep.2017.07.002
Bayar S, Yıldız YŞ, Yılmaz AE, İrdemez Ş (2011) The effect of stirring speed and current density on removal efficiency of poultry slaughterhouse wastewater by electrocoagulation method. Desalination 280:103–107. https://doi.org/10.1016/j.desal.2011.06.061
Bayramoglu M, Eyvaz M, Kobya M (2007) Treatment of the textile wastewater by electrocoagulation: economical evaluation. Chem Eng J 128:155–161. https://doi.org/10.1016/j.cej.2006.10.008
Bazrafshan E, Moein H, Kord Mostafapour F, Nakhaie S (2012) Application of electrocoagulation process for dairy wastewater treatment. J Chem 2013:e640139. https://doi.org/10.1155/2013/640139
Bazrafshan E, Mohammadi L, Ansari-Moghaddam A, Mahvi AH (2015) Heavy metals removal from aqueous environments by electrocoagulation process–a systematic review. J Environ Health Sci Eng 13:1–16. https://doi.org/10.1186/s40201-015-0233-8
Bejan D, Sagitova F, Bunce NJ (2005) Evaluation of electrolysis for oxidative deodorization of hog manure. J Appl Electrochem 35:897–902. https://doi.org/10.1007/s10800-005-4722-9
Bejan D, Rabson LM, Bunce NJ (2007) Electrochemical deodorization and disinfection of hog manure. Can J Chem Eng 85:929–935. https://doi.org/10.1002/cjce.5450850615
Benaissa F, Kermet-Said H, Moulai-Mostefa N (2016) Optimization and kinetic modeling of electrocoagulation treatment of dairy wastewater. Desalin Water Treat 57:5988–5994. https://doi.org/10.1080/19443994.2014.985722
Benazzi TL, Di Luccio M, Dallago RM et al (2016) Continuous flow electrocoagulation in the treatment of wastewater from dairy industries. Water Sci Technol 73:1418–1425. https://doi.org/10.2166/wst.2015.620
Bensadok K, El Hanafi N, Lapicque F (2011) Electrochemical treatment of dairy effluent using combined Al and Ti/Pt electrodes system. Desalination 280:244–251. https://doi.org/10.1016/j.desal.2011.07.006
Bergmann MEH, Rollin J (2007) Product and by-product formation in laboratory studies on disinfection electrolysis of water using boron-doped diamond anodes. Catal Today 124:198–203. https://doi.org/10.1016/j.cattod.2007.03.038
Bersier PM, de León CP, Walsh FC (2008) Electrochemical approaches to environmental treatment and recycling. Encycl life support syst (EOLSS), vol II. UNESCO Publishing-Eolss Publishers, Oxford, pp 340–381
Bhushan L, Kulkarni AD (2016) Performance of electrocoagulation technique for the treatment of dairy wastewater in a batch process. Int J Sci Res 5:2574–2578
Bonnin EP, Biddinger EJ, Botte GG (2008) Effect of catalyst on electrolysis of ammonia effluents. J Power Sources 182:284–290. https://doi.org/10.1016/j.jpowsour.2008.03.046
Borbón B, Guzmán MTO, Ho SWL, Hernandez GA (2011) Evaluation of an electrochemical advanced oxidation process for the organic matter removal from dairy wastewater. ECS Trans 36:323
Borbón B, Oropeza-Guzman MT, Brillas E, Sirés I (2014) Sequential electrochemical treatment of dairy wastewater using aluminum and DSA-type anodes. Environ Sci Pollut Res 21:8573–8584. https://doi.org/10.1007/s11356-014-2787-x
Burkholder J, Libra B, Weyer P et al (2007) Impacts of waste from concentrated animal feeding operations on water quality. Environ Health Perspect 115:308–312. https://doi.org/10.1289/ehp.8839
Butler E, Hung Y-T, Yeh RY-L, Suleiman Al Ahmad M (2011) Electrocoagulation in wastewater treatment. Water 3:495–525. https://doi.org/10.3390/w3020495
Cabeza A, Urtiaga A, Rivero M-J, Ortiz I (2007) Ammonium removal from landfill leachate by anodic oxidation. J Hazard Mater 144:715–719. https://doi.org/10.1016/j.jhazmat.2007.01.106
Can OT, Kobya M, Demirbas E, Bayramoglu M (2006) Treatment of the textile wastewater by combined electrocoagulation. Chemosphere 62:181–187. https://doi.org/10.1016/j.chemosphere.2005.05.022
Canizares P, Martınez F, Dıaz M et al (2002) Electrochemical oxidation of aqueous phenol wastes using active and nonactive electrodes. J Electrochem Soc 149:D118
Canizares P, Lobato J, Paz R et al (2005) Electrochemical oxidation of phenolic wastes with boron-doped diamond anodes. Water Res 39:2687–2703. https://doi.org/10.1016/j.watres.2005.04.042
Chae KJ, Yim SK, Choi KH et al (2004) Integrated biological and electro-chemical treatment of swine manure. Water Sci Technol 49:427–434. https://doi.org/10.2166/wst.2004.0784
Chakchouk I, Elloumi N, Belaid C et al (2017) A combined electrocoagulation-electrooxidation treatment for dairy wastewater. Braz J Chem Eng 34:109–117. https://doi.org/10.1590/0104-6632.20170341s20150040
Chen G (2004) Electrochemical technologies in wastewater treatment. Sep Purif Technol 38:11–41. https://doi.org/10.1016/j.seppur.2003.10.006
Chen X, Chen G, Yue PL (2000) Separation of pollutants from restaurant wastewater by electrocoagulation. Sep Purif Technol 19:65–76. https://doi.org/10.1016/S1383-5866(99)00072-6
Chen X, Chen G, Yue PL (2003) Anodic oxidation of dyes at novel Ti/B-diamond electrodes. Chem Eng Sci 58:995–1001. https://doi.org/10.1016/S0009-2509(02)00640-1
Chen JP, Chang S-Y, Hung Y-T (2005) Electrolysis. In: Wang LK, Hung Y-T, Shammas NK (eds) Physicochemical treatment processes. Humana Press, Totowa, NJ, pp 359–378
Chen R-F, Liu T, Rong H-W et al (2021a) Effect of organic substances on nutrients recovery by struvite electrochemical precipitation from synthetic anaerobically treated swine wastewater. Membranes 11:594. https://doi.org/10.3390/membranes11080594
Chen R-F, Wu L, Zhong H-T et al (2021b) Evaluation of electrocoagulation process for high-strength swine wastewater pretreatment. Sep Purif Technol 272:118900. https://doi.org/10.1016/j.seppur.2021.118900
Chezeau B, Boudriche L, Vial C, Boudjemaa A (2020) Treatment of dairy wastewater by electrocoagulation process: advantages of combined iron/aluminum electrodes. Sep Sci Technol 55:2510–2527. https://doi.org/10.1080/01496395.2019.1638935
Chiang L-C, Chang J-E, Wen T-C (1995) Indirect oxidation effect in electrochemical oxidation treatment of landfill leachate. Water Res 29:671–678. https://doi.org/10.1016/0043-1354(94)00146-X
Cho J, Lee J, Ra C (2010) Effects of electric voltage and sodium chloride level on electrolysis of swine wastewater. J Hazard Mater 180:535–541. https://doi.org/10.1016/j.jhazmat.2010.04.067
Chung S-H (2004) A novel application of advanced treatment in livestock wastewater by electrolysis. J Environ Sanit Eng 19:31–39
Comninellis C (1994) Electrocatalysis in the electrochemical conversion/combustion of organic pollutants for waste water treatment. Electrochim Acta 39:1857–1862. https://doi.org/10.1016/0013-4686(94)85175-1
Comninellis C, Chen G (2010) Electrochemistry for the environment. Springer, New York
Comninellis Ch, Pulgarin C (1993) Electrochemical oxidation of phenol for wastewater treatment using SnO2, anodes. J Appl Electrochem 23:108–112. https://doi.org/10.1007/BF00246946
Costa CR, Olivi P (2009) Effect of chloride concentration on the electrochemical treatment of a synthetic tannery wastewater. Electrochim Acta 54:2046–2052. https://doi.org/10.1016/j.electacta.2008.08.033
Cotillas S, Llanos J, Cañizares P et al (2013) Optimization of an integrated electrodisinfection/electrocoagulation process with Al bipolar electrodes for urban wastewater reclamation. Water Res 47:1741–1750. https://doi.org/10.1016/j.watres.2012.12.029
Daghrir R, Drogui P, Tshibangu J (2014) Efficient treatment of domestic wastewater by electrochemical oxidation process using bored doped diamond anode. Sep Purif Technol 131:79–83. https://doi.org/10.1016/j.seppur.2014.04.048
Daneshvar N, Sorkhabi HA, Kasiri MB (2004) Decolorization of dye solution containing Acid Red 14 by electrocoagulation with a comparative investigation of different electrode connections. J Hazard Mater 112:55–62. https://doi.org/10.1016/j.jhazmat.2004.03.021
Daneshvar N, Oladegaragoze A, Djafarzadeh N (2006) Decolorization of basic dye solutions by electrocoagulation: an investigation of the effect of operational parameters. J Hazard Mater 129:116–122. https://doi.org/10.1016/j.jhazmat.2005.08.033
Davarnejad R, Nikseresht M (2016) Dairy wastewater treatment using an electrochemical method: experimental and statistical study. J Electroanal Chem 775:364–373. https://doi.org/10.1016/j.jelechem.2016.06.016
Demirci Y, Pekel LC, Alpbaz M (2015) Investigation of different electrode connections in electrocoagulation of textile wastewater treatment. Int J Electrochem Sci 10:2685–2693
Den W, Huang C (2006) Electrocoagulation of silica nanoparticles in wafer polishing wastewater by a multichannel flow reactor: a kinetic study. J Environ Eng 132:1651–1658
Deng Y, Englehardt JD (2007) Electrochemical oxidation for landfill leachate treatment. Waste Manag 27:380–388. https://doi.org/10.1016/j.wasman.2006.02.004
Diao HF, Li XY, Gu JD et al (2004) Electron microscopic investigation of the bactericidal action of electrochemical disinfection in comparison with chlorination, ozonation and Fenton reaction. Process Biochem 39:1421–1426. https://doi.org/10.1016/S0032-9592(03)00274-7
Diaz LA, Botte GG (2012) Electrochemical deammonification of synthetic swine wastewater. Ind Eng Chem Res 51:12167–12172. https://doi.org/10.1021/ie3015022
Ding L, Lin H, Hetchler B et al (2021) Electrochemical mitigation of hydrogen sulfide in deep-pit swine manure storage. Sci Total Environ 777:146048. https://doi.org/10.1016/j.scitotenv.2021.146048
Drogui P, Blais J-F, Mercier G (2007) Review of electrochemical technologies for environmental applications. Recent Pat Eng 1:257–272. https://doi.org/10.2174/187221207782411629
Drogui P, Asselin M, Brar SK et al (2008) Electrochemical removal of pollutants from agro-industry wastewaters. Sep Purif Technol 61:301–310. https://doi.org/10.1016/j.seppur.2007.10.013
Drouiche N, Aoudj S, Hecini M et al (2009) Study on the treatment of photovoltaic wastewater using electrocoagulation: Fluoride removal with aluminium electrodes—characteristics of products. J Hazard Mater 169:65–69. https://doi.org/10.1016/j.jhazmat.2009.03.073
Duan Y, Du J, Cai Y, Han Z (2019) Advanced treatment of simulated bio-treated piggery wastewater by a novel electrolysis process. In: 2019 ASABE Annual international meeting. American Society of Agricultural and Biological Engineers, pp 1–5. https://doi.org/10.13031/aim.201900733
Eulmi A, Hazourli S, Abrane R et al (2019) Evaluation of electrocoagulation and activated carbon adsorption techniques used separately or coupled to treat wastewater from industrial dairy. Int J Chem React Eng 17:1–12. https://doi.org/10.1515/ijcre-2018-0229
Feng C, Sugiura N, Shimada S, Maekawa T (2003) Development of a high performance electrochemical wastewater treatment system. J Hazard Mater 103:65–78. https://doi.org/10.1016/S0304-3894(03)00222-X
Ferro G, Fiorentino A, Alferez MC et al (2015) Urban wastewater disinfection for agricultural reuse: effect of solar driven AOPs in the inactivation of a multidrug resistant E. coli strain. Appl Catal B Environ 178:65–73. https://doi.org/10.1016/j.apcatb.2014.10.043
Fouad YOA, Konsowa AH, Farag HA, Sedahmed GH (2009) Performance of an electrocoagulation cell with horizontally oriented electrodes in oil separation compared to a cell with vertical electrodes. Chem Eng J 145:436–440. https://doi.org/10.1016/j.cej.2008.04.027
Geraldino HCL, Simionato JI, de Souza Freitas TKF et al (2015) Efficiency and operating cost of electrocoagulation system applied to the treatment of dairy industry wastewater. Acta Sci Technol 37:401–408. https://doi.org/10.4025/actascitechnol.v37i3.26452
Gerischer H, Mauerer A (1970) Untersuchungen Zur anodischen Oxidation von Ammoniak an Platin-Elektroden. J Electroanal Chem Interfacial Electrochem 25:421–433. https://doi.org/10.1016/S0022-0728(70)80103-6
Ghahremani H, Bagheri S, Hassani SM, Khoshchehreh MR (2012) Treatment of dairy industry wastewater using an electrocoagulation process. Adv Environ Biol 6:1897–1901
Ghazouani M, Akrout H, Jellali S, Bousselmi L (2019) Comparative study of electrochemical hybrid systems for the treatment of real wastewaters from agri-food activities. Sci Total Environ 647:1651–1664. https://doi.org/10.1016/j.scitotenv.2018.08.023
Ghernaout D, Ghernaout B (2010) From chemical disinfection to electrodisinfection: The obligatory itinerary? Desalination Water Treat 16:156–175. https://doi.org/10.5004/dwt.2010.1085
Ghernaout D, Ghernaout B, Kellil A (2009) Natural organic matter removal and enhanced coagulation as a link between coagulation and electrocoagulation. Desalination Water Treat 2:203–222. https://doi.org/10.5004/dwt.2009.116
Ghernaout D, Ghernaout B, Naceur MW (2011) Embodying the chemical water treatment in the green chemistry—a review. Desalination 271:1–10. https://doi.org/10.1016/j.desal.2011.01.032
Gilboa Y, Friedler E (2008) UV disinfection of RBC-treated light greywater effluent: kinetics, survival and regrowth of selected microorganisms. Water Res 42:1043–1050. https://doi.org/10.1016/j.watres.2007.09.027
Greger M, Koneswaran G (2010) The public health impacts of concentrated animal feeding operations on local communities. Fam Community Health 33:11–20. https://doi.org/10.1097/FCH.0b013e3181c4e22a
Guitaya L, Drogui P, Blais JF (2015) In situ reactive oxygen species production for tertiary wastewater treatment. Environ Sci Pollut Res 22:7025–7036. https://doi.org/10.1007/s11356-014-3907-3
Gupta S, Kumar A, Mathur S (2016) Optimization of electrocoagulation process for the treatment of dairy wastewater using response surface methodology. Int J Eng Res 4:1–4
Gürses A, Yalçin M, Doğar C (2002) Electrocoagulation of some reactive dyes: a statistical investigation of some electrochemical variables. Waste Manag 22:491–499. https://doi.org/10.1016/S0956-053X(02)00015-6
Hakizimana JN, Gourich B, Chafi M et al (2017) Electrocoagulation process in water treatment: a review of electrocoagulation modeling approaches. Desalination 404:1–21. https://doi.org/10.1016/j.desal.2016.10.011
Han Z, Wang L, Duan L et al (2015) The electrocoagulation pretreatment of biogas digestion slurry from swine farm prior to nanofiltration concentration. Sep Purif Technol 156:817–826. https://doi.org/10.1016/j.seppur.2015.10.054
Harif T, Adin A (2011) Size and structure evolution of kaolin–Al(OH)3 flocs in the electroflocculation process: a study using static light scattering. Water Res 45:6195–6206. https://doi.org/10.1016/j.watres.2011.09.027
Harif T, Khai M, Adin A (2012) Electrocoagulation versus chemical coagulation: Coagulation/flocculation mechanisms and resulting floc characteristics. Water Res 46:3177–3188. https://doi.org/10.1016/j.watres.2012.03.034
Hashim KS, Shaw A, Al Khaddar R et al (2017) Iron removal, energy consumption and operating cost of electrocoagulation of drinking water using a new flow column reactor. J Environ Manag 189:98–108. https://doi.org/10.1016/j.jenvman.2016.12.035
Hijnen WAM, Beerendonk EF, Medema GJ (2006) Inactivation credit of UV radiation for viruses, bacteria and protozoan (oo)cysts in water: a review. Water Res 40:3–22. https://doi.org/10.1016/j.watres.2005.10.030
Holt PK, Barton GW, Mitchell CA (2005) The future for electrocoagulation as a localised water treatment technology. Chemosphere 59:355–367. https://doi.org/10.1016/j.chemosphere.2004.10.023
Hossain F, Perales-Perez Oscar J, Hwang S, Román F (2014) Antimicrobial nanomaterials as water disinfectant: Applications, limitations and future perspectives. Sci Total Environ 466–467:1047–1059. https://doi.org/10.1016/j.scitotenv.2013.08.009
Hu Y, Cheng H, Tao S (2017) Environmental and human health challenges of industrial livestock and poultry farming in China and their mitigation. Environ Int 107:111–130. https://doi.org/10.1016/j.envint.2017.07.003
Huang H, Zhang D, Guo G et al (2018a) Dolomite application for the removal of nutrients from synthetic swine wastewater by a novel combined electrochemical process. Chem Eng J 335:665–675. https://doi.org/10.1016/j.cej.2017.11.013
Huang K-L, Wei K-C, Chen M-H, Ma C-Y (2018b) Removal of organic and ammonium nitrogen pollutants in swine wastewater using electrochemical advanced oxidation. Int J Electrochem Sci 13:11418–11431. 1020964/2018.12.32
Huang K-L, Liu C-C, Ma C-Y, Chen T-T (2019) Effects of operating parameters on electrochemical treatment of swine wastewater. Int J Electrochem Sci. https://doi.org/10.20964/2019.12.43
Huber R, Whitelaw CBA, Olsson IAS (2010) Transgenic livestock: a contribution to global food security? Global food security: ethical and legal challenges. Wageningen Academic Publishers, Bilbao, pp 159–164
Ibanez JG, Fitch A, Frontana-Uribe BA, Vasquez-Medrano R (2014) Green electrochemistry. In: Encyclopedia of applied electrochemistry. Springer, New York, pp 964–971
Ihara I, Umetsu K, Kanamura K, Watanabe T (2006) Electrochemical oxidation of the effluent from anaerobic digestion of dairy manure. Bioresour Technol 97:1360–1364. https://doi.org/10.1016/j.biortech.2005.07.007
Ikematsu M, Kaneda K, Takaoka D, Yasuda M (2007) Laboratory studies on nitrogen and phosphorus removal from swine wastewater by iron electrolysis. Environ Technol 28:521–528. https://doi.org/10.1080/09593332808618809
Inan H, Dimoglo A, Şimşek H, Karpuzcu M (2004) Olive oil mill wastewater treatment by means of electro-coagulation. Sep Purif Technol 36:23–31. https://doi.org/10.1016/S1383-5866(03)00148-5
İrdemez Ş, Yildiz YŞ, Tosunoğlu V (2006) Optimization of phosphate removal from wastewater by electrocoagulation with aluminum plate electrodes. Sep Purif Technol 52:394–401. https://doi.org/10.1016/j.seppur.2006.05.020
Jafvert CT, Valentine RL (1992) Reaction scheme for the chlorination of ammoniacal water. Environ Sci Technol 26:577–586
Jagadal CB, Hiremath MN, Shivayogimath CB (2017) Study of dairy wastewater treatment using monopolar series system of electrocoagulation process with aluminium electrodes. Int Res J Eng Technol 04:1188–1192
Jeong J, Kim JY, Cho M et al (2007) Inactivation of Escherichia coli in the electrochemical disinfection process using a Pt anode. Chemosphere 67:652–659. https://doi.org/10.1016/j.chemosphere.2006.11.035
Jeong J, Kim C, Yoon J (2009) The effect of electrode material on the generation of oxidants and microbial inactivation in the electrochemical disinfection processes. Water Res 43:895–901. https://doi.org/10.1016/j.watres.2008.11.033
Jiang J-Q, Graham N, André C et al (2002) Laboratory study of electro-coagulation–flotation for water treatment. Water Res 36:4064–4078. https://doi.org/10.1016/S0043-1354(02)00118-5
Jiménez C, Sáez C, Martínez F et al (2012) Electrochemical dosing of iron and aluminum in continuous processes: a key step to explain electro-coagulation processes. Sep Purif Technol 98:102–108. https://doi.org/10.1016/j.seppur.2012.07.005
Kabdaşlı I, Arslan-Alaton I, Ölmez-Hancı T, Tünay O (2012) Electrocoagulation applications for industrial wastewaters: a critical review. Environ Technol Rev 1:2–45. https://doi.org/10.1080/21622515.2012.715390
Kapałka A, Katsaounis A, Michels N-L, et al (2010) Ammonia oxidation to nitrogen mediated by electrogenerated active chlorine on Ti/PtOx-IrO2. Electrochem Commun 12:1203–1205. https://doi.org/10.1016/j.elecom.2010.06.019
Katal R, Pahlavanzadeh H (2011) Influence of different combinations of aluminum and iron electrode on electrocoagulation efficiency: application to the treatment of paper mill wastewater. Desalination 265:199–205. https://doi.org/10.1016/j.desal.2010.07.052
Kerwick MI, Reddy SM, Chamberlain AHL, Holt DM (2005) Electrochemical disinfection, an environmentally acceptable method of drinking water disinfection? Electrochim Acta 50:5270–5277. https://doi.org/10.1016/j.electacta.2005.02.074
Khaled B, Wided B, Béchir H et al (2019) Investigation of electrocoagulation reactor design parameters effect on the removal of cadmium from synthetic and phosphate industrial wastewater. Arab J Chem 12:1848–1859. https://doi.org/10.1016/j.arabjc.2014.12.012
Khandegar V, Saroha AK (2012) Electrochemical treatment of distillery spent wash using aluminum and iron electrodes. Chin J Chem Eng 20:439–443. https://doi.org/10.1016/S1004-9541(11)60204-8
Khandegar V, Saroha AK (2013a) Electrochemical treatment of effluent from small-scale dyeing unit. Indian Chem Eng 55:112–120. https://doi.org/10.1080/00194506.2013.798889
Khandegar V, Saroha AK (2013b) Electrocoagulation for the treatment of textile industry effluent–a review. J Environ Manag 128:949–963. https://doi.org/10.1016/j.jenvman.2013.06.043
Khandegar V, Saroha AK (2014) Electrochemical treatment of textile effluent containing acid red 131 dye. J Hazard Toxic Radioact Waste 18:38–44. https://doi.org/10.1061/(ASCE)HZ.2153-5515.0000194
Khandegar V, Saroha AK (2016) Effect of electrode shape and current source on performance of electrocoagulation. J Hazard Toxic Radioact Waste 20:06015001. https://doi.org/10.1061/(ASCE)HZ.2153-5515.0000278
Khandegar V, Acharya S, Jain AK (2018) Data on treatment of sewage wastewater by electrocoagulation using punched aluminum electrode and characterization of generated sludge. Data Brief 18:1229–1238. https://doi.org/10.1016/j.dib.2018.04.020
Kim S, Kim T-H, Park C, Shin E-B (2003) Electrochemical oxidation of polyvinyl alcohol using a RuO2/Ti anode. Desalination 155:49–57. https://doi.org/10.1016/S0011-9164(03)00238-8
Kobya M, Can OT, Bayramoglu M (2003) Treatment of textile wastewaters by electrocoagulation using iron and aluminum electrodes. J Hazard Mater 100:163–178. https://doi.org/10.1016/S0304-3894(03)00102-X
Kobya M, Senturk E, Bayramoglu M (2006) Treatment of poultry slaughterhouse wastewaters by electrocoagulation. J Hazard Mater 133:172–176. https://doi.org/10.1016/j.jhazmat.2005.10.007
Kraft A (2008) Electrochemical water disinfection: a short review. Platin Met Rev 52:177–185. https://doi.org/10.1595/147106708X329273
Kranert M, Kusch S, Huang J, Fischer K (2012) Anaerobic digestion of waste. In: Karagiannidis A (ed) Waste to energy: opportunities and challenges for developing and transition economies. Springer, London, pp 107–135
Kumari P, Kushwaha JP, Sangal VK, Singh N (2019) Dairy wastewater treatment in continuous stirred tank electrochemical reactor (cster): parametric optimization and kinetics. Environ Eng Manag J; 18:1219–1230. https://doi.org/10.30638/eemj.2019.117
Kuokkanen V, Kuokkanen T, Rämö J et al (2015) Removal of phosphate from wastewaters for further utilization using electrocoagulation with hybrid electrodes—techno-economic studies. J Water Process Eng 8:e50–e57. https://doi.org/10.1016/j.jwpe.2014.11.008
Kuroda Y, Kawada Y, Takahashi T et al (2003) Effect of electrode shape on discharge current and performance with barrier discharge type electrostatic precipitator. J Electrost 57:407–415. https://doi.org/10.1016/S0304-3886(02)00177-8
Kushwaha JP, Srivastava VC, Mall ID (2010) Organics removal from dairy wastewater by electrochemical treatment and residue disposal. Sep Purif Technol 76:198–205. https://doi.org/10.1016/j.seppur.2010.10.008
Lahav O, Schwartz Y, Nativ P, Gendel Y (2013) Sustainable removal of ammonia from anaerobic-lagoon swine waste effluents using an electrochemically-regenerated ion exchange process. Chem Eng J 218:214–222. https://doi.org/10.1016/j.cej.2012.12.043
Laridi R, Drogui P, Benmoussa H et al (2005) Removal of refractory organic compounds in liquid swine manure obtained from a biofiltration process using an electrochemical treatment. J Environ Eng 131:1302–1310. https://doi.org/10.1061/(ASCE)0733-9372(2005)131:9(1302)
Lee H, Lee E, Lee C-H, Lee K (2011) Degradation of chlorotetracycline and bacterial disinfection in livestock wastewater by ozone-based advanced oxidation. J Ind Eng Chem 17:468–473. https://doi.org/10.1016/j.jiec.2011.05.006
Lei X, Maekawa T (2007) Electrochemical treatment of anaerobic digestion effluent using a Ti/Pt–IrO2 electrode. Bioresour Technol 98:3521–3525. https://doi.org/10.1016/j.biortech.2006.11.018
Lekhlif B, Oudrhiri L, Zidane F et al (2014) Study of the electrocoagulation of electroplating industry wastewaters charged by nickel (II) and chromium (VI). J Mater Env Sci 5:111–120
Levenspiel O (1998) Chemical reaction engineering. Wiley, New York
Li L, Liu Y (2009) Ammonia removal in electrochemical oxidation: mechanism and pseudo-kinetics. J Hazard Mater 161:1010–1016. https://doi.org/10.1016/j.jhazmat.2008.04.047
Lin X, Han Z, Yu H et al (2018) Struvite precipitation from biogas digestion slurry using a two-chamber electrolysis cell with a magnesium anode. J Clean Prod 174:1598–1607. https://doi.org/10.1016/j.jclepro.2017.10.224
Linares-Hernández I, Barrera-Díaz C, Roa-Morales G et al (2009) Influence of the anodic material on electrocoagulation performance. Chem Eng J 148:97–105. https://doi.org/10.1016/j.cej.2008.08.007
Liu Y, Li L, Goel R (2009) Kinetic study of electrolytic ammonia removal using Ti/IrO2 as anode under different experimental conditions. J Hazard Mater 167:959–965. https://doi.org/10.1016/j.jhazmat.2009.01.082
Liu Y, Kumar S, Kwag J et al (2011a) Recycle of electrolytically dissolved struvite as an alternative to enhance phosphate and nitrogen recovery from swine wastewater. J Hazard Mater 195:175–181. https://doi.org/10.1016/j.jhazmat.2011.08.022
Liu Y, Kwag J-H, Kim J-H, Ra C (2011b) Recovery of nitrogen and phosphorus by struvite crystallization from swine wastewater. Desalination 277:364–369. https://doi.org/10.1016/j.desal.2011.04.056
Liu H, Zhao X, Qu J (2010) Electrocoagulation in water treatment. In: Electrochemistry for the Environment. Springer, pp 245–262. https://doi.org/10.1007/978-0-387-68318-8_10
Logan BE (2005) Simultaneous wastewater treatment and biological electricity generation. Water Sci Technol 52:31–37. https://doi.org/10.2166/wst.2005.0495
Lourinho G, Brito PSD (2021) Electrolytic treatment of swine wastewater: recent progress and challenges. Waste Biomass Valorization 12:553–576. https://doi.org/10.1007/s12649-020-00951-4
Lourinho G, Santos DMF, Brito PSD (2021) Electrooxidation studies of swine effluents before and after the anaerobic digestion process. J Environ Chem Eng 9:104712. https://doi.org/10.1016/j.jece.2020.104712
Mahesh S, Prasad B, Mall ID, Mishra IM (2006a) Electrochemical degradation of pulp and paper mill wastewater. Part 1. COD and color removal. Ind Eng Chem Res 45:2830–2839. https://doi.org/10.1021/ie0514096
Mahesh S, Prasad B, Mall ID, Mishra IM (2006b) Electrochemical degradation of pulp and paper mill wastewater. Part 2. characterization and analysis of sludge. Ind Eng Chem Res 45:5766–5774. https://doi.org/10.1021/ie0603969
Majlessi-Nasr M, Rafiee M, Amereh F et al (2020) Multi-objective optimization of electrocoagulation-flotation (ECF) process for treatment of real dairy wastewater. Desalin Water Treat 206:44–57. https://doi.org/10.5004/dwt.2020.26212
Majlessi-Nasra M, Rafieea M, Amereha F et al (2020) Multi-objective optimization of electrocoagulation-flotation (ECF) process for treatment of real dairy wastewater. Desalin Water Treat 206:44–57. https://doi.org/10.5004/dwt.2020.26212
Maljaei A, Arami M, Mahmoodi NM (2009) Decolorization and aromatic ring degradation of colored textile wastewater using indirect electrochemical oxidation method. Desalination 249:1074–1078. https://doi.org/10.1016/j.desal.2009.05.016
Maniakova G, Salmerón I, Nahim-Granados S et al (2021) Sunlight advanced oxidation processes vs ozonation for wastewater disinfection and safe reclamation. Sci Total Environ 787:147531. https://doi.org/10.1016/j.scitotenv.2021.147531
Markou V, Kontogianni M-C, Frontistis Z et al (2017) Electrochemical treatment of biologically pre-treated dairy wastewater using dimensionally stable anodes. J Environ Manag 202:217–224. https://doi.org/10.1016/j.jenvman.2017.07.046
Marol C, Puneet K, Prashant Y et al (2017) Treatment of dairy industry waste water by electrocoagulation (EC) technique removal of BOD, COD, turbidity and color. Int J Innov Res Sci Technol 4:246–251
Marriaga-Cabrales N, Machuca-Martínez F (2014) Fundamentals of electrocoagulation. Peralta-Hernández JM, Rodrigo-Rodrigo MA, Martínez-Huit CA (eds.) Evaluation of Electrochemical Reactors as a New Way to Environmental Protection, Research Signpost, Kerala, pp. 1–16.
Martinez-Huitle CA, Ferro S (2006) Electrochemical oxidation of organic pollutants for the wastewater treatment: direct and indirect processes. Chem Soc Rev 35:1324–1340. https://doi.org/10.1039/B517632H
Melchiors MS, Piovesan M, Becegato VR et al (2016) Treatment of wastewater from the dairy industry using electroflocculation and solid whey recovery. J Environ Manag 182:574–580. https://doi.org/10.1016/j.jenvman.2016.08.022
Mohammadi A, Khadir A, M.A. Tehrani R, (2019) Optimization of nitrogen removal from an anaerobic digester effluent by electrocoagulation process. J Environ Chem Eng 7:103195. https://doi.org/10.1016/j.jece.2019.103195
Mollah MYA, Schennach R, Parga JR, Cocke DL (2001) Electrocoagulation (EC)—science and applications. J Hazard Mater 84:29–41. https://doi.org/10.1016/S0304-3894(01)00176-5
Mollah MYA, Morkovsky P, Gomes JAG et al (2004) Fundamentals, present and future perspectives of electrocoagulation. J Hazard Mater 114:199–210. https://doi.org/10.1016/j.jhazmat.2004.08.009
Mook WT, Chakrabarti MH, Aroua MK et al (2012) Removal of total ammonia nitrogen (TAN), nitrate and total organic carbon (TOC) from aquaculture wastewater using electrochemical technology: a review. Desalination 285:1–13. https://doi.org/10.1016/j.desal.2011.09.029
Mook WT, Aroua MK, Issabayeva G (2014) Prospective applications of renewable energy based electrochemical systems in wastewater treatment: a review. Renew Sustain Energy Rev 38:36–46. https://doi.org/10.1016/j.rser.2014.05.042
Moreno-Casillas HA, Cocke DL, Gomes JAG et al (2007) Electrocoagulation mechanism for COD removal. Sep Purif Technol 56:204–211. https://doi.org/10.1016/j.seppur.2007.01.031
Mores R, Kunz A, Steffens J et al (2016a) Swine manure digestate treatment using electrocoagulation. Sci Agric 73:439–443. https://doi.org/10.1590/0103-9016-2015-0269
Mores R, Treichel H, Zakrzevski CA et al (2016b) Remove of phosphorous and turbidity of swine wastewater using electrocoagulation under continuous flow. Sep Purif Technol 171:112–117. https://doi.org/10.1016/j.seppur.2016.07.016
Mores R, Kunz A, Chini A et al (2017) Eletro-oxidation as an alternative in the removal of ammonia from swine wastewater pretreated by electrocoagulation. Simpósio Internacional Sobre Gerenciamento De Resíduos Agropecuários E Agroindustriais 5:344–348
Mores R, de Mello P, A, Zakrzevski CA, et al (2018) Reduction of soluble organic carbon and removal of total phosphorus and metals from swine wastewater by electrocoagulation. Braz J Chem Eng 35:1231–1240. https://doi.org/10.1590/0104-6632.20180354s20170300
Mouedhen G, Feki M, Wery MDP, Ayedi HF (2008) Behavior of aluminum electrodes in electrocoagulation process. J Hazard Mater 150:124–135. https://doi.org/10.1016/j.jhazmat.2007.04.090
Moussa DT, El-Naas MH, Nasser M, Al-Marri MJ (2017) A comprehensive review of electrocoagulation for water treatment: potentials and challenges. J Environ Manag 186:24–41. https://doi.org/10.1016/j.jenvman.2016.10.032
Muddemann T, Haupt D, Sievers M, Kunz U (2019) Electrochemical reactors for wastewater treatment. ChemBioEng Rev 6:142–156. https://doi.org/10.1002/cben.201900021
Naje AS, Abbas SA (2013) Combination of electrocoagulation and electro-oxidation processes of textile waste waters treatment. Civ Env Res 3:61–74
Naje AS, Chelliapan S, Zakaria Z et al (2017) A review of electrocoagulation technology for the treatment of textile wastewater. Rev Chem Eng 33:263–292. https://doi.org/10.1515/revce-2016-0019
Nasrullah M, Singh L, Wahid ZA (2012) Treatment of sewage by electrocoagulation and the effect of high current density. Energy Env Eng J 1:27–31
Nassef EMR (2014) Removal of polyaromatic hydrocarbons from waste water by electrocoagulation. J Pet Gas Eng 5:32–42. https://doi.org/10.5897/JPGE2014.0192
Panizza M, Martinez-Huitle CA (2013) Role of electrode materials for the anodic oxidation of a real landfill leachate—comparison between Ti–Ru–Sn ternary oxide, PbO2 and boron-doped diamond anode. Chemosphere 90:1455–1460. https://doi.org/10.1016/j.chemosphere.2012.09.006
Petsriprasit C, Namboonmee J, Hunsom M (2010) Application of the electrocoagulation technique for treating heavy metals containing wastewater from the pickling process of a billet plant. Korean J Chem Eng 27:854–861. https://doi.org/10.1007/s11814-010-0145-3
Rahman S, Borhan MS (2014) Electrolysis of swine manure effluents using three different electrodes Fe-Fe, Al-Al and Fe-Al. Am J Agric Biol Sci 9:490–502. https://doi.org/10.3844/ajabssp.2014.490.502
Rajeshwar K, Ibanez JG, Swain GM (1994) Electrochemistry and the environment. J Appl Electrochem 24:1077–1091. https://doi.org/10.1007/BF00241305
Rodriguez J, Stopić S, Krause G, Friedrich B (2007) Feasibility assessment of electrocoagulation towards a new sustainable wastewater treatment. Environ Sci Pollut Res-Int 14:477–482. https://doi.org/10.1065/espr2007.05.424
Ruotolo LAM, Gubulin JC (2011) A mathematical model to predict the electrode potential profile inside a polyaniline-modified reticulate vitreous carbon electrode operating in the potentiostatic reduction of Cr (VI). Chem Eng J 171:1170–1177. https://doi.org/10.1016/j.cej.2011.05.017
Rutala WA, Weber DJ (2008) Guideline for disinfection and sterilization in healthcare facilities. https://stacks.cdc.gov/view/cdc/47378
Sahu O, Mazumdar B, Chaudhari PK (2014) Treatment of wastewater by electrocoagulation: a review. Environ Sci Pollut Res 21:2397–2413. https://doi.org/10.1007/s11356-013-2208-6
Schmalz V, Dittmar T, Haaken D, Worch E (2009) Electrochemical disinfection of biologically treated wastewater from small treatment systems by using boron-doped diamond (BDD) electrodes—contribution for direct reuse of domestic wastewater. Water Res 43:5260–5266. https://doi.org/10.1016/j.watres.2009.08.036
Şengil İA, özacar M (2006) Treatment of dairy wastewaters by electrocoagulation using mild steel electrodes. J Hazard Mater 137:1197–1205. https://doi.org/10.1016/j.jhazmat.2006.04.009
Sharma D (2014) Treatment of dairy waste water by electro coagulation using aluminum electrodes and settling, filtration studies. Int J ChemTech Res 6:591–599
Shi L, Simplicio WS, Wu G et al (2018) Nutrient recovery from digestate of anaerobic digestion of livestock manure: a review. Curr Pollut Rep 4:74–83. https://doi.org/10.1007/s40726-018-0082-z
Shim S, Reza A, Kim S et al (2020) Simultaneous removal of pollutants and recovery of nutrients from high-strength swine wastewater using a novel integrated treatment process. Animals 10:835. https://doi.org/10.3390/ani10050835
Shim S, Reza A, Kim S et al (2021) Nutrient recovery from swine wastewater at full-scale: an integrated technical, economic and environmental feasibility assessment. Chemosphere 277:130309. https://doi.org/10.1016/j.chemosphere.2021.130309
Shivayogimath DCB, Naik VR (2014) Treatment of dairy industry wastewater using electrocoagulation technique. Int J Eng Res 3:971–974
Simas A, Mores R, Steffens J et al (2019) Electrodisinfection of real swine wastewater for water reuse. Environ Chem Lett 17:495–499. https://doi.org/10.1007/s10311-018-0782-z
Smoczynski L, Munska K, Pierozynski B (2013) Electrocoagulation of synthetic dairy wastewater. Water Sci Technol 67:404–409. https://doi.org/10.2166/wst.2012.585
Song Y, Hahn HH, Hoffmann E (2002) Effects of solution conditions on the precipitation of phosphate for recovery: a thermodynamic evaluation. Chemosphere 48:1029–1034. https://doi.org/10.1016/S0045-6535(02)00183-2
Stasinakis AS (2008) Use of selected advanced oxidation processes (AOPs) for wastewater treatment–a mini review. Glob NEST J 10:376–385
Stylianou M, Montel E, Dermentzis K, Agapiou A (2020) Electrochemical treatment of cattle wastewater samples. Waste Biomass Valorization 11:5185–5196. https://doi.org/10.1007/s12649-020-01056-8
Tak B, Tak B, Kim Y et al (2015) Optimization of color and COD removal from livestock wastewater by electrocoagulation process: application of box-behnken design (BBD). J Ind Eng Chem 28:307–315. https://doi.org/10.1016/j.jiec.2015.03.008
Tchamango S, Nanseu-Njiki CP, Ngameni E et al (2010) Treatment of dairy effluents by electrocoagulation using aluminium electrodes. Sci Total Environ 408:947–952. https://doi.org/10.1016/j.scitotenv.2009.10.026
Thirugnanasambandham K, Sivakumar V, Prakash Maran J (2015) Optimization of process parameters in electrocoagulation treating chicken industry wastewater to recover hydrogen gas with pollutant reduction. Renew Energy 80:101–108. https://doi.org/10.1016/j.renene.2015.01.030
Tir M, Moulai-Mostefa N (2008) Optimization of oil removal from oily wastewater by electrocoagulation using response surface method. J Hazard Mater 158:107–115. https://doi.org/10.1016/j.jhazmat.2008.01.051
Torres-Sanchez AL, Lopez-Cervera SJ, de la Rosa C et al (2014) Electrocoagulation process coupled with advance oxidation techniques to treatment of dairy industry wastewater. Int J Electrochem Sci 9:6103–6112
Tran L-H, Drogui P, Mercier G, Blais J-F (2009) Electrolytic oxidation of polynuclear aromatic hydrocarbons from creosote solution using Ti/ IrO2 and Ti/ SnO2 circular mesh electrodes. J Environ Eng 135:1051–1062. https://doi.org/10.1061/(ASCE)EE.1943-7870.0000064
Troster I, Schafer L, Fryda M (2002) Recent developments in production and application of DiaChem®-electrodes for wastewater treatment (NDFCT 380). New Diam Front Carbon Technol 12:89–98
Turan NB (2021) The application of hybrid electrocoagulation–electrooxidation system for the treatment of dairy wastewater using different electrode connections. Sep Sci Technol 56:1788–1801. https://doi.org/10.1080/01496395.2020.1788596
Uğurlu M, Gürses A, Doğar Ç, Yalçın M (2008) The removal of lignin and phenol from paper mill effluents by electrocoagulation. J Environ Manage 87:420–428. https://doi.org/10.1016/j.jenvman.2007.01.007
Valente GFS, Santos Mendonça RC, Pereira JAM, Felix LB (2012) The efficiency of electrocoagulation in treating wastewater from a dairy industry, part I: iron electrodes. J Environ Sci Health Part B 47:355–361. https://doi.org/10.1080/03601234.2012.646174
Valente GFS, Mendonça RCS, Pereira JAM, Felix LB (2014) Artificial neural network prediction of chemical oxygen demand in dairy industry effluent treated by electrocoagulation. Sep Purif Technol 132:627–633. https://doi.org/10.1016/j.seppur.2014.05.053
Varank G, Sabuncu ME (2015) Application of Central Composite Design approach for dairy wastewater treatment by electrocoagulation using iron and aluminum electrodes: modeling and optimization. Desalin Water Treat 56:33–54. https://doi.org/10.1080/19443994.2014.934731
Vasudevan S, Lakshmi J, Jayaraj J, Sozhan G (2009) Remediation of phosphate-contaminated water by electrocoagulation with aluminium, aluminium alloy and mild steel anodes. J Hazard Mater 164:1480–1486. https://doi.org/10.1016/j.jhazmat.2008.09.076
Vasudevan S, Lakshmi J, Sozhan G (2011) Effects of alternating and direct current in electrocoagulation process on the removal of cadmium from water. J Hazard Mater 192:26–34. https://doi.org/10.1016/j.jhazmat.2011.04.081
Vasudevan S, Lakshmi J, Sozhan G (2012) Electrocoagulation studies on the removal of copper from water using mild steel electrode. Water Environ Res 84:209–219. https://doi.org/10.2175/106143011X13225991083640
Vik EA, Carlson DA, Eikum AS, Gjessing ET (1984) Electrocoagulation of potable water. Water Res 18:1355–1360. https://doi.org/10.1016/0043-1354(84)90003-4
Walsh F, Mills G (1994) Electrochemical methods for pollution control. Chem Technol Eur 1:13–18
Wang C-T, Chou W-L, Kuo Y-M (2009) Removal of COD from laundry wastewater by electrocoagulation/electroflotation. J Hazard Mater 164:81–86. https://doi.org/10.1016/j.jhazmat.2008.07.122
Wang Y, Gao P, Fan M, Jin H (2011) Preliminary study of purification for livestock wastewater of immobilized microcystis aeruginosa. Procedia Environ Sci 11:1316–1321. https://doi.org/10.1016/j.proenv.2011.12.197
Wang M, Wu Y, Li B et al (2015a) Pretreatment of poultry manure anaerobic-digested effluents by electrolysis, centrifugation and autoclaving process for Chlorella vulgaris growth and pollutants removal. Environ Technol 36:837–843. https://doi.org/10.1080/09593330.2014.963695
Wang M, Cao W, Wu Y et al (2016) Electrochemical oxidation of the poultry manure anaerobic digested effluents for enhancing pollutants removal by Chlorella vulgaris. Environ Technol 37:1451–1460. https://doi.org/10.1080/09593330.2015.1119199
Wang F, Fu R, Lv H et al (2019a) Phosphate recovery from swine wastewater by a struvite precipitation electrolyzer. Sci Rep 9:1–10. https://doi.org/10.1038/s41598-019-45085-3
Wang F, Wei J, Zou X et al (2019b) Enhanced electrochemical phosphate recovery from livestock wastewater by adjusting pH with plant ash. J Environ Manage 250:109473. https://doi.org/10.1016/j.jenvman.2019.109473
Wang X, Lin L, Zhang L et al (2020) Combination of electrolysis and microalgae cultivation to beneficial reuse fertilizer wastewater from poultry manure anaerobic digestion effluent. Desalin Water Treat 183:139–148. https://doi.org/10.5004/dwt.2020.25042
Wang X, Lu H, Zhang L, et al (2015b) Combination of Electrolysis and Microalgae cultivation to treat effluent from anaerobic digestion of poultry manure. Int Symp Anim Env Welf 179–186.
Wang M, Cao W, Wu Y, et al (2017) Electrochemical disinfection of effluents from poultry waste anaerobic digestion. Environ Eng Manag J EEMJ; 16:329–335. https://doi.org/10.30638/eemj.2017.033
Won S-G, Jeon D-Y, Rahman MM et al (2016) Optimization of electrochemical reaction for nitrogen removal from biological secondary-treated milking centre wastewater. Environ Technol 37:1510–1519. https://doi.org/10.1080/09593330.2015.1119205
Yavuz Y, Öcal E, Koparal AS, Öğütveren ÜB (2011) Treatment of dairy industry wastewater by EC and EF processes using hybrid Fe–Al plate electrodes. J Chem Technol Biotechnol 86:964–969. https://doi.org/10.1002/jctb.2607
Yetilmezsoy K, Ilhan F, Sapci-Zengin Z et al (2009) Decolorization and COD reduction of UASB pretreated poultry manure wastewater by electrocoagulation process: a post-treatment study. J Hazard Mater 162:120–132. https://doi.org/10.1016/j.jhazmat.2008.05.015
Yi X, Li X, Liu R et al (2016) Removal of ammonia nitrogen from swine wastewater by electrooxidation using Ti/Mn-Ni/SnO2-Sb-CeO2 anode. Environ Eng Manag J 15:2147–2153
Zakeri HR, Yousefi M, Mohammadi AA et al (2021) Chemical coagulation-electro fenton as a superior combination process for treatment of dairy wastewater: performance and modelling. Int J Environ Sci Technol 18:3929–3942. https://doi.org/10.1007/s13762-021-03149-w
Zaroual Z, Chaair H, Essadki AH et al (2009) Optimizing the removal of trivalent chromium by electrocoagulation using experimental design. Chem Eng J 148:488–495. https://doi.org/10.1016/j.cej.2008.09.040
Zhang H, Li Y, Wu X et al (2010) Application of response surface methodology to the treatment landfill leachate in a three-dimensional electrochemical reactor. Waste Manag 30:2096–2102. https://doi.org/10.1016/j.wasman.2010.04.029
Zhang X, Lin H, Hu B (2016) Phosphorus removal and recovery from dairy manure by electrocoagulation. RSC Adv 6:57960–57968. https://doi.org/10.1039/C6RA06568F
Zhang X, Lin H, Hu B (2018) The effects of electrocoagulation on phosphorus removal and particle settling capability in swine manure. Sep Purif Technol 200:112–119. https://doi.org/10.1016/j.seppur.2018.02.025
Funding
This work was financially supported partially by the USDA National Institute of Food and Agriculture (NIFA), Hatch Project (Project No. IDA01604, Accession No. 1019082), and the USDA NIFA Sustainable Agricultural Systems Project (Award No. 2020-69012-31871).
Author information
Authors and Affiliations
Contributions
AR and LC contributed to conceptualization and methodology; AR performed data curation, writing—original draft preparation, and visualization; LC and AR helped in writing—review and editing; LC was involved in supervision, project administration, and funding acquisition.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Reza, A., Chen, L. Electrochemical treatment of livestock waste streams. A review. Environ Chem Lett 20, 1863–1895 (2022). https://doi.org/10.1007/s10311-022-01393-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-022-01393-1