Abstract
This study investigated the electrochemical oxidation of organic matter present in shrimp farming effluent using three types of electrocatalytic materials: Ti/Ru0.34Ti0.66O2, Ti/Pt, and boron-doped diamond (BDD). An electrochemical cell with 300 mL under stirring agitation was used by applying 20, 40, and 60 mA cm−2. A Ti/Ru0.34Ti0.66O2 anode showed a reduction of chemical oxygen demand (COD) about 84 % after 1 h of electrolysis, while at the same time, 71 % of COD decay was achieved at Ti/Pt. Conversely, only 71 % of COD was removed after 2 h with a BDD anode. Regarding the temperature effect, BDD showed better performances than those achieved for Ti/Ru0.34Ti0.66O2 and Ti/Pt anodes during an electrochemical treatment of a shrimp farming effluent, obtaining 72 % of COD removal by applying 20 mA cm−2 at 40 °C after 15 min. Energy consumption and cost were estimated in order to established the engineering applicability of this alternative process.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The activity of shrimp aquaculture is a branch that corresponds to shrimp farming in tank producers, usually excavated in estuarine ecosystems, a technique inspired by the model spread widely through Asia, where shrimp are raised in small pens made from handcrafted bamboo on the banks of mangroves. In Brazil, shrimp farming began in the 1970s, as a commercial activity, and since 2003, it has had a production of 90.19 million tons, arriving to 80 tons in 2010, using an area of 18,500 ha (ABCC 2013).
Water is a decisive factor for the activity of shrimp farming, so that, for every ton of shrimp produced, between 50 and 60 million l of water is needed (Mello 2007). The effluents of shrimp farming cause high impact on water quality when discharged in natural aquatic ecosystems; in extreme cases, it can cause the death of many species (Brugger 2003) because of a high concentration of organic material (mainly from the remains of food supplied to shrimp), excretion, phytoplankton, and fertilizers, creating a problem for eutrophication of coastal water.
There are several studies regarding the impacts of shrimp farming, particularly in regions where this activity is developed, such as China (Herbeck et al. 2012), India (Ronnback et al. 2003), and Mexico (Berlanga-Robles et al. 2011). Meanwhile, several authors have also described the impacts of this commercial activity in different Brazilian regions (Dias et al. 2012; Joventino and Mayorga 2008; Sampaio et al. 2008; Freitas et al. 2008; Figueiredo et al. 2005; Ormond et al. 2004; Meireles and Marques 2004; Shanahan 2003; Brugger 2003; Meireles and Vicente da Silva 2002; Paez-Ozuma 2001; Coelho Junior and Schaeffer-Novelli 2000).
For that reasons, the development of an efficient and not harmful methodology for treating effluents produced by farming shrimp is essential to ensure environmental sustainability.
Biological treatment is not as effective for wastewater with high volumes and high salinity because a important problem is the acclimation of microorganisms as well as the high time to achieve significant results (Schroeder et al. 2011; Dias et al. 2012). Moreover, in intensive aquaculture systems where the recirculation water mode is applied, there is an significant accumulation of intermediates, products of metabolism and toxic organic compounds that are refractory at the treatment, requiring a more efficient decontamination approach of water, seeking to maintain their quality (Schroeder et al. 2011).
In recent years, promising alternatives for the treatment of various effluents have been proposed (Martínez-Huitle and Brillas 2009; Panizza and Cerisola 2009; Martínez-Huitle et al. 2015), electrochemical technologies. Among them, the electrochemical advanced oxidant processes (EAOPs) demonstrate high efficiencies on the mineralization of highly persistent organic pollutants (POPs). These processes use different reaction systems, all characterized by the electrogeneration of strong oxidants (e.g., ·OH radicals, active chlorine, ozone, hydrogen peroxide, and so on; Martínez-Huitle et al. 2015) for treating effluents such as textile (Martínez-Huitle et al. 2012), wastewater generated by petrochemical industry (Rocha et al. 2012; Santos et al. 2010), and aquaculture (Díaz et al. 2011; Virkutyte and Jegatheesan 2009).
The simplest and most popular EAOP is electrochemical oxidation (EO) where organic pollutants in solution are oxidized by direct charge transfer at the anode (M) or extensively destroyed by reactive oxygen species and/or active chlorine species electrogenerated by oxidation reactions onto the anode surface (Martínez-Huitle et al. 2015). In the absence of chloride in solution, only reactive oxygen species are electrogenerated, such as hydroxyl (·OH) radical. This oxidant specie can react with most POPs up to complete combustion due to the high standard reduction potential (E°(·OH/H2O) = 2.80 V/SHE) and its being non-selective to attack the organic compounds. In EO, the ·OH radical is produced as an intermediate from water oxidation on anode materials with high over-potential of O2 evolution from Eq. 1 (Martínez-Huitle et al. 2015), remaining adsorbed (physically or chemically, depending on the nature of electrode material) onto the anode surface that afterwards reacts with the pollutants in solution promoting to their electrochemical conversion or electrochemical mineralization to CO2.
Meanwhile, in the presence of chloride, active chlorine species can be electrogenerated by Cl− ion oxidation onto the anode releasing Cl2 from Eq. 2 (Martínez-Huitle et al. 2015). Afterwards, from the disproportionation of chlorine according to Eq. 3, HClO and Cl− are released into the bulk. Due to the acid-base equilibrium (Eq. 4) with pK a = 7.55, ClO− could remain as predominant species in solution depending on the effluent pH. Besides, ClO− could be directly yielded from chloride reaction with M(·OH) according to Eq. 5.
The quasi-ubiquitous presence of chloride ions in water effluents highlights the possible usage of EO technology in order to promote the generation of active chlorine species by using particular electrocatalytic anodes, such as dimensionally stable anodes (DSAs). Likewise, the correct selections of DSA as well as suitable operating conditions avoid the production of chlorate and perchlorate ions (Martínez-Huitle et al. 2015; Garcia-Segura et al. 2015).
Then, the main objective of this study is to investigate the influence of the operating parameters (current density and temperature) on the chemical oxygen demand (COD) removal of shrimp farming effluent, at different electrocatalytic materials (Ti/Pt, Ti/Ru0.34Ti0.66O2, and boron-doped diamond (BDD)) in order to identify the optimal experimental conditions which give high current efficiency and need low energy requirements.
Experimental procedure
Reagents and solutions
All analyses were performed with reagents and analytical standard solutions and deionized water, employing chemical procedures described in the Standard Methods for the Examination of Water and Wastewater (APHA 2005).
Effluent samples
The samples were supplied by a shrimp farm located at the northwest Brazilian region, and these samples were used without any treatment or addition products.
Sample characterization
Physical-chemical characterization of the effluent was carried out (turbidity, chloride, nitrate, nitrite, COD, and so on) in order to establish its chemical composition and compare the results with the values permitted by the Brazilian environmental laws (Conselho Nacional do Meio Ambiente (CONAMA) 2012).
Electrooxidation experiments
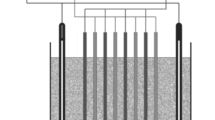
The anodic oxidations were carried out in a single-compartment electrochemical cell of 0.3 L with magnetic stirring. For the application of current, a power source Minipa MLP-3305 was used. In this study, three different anode materials were used: Ti/Pt, Ti/Ru0.34Ti0.66O2, and BDD. As cathodes, two plates of steel 316 were employed, with an area about 20 cm2, with a separation of 0.5 cm between the electrodes. Initially, the influence of the applied current density (20, 40, and 60 mA cm−2) to treat the effluent was analyzed, maintaining the temperature constant at 25 °C by using a thermostated cell. During the bulk electrolysis, the samples were collected for COD measurements. After that, by applying a current density of 20 mA cm−2, the influence of temperature at 40 °C during the electrochemical treatment of the shrimp effluent was evaluated.
Analytical methods
The efficiency of the treatment was available by means of COD removal, according to the standard methods for water analyses (APHA 2005), using the photometer HI83099.
The total current efficiency for the electrochemical treatment was calculated, using the following relation:
where COD t and COD t + Δt are the chemical oxygen demands (g dm−3) at times t = 0 (initial) and f (final), respectively; I is the current (A); F is the Faraday constant (96,487 C mol−1); V is the volume of electrolyte (dm3); and 8 is the oxygen equivalent mass (g eq−1).
The energy consumption per volume of the treated effluent was calculated and expressed in kilowatts per hour per cubic meter. The average cell voltage, during the electrolysis, is taken for calculating the energy consumption, as follows (Martínez-Huitle and Brillas 2009):
where t is the time of electrolysis (h); V and A are the average cell voltage (in volts) and the electrolysis current (in amperes), respectively; and V s is the sample volume (m3). Afterwards, the cost of treatment per unit of volume of the treated effluent was estimated, considering the electric energy cost in Brazil (Ramalho et al. 2010). To determine the active chlorine species concentration produced (chlorine (Cl2(aq)), chlorite (ClO2 −), chlorine dioxide (ClO2), and chlorate (ClO3 −)) at Ti/Ru0.34Ti0.66O2, Ti/Pt, and BDD electrocatalytic materials during 4 h of electrolysis by applying 60 mA cm−2 in the presence of NaCl (5 g L−1), a sequence of iodometric titration was performed, where 5-mL aliquots were sampled and chemically analyzed, as reported by Neodo et al. (2012) and De Moura et al. (2014). The different species were discriminated on the basis of their reactivity, which was emphasized by either changing the pH or adding specific reagents. In the case of the chlorate and perchlorate content, it was also assessed by ion chromatography (IC), using an IonPac AS16 column (4 mm × 250 mm) and a 1-mL injection loop (De Moura et al. 2014).
Results and discussion
Sample characterization
The physical-chemical characterization of the effluent is reported in Table 1. From these results, we can observe that several parameters have higher values than those permitted by the Brazilian environmental laws (Conselho Nacional do Meio Ambiente (CONAMA) 2012). For high values of turbidity and chloride and COD content, low biodegradability of this effluent is expected, limiting its treatment by a biological process. At the same time, high nitrate content in the effluent could be easily converted in nitrite and ammonium by biochemical processes of microorganisms, increasing the poor quality of water and consequently diminishing the life conditions. Also, total suspended solids avoid the light penetration, blocking the photosynthesis routes for aquatic plants. Nevertheless, the applicability of indirect electrochemical process could be considered as a good alternative, avoiding the addition of chemical products.
Influence of applied current density
Figure 1 shows the influence of applied current density (20, 40, and 60 mA cm−2) on the removal of COD, as a function of time, at different anode materials. During the electrolysis with Ti/Pt and Ti/Ru0.34Ti0.66O2 anodes, a significant COD decay in the beginning of electrolysis was obtained, remaining practically constant throughout the reaction (mass transport limitations). In the case of Ti/Pt, 89.2, 92.3, and 93.0 % of COD removal were achieved after 4 h of electrolysis by applying 20, 40, and 60 mA cm−2, respectively. For Ti/Ru0.34Ti0.66O2, COD removals of about 92.6, 98.7, and 91.7 % were achieved after 4 h of electrolytic treatment at 20, 40, and 60 mA cm−2, respectively. Conversely, a BDD anode showed lower COD removals (42.3, 61.8, and 67.2 % for 20, 40, and 60 mA cm−2, respectively) under similar experimental conditions. The results clearly indicate that the DSAs are the most efficient materials for treating this kind of effluent. It is due to the active electrocatalytic nature of Ti/Ru0.34Ti0.66O2 and Ti/Pt anodes (Martínez-Huitle and Ferro 2006; Panizza and Cerisola 2009) where the oxidation of organic materials occurs by a direct interaction with the so-called higher oxides (adsorbed hydroxyl radicals in the lattice of the oxide anode: MO x + 1, Eq. 8) at the electrode surface (Eq. 9)
as well as mainly by active chlorine species formed when the effluents contain chlorides (Eqs. 2–4). Theoretically (Aquino et al. 2011, 2012; Ferro et al. 1998; De Battisti et al. 2000; Bonfatti et al. 2000a, b; Martínez-Huitle et al. 2008), the electrochemical treatment can be carried out at lower potentials in the presence of chlorides, compared with those required for the direct anodic oxidation (Bonfatti et al. 2000a). It is due to the increase in the conductivity, affecting the cell potential and consequently promoting the active chlorine production which favors a faster COD abatement, at active anodes. However, the electrogeneration of these oxidants is strongly dependent on pH and in competition with side reactions such as oxygen and chlorine evolution reactions
In fact, in the case of Ti/Ru0.34Ti0.66O2 and Ti/Pt anodes, when total current efficiency (TCE) values are estimated (Fig. 2), it is evident that, when an increase in the applied current density was attained, the electrical charge furnished to the system decreased. This behavior is because the undesired reactions are favored (such as oxygen evolution reaction (Eq. 10) and, probably, chlorine evolution reaction (Eq. 11)). Conversely, at a BDD electrode, an increase in the applied current density promotes a slight increase in the TCE due to the production of active chlorine species that participate in the elimination of organic matter.
For this reason, the behavior of pH conditions during the electrochemical treatment of shrimp farming effluent using Ti/Pt, BDD, and Ti/Ru0.34Ti0.66O2 anodes was investigated (Fig. 3). When the solution pH, originally around 7.5, was analyzed, it decreases to values of 6.3–6.5 after 2.5 h of electrolysis; after that, the pH around 7.5 is restored in the last phase of the electrochemical process by applying 20, 40, and 60 mA cm−2 when Ti/Pt was used (Fig. 3). In the case of Ti/Ru0.34Ti0.66O2 anode, pH passes from 7.5 to 8.1 after 1.5 h of electrolysis, remaining constant around 8.2–8.3 during all bulk tests, under similar experimental conditions. Thus, in both cases, ClO− prevails in all stages of the electrolysis, but HClO can be active at the Nernst layer on the anode surface, as a consequence of the large acidity (pH is slightly acidic) due to the concomitant oxygen evolution (Eq. 10), as indicated previously by other authors (Bonfatti et al. 2000a). Based on these results, we can assume that the production of species like hypochlorite and hypochlorous acid confirms that the organic compounds dissolved in the effluent and the by-products generated are oxidized in the bulk of the solution by them. Assuming these statements, the COD removal is independent of the applied current density, but it depends on the active chlorine species formed as well as the nature of electrode material. These outcomes are in agreement with the results reported by De Battisti and co-workers studying the oxidation of glucose in the presence of chlorides at Pt anodes (Ferro et al. 1998; De Battisti et al. 2000; Bonfatti et al. 2000a, b). Additionally, in consideration of the fact that both chloro and oxychloro radicals react in solution, these must be considered in the mechanism of the electrochemical destruction, thus representing an extension (Bonfatti et al. 2000a) of the model initially proposed by Comninellis (1994) for the direct electrochemical oxidation.
Another important feature of the Cl-mediated oxidation is that secondary oxidants such as Cl2(aq), chlorite, chlorine dioxide, and chlorate can be formed from the reactions between primary oxidants (HClO, ClO−, oxygen, and hydroxyl radicals (at lower concentrations)) or from direct oxidation reactions at anode surfaces. After that, as reported in the literature (Martínez-Huitle and Brillas 2009; Panizza and Cerisola 2009; Martínez-Huitle et al. 2015), primary and secondary oxidants are quite stable and migrate in the solution bulk, depending on the diffusion rate and pH conditions (Martínez-Huitle and Brillas 2009; Aquino et al. 2011, 2012; Bonfatti et al. 2000a), where these indirectly oxidize the organic matter (as shown in Fig. 1 during COD decay achieved at Ti/Pt and Ti/Ru0.34Ti0.66O2 anodes).
Taking into consideration the above information, oxidation reactions should be mainly a set of volume rather surface reactions; the change from active anodes (by the way also a bad catalyst for oxygen evolution reaction (o.e.r)) to BDD should not involve dramatic changes in the incineration mechanism. However, when BDD was used (non-active anode), lower COD removals were achieved, as shown in Fig. 1. This behavior can be due to the coexistence of different active chlorine species than those electrochemically generated at Ti/Pt and Ti/Ru0.34Ti0.66O2 anodes. In fact, it is confirmed by the pH conditions which quickly decrease to 6.7 after 10 min of electrolysis, and after that, pH is slowly restored to values around 7.3 (see Fig. 3). For this reason, a new set of experiments was performed at 60 mA cm−2 at 25 °C by using Ti/Pt, Ti/Ru0.34Ti0.66O2, and BDD anodes to understand and determine the electrochemical production of reactive oxidant species (Cl2(aq), HClO, ClO−, chlorite, chlorine dioxide, and chlorate) when a synthetic solution with similar chloride concentration was electrolyzed. Figure 4 shows the trends of active chlorine species produced at Ti/Ru0.3Ti0.7O2, Ti/Pt, and BDD anodes as a function of concentration and electrolysis time. The results clearly demonstrated that the concentration of active chlorine species depends on the pH conditions as well as the electrode material. For the Ti/Ru0.34Ti0.66O2 anode, it is worth to note the evident production of Cl2(aq), ClO2, and ClO2 − species at 60 mA cm−2, when compared to the concentration of active chlorine species produced at Ti/Pt and BDD anodes. In the case of BDD electrode, Cl2(aq), ClO2 −, and ClO3 − are preferentially formed; however, the concentration of Cl2(aq) is lower than that achieved at the Ti/Ru0.34Ti0.66O2 anode. Conversely, the production of Cl2(aq) increases slightly with electrolysis time at Ti/Pt electrode. It may be due to the Cl− conversion to Cl2(g) (Eq. 11) as a consequence of the use of an undivided electrochemical cell at higher applied current density. However, a significant concentration of ClO3 − was assessed at Ti/Pt and BDD anodes in the course of the electrolytic processes (Fig. 4), as confirmed by titrimetric and chromatographic analyses. It is related to the conversion of Cl− to more oxidative states after the production of ClO2 − and ClO2.
For the Ti/Ru0.34Ti0.66O2 anode, the Cl2(aq) concentration reaches a maximum at around 170 ppm when 60 mA cm−2 was applied; after that, the concentration of these species varied depending on the pH conditions (Fig. 4). In the case of ClO2 and ClO2 −, the concentrations achieved a maximum production at 120 and 240 min, in that order, reaching 45 and 65 ppm, respectively. Meanwhile, at BDD, a significant production of Cl2(aq) and ClO3 − species is attained, reaching higher concentrations in 240 min (up to 94 ppm for Cl2(aq)) and 180 min (up to 112 ppm for ClO3 −) of electrolysis, respectively. In contrast, no significant ClO2 − concentrations were measured. This behavior occurs with the establishment of adsorptive interactions between the electrode and some active chlorine species formed, and consequently, catalytic mechanisms may proceed to higher electron-state species, such as ClO3 −.
In the case of Cl2(aq), the accumulation of higher concentrations may promote its conversion to other active chlorine species in the bulk of solution, preferentially, the formation of HClO(aq) and ClO− (aq) by Eqs. 2 to 5. The reactivity of these species occurs in the proximity of Nernst layer due to the poor coexistence of one of these species (HClO(aq)/ClO− (aq) ratio). Conversely, these species are found at neutral pH conditions at higher concentrations, favoring a rapid homogenous catalytic reaction with organic pollutants. Therefore, significant COD removal efficiencies were achieved at Ti/Pt and Ti/Ru0.34Ti0.66O2 anodes when Cl-mediated oxidation was performed. This behavior decreases at BDD. In the former cases, the significant concentration of Cl2(aq) favors its conversion to HClO(aq) and ClO− (aq) in the proximity of Nernst layer, and consequently, the oxidation of organic matter is efficiently attained. In the latter case, poor organic removal was obtained due to the loss of Cl− in solution by Cl2(g) formation (Eq. 11).
It is also important to consider that BDD produces large quantities of hydroxyl radicals by water discharge (Eq. 1) and, in the presence of chlorides in aqueous solutions, ·OH can oxidize this raw material successively to different oxochlorinated compounds (Eqs. 12 to 16):
In fact, ClO3 − was determined and quantified, while the production of ClO4 − was not detected. It is relevant information which indicates that the use of active chlorine may result in the generation of undesired by-products at the end of electrochemical treatment.
Temperature effect
The effect of temperature during the electrochemical treatment of an actual shrimp effluent was also studied by applying 20 mA cm−2, varying the temperature from 25 to 40 °C. The latter temperature was selected because it mimics the real temperature of the shrimp farming discharges (40 °C). It was observed (Fig. 5) that changes in temperature have a strong influence on oxidation rate by applying 20 mA cm−2 and varying the temperature from 25 to 40 °C. By using the BDD anode, COD removal reaches about 73 % after 30 min of treatment, remaining constant due to the mass transport limitations. Meanwhile, a significant decrease in the COD decay rates was noticeably attained at Ti/Pt and Ti/Ru0.34Ti0.66O2 anodes when an increase in temperature was achieved, obtaining 77 and 73 %, respectively (Fig. 4), after 3 h of electrolysis. This behavior indicates that a remarkable decay on the production of active chlorine species is attained when active anodes were used because a part of Cl− in solution is converted to Cl2(g) at 40 °C (Neodo et al. 2012; Martínez-Huitle et al. 2015), consequently disfavoring the indirect reaction of organic pollutants with electrogenerated oxidizing agents. It is confirmed by the TCE values reported in the inset of Fig. 5 where it is clear that the electrical energy furnished to the electrochemical system decreased when an increase in the temperature was attained (see Fig. 2). In fact, TCE values lower than 30 % were achieved.
In the case of non-active anodes (BDD), the efficient production of ·OH radicals promotes the formation of active chlorine species (Eqs. 10–14), consequently increasing the TCE (inset in Fig. 5). It is also confirmed by the pH conditions achieved during electrochemical treatment at 40 °C (Fig. 6). Conversely, at Ti/Pt and Ti/Ru0.34Ti0.66O2 anodes (active anodes), undesired reactions are favored under these pH conditions (Fig. 6), as oxygen evolution reaction (Eq. 10) and production of Cl2(gas) (Eq. 11), respectively, limiting the oxidation of organic matter and consequently decreasing the TCE (inset in Fig. 5) with respect to the values obtained at 25 °C (Fig. 2). Thus, these results put into evidence the difference in the mechanisms involved when active and non-active anodes were used, mainly in a medium containing chloride at higher temperatures.
Estimation of energy consumption
Key points for the development of clean methodologies are the energetic and economic aspects, allowing indications about the feasibility of the electrochemical process. Table 2 summarizes the data about energy consumption, and costs of treatment per unit volume of the treated effluent.
The energy consumption per unit volume appeared proportional to the applied current density during electrolysis for all electrocatalytic materials. For example, in the case of Ti/Ru0.34Ti0.66O2, there was increased consumption of 6.00, 16.10, and 27.31 kWh m−3 of the treated effluent, when the current density was 20, 40, and 60 mA cm−2, respectively. The current density of 20 mA cm−2 required a lower cost per unit volume, and good efficiencies of current during the electrolysis was achieved, except for the BDD anode (Table 2).
Conclusions
On basis of the results obtained for anodic oxidation of a real effluent, the electrochemical technology can be suitable as an alternative for treatment under the real discharge conditions to accomplish the Brazilian legal requirements. Considering that using biological treatment for depuration of shrimp effluent is accomplished after 5 or 6 days with a subsequent physical-chemical treatment (under specific pH and temperature conditions), the electrochemical treatment could be considered as a promising alternative because higher organic matter removal is achieved in short times. Also, assuming that the effluent is discharged at 40 °C, the efficiency of electrochemical treatment can be improved without the addition of other chemical reagents or pre-treatment waste.
Particular attention and experimental observations must be taken into consideration when using non-active anodes in the presence of higher concentrations of chloride because these anodes can favor undesired reactions (oxygen and chlorine evolution), limiting organic matter oxidation (up to 85 % of COD removal). Nevertheless, at Ti/Ru0.34Ti0.66O2 and Ti/Pt anodes, a quasi-complete COD removal (more than 95 % of organic matter) is attained in short times by applying lower current densities, confirming the applicability of this electrochemical technology. It is due to the efficient production of active chlorine species, as confirmed by the study of the strong oxidants formed at similar chloride concentrations of the real effluent, without the production of perchlorate.
Finally, the results reported in the present work have recently allowed to start the design and implementation of a pilot industrial electrochemical cell in a shrimp farm. These experiments are in progress, and their results will be reported in detail in a separate paper in the near future.
References
ABCC. Brazilian Association of Shrimp Farmers (2013) Story shrimp farming in Brazil. Available at: <http://www.abccam.com.br/historico2.html>. Accessed Jan 2013. (Portuguese)
APHA (2005) Standard methods for the examination of water and wastewater, 21 edn. American Public Health Association, Washington, DC
Aquino JM, Pereira GF, Rocha-Filho RC, Bocchi N, Biaggio SR (2011) Electrochemical degradation of a real textile effluent using boron-doped diamond or β-PbO2 as anode. Journal Hazardous Materials 192(3):1275–1282
Aquino JM, Rodrigo MA, Rocha-Filho RC, Sáez C, Cañizares P (2012) Influence of the supporting electrolyte on the electrolyses of dyes with conductive-diamond anodes. Chem Eng J 184:221–227
Berlanga-Robles CA, Ruiz-Luna A, Bocco G, Vekerdy Z (2011) Spatial analysis of the impact of shrimp culture on the coastal wetlands on the northern coast of Sinaloa, Mexico. Ocean & Coastal Management 54:535–543
Bonfatti F, De Battisti A, Ferro S, Lodi G, Osti S (2000b) Anodic mineralization of organic substrates in chlorine-containing aqueous media. Electrochim Acta 46:305–314
Bonfatti F, Ferro S, Lavezzo F, Malacarne M, Lodi G, Battisti A (2000a) Electrochemical incineration of glucose as a model organic substrate. II. Role of active chlorine mediation. Jounal of the Electrochemical Society 147(2):592–596
Brugger RE (2003) Aquaculture and society in the new millennium. World Aquaculture 34(1):51–59
Coelho Junior, C.; Schaeffer-Novelli, Y., 2000. Considerações teóricas e práticas sobre o impacto da carcinicultura nos ecossistemas costeiros brasileiros, com ênfase no ecossistema manguezal. In: Proceeding of Mangrove. International Society for Mangrove Ecosystems – Mangrove. Recife, 2000.
Comninellis C (1994) Electrocatalysis in the electrochemical conversion/combustion of organic pollutants for waste water treatment. Electrochim Acta 39(11–12):1857–1862
Companhia Energética do Rio Grande do Norte (COSERN) (2012). Serviço ao cliente; Cosern Serviços. Access in 3 February 2015. Available in: http://www.cosern.com.br
Conselho Nacional do Meio Ambiente (CONAMA) (2012) Resoluções do Conama: Resoluções vigentes publicadas entre setembro de 1984 e janeiro de 2012. Brasília: MMA. Acesso em 01 julho 2012. Disponivel em: http://www.mma.gov.br/port/conama/index.cfm
De Battisti A, Ferro S, Lavezzo F, Lodi G, Osti S (2000) A study on the optimization of the mediated electrochemical incineration of glucose. Proceedings–Electrochemical Society 99-39:49–65
De Moura DC, Kerzia Costa de Araujo C, Zanta CLPS, Salazar R, Martinez-Huitle CA (2014) Active chlorine species electrogenerated on Ti/Ru0.3Ti0.7O2 surface: electrochemical behavior, concentration determination and their application. J Electroanal Chem 731:145–152
Dias HM, Soares MLG, Neffa E (2012) Conflitos socioambientais: o caso da carcinicultura no complexo estuarino Caravelas - Nova Viçosa/Bahia-Brasil. Ambiente & Sociedade 15:111–130
Díaz V, Ibáñez R, Gómez P, Urtiaga AM, Ortiz I (2011) Kinetic of electro-oxidation of ammonia-N, nitrites and COD from a recirculating aquaculture saline water sys-tem using BDD anodes. Water Res 45(1):125–134
Ferro S, Lavezzo F, Lodi G, De Battisti A, Comninellis C (1998) Electrochemical incineration (mineralization) of glucose as a model organic substrate. Role of the electrode material and of active chlorine mediation. Proceedings – Electrochemical Society 98-5:75–90
Figueiredo MCB, Araujo LFP, Gomes RB, Rosa MF, Paulino WD, Morais LFS (2005) Environmental impacts of inland shrimp farming effluents. Engenharia Sanitária Ambiental 10(2):167–174 Portuguese
Freitas U, Niencheski LFH, Zarzur S, Manzolli RP, Vieira JPP, Rosa LC (2008) Influência de um cultivo de camarão sobre o metabolismo bêntico e a qualidade da água. Revista Brasileira de Engenharia Agrícola e Ambiental 12:293–301
Garcia-Segura S, Vieira Dos Santos E, Martínez-Huitle CA (2015) Role of sp3/sp2 ratio on the electrocatalytic properties of boron-doped diamond electrodes: a mini review. Electrochem Commun 59:52–55
Herbeck LS, Unger D, Wu Y, Jennerjahn TC (2012) Effluent, nutrient and organic matter export from shrimp and fish ponds causing eutrophication in coastal and back-reef waters of NE Hainan, tropical China. Cont Shelf Res. doi:10.1016/j.csr.2012.05.006
Joventino FKP, Mayorga MIO (2008) Diagnóstico socioambiental e tecnológico da carcinicultura no município de Fortim, Ceará, Brasil. Revista eletrônica do PRODEMA, Fortaleza 2(1):80–96
Martínez-Huitle CA, Brillas E (2009) Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods: a general review. Appl Catal B Environ 87(3–4):105–145
Martínez-Huitle CA, Ferro S (2006) Electrochemical oxidation of organic pollutants for the wastewater treatment: direct and indirect processes. Chem Soc Rev 35(12):1324–1340
Martínez-Huitle CA, Ferro S, Reyna S, Cerro-López M, De Battisti A, Quiroz JM (2008) Electrochemical oxidation of oxalic acid in the presence of halides at boron doped diamond electrode. Journal Brazilian Chemical Society 19(1):150–156
Martínez-Huitle CA, Rodrigo MA, Sirés I, Scialdone O (2015) Single and coupled electrochemical processes and reactors for the abatement of organic water pollutants: a critical review. Chem Rev 115:13362–13407
Martínez-Huitle CA, Santos EV, Araújo DM, Panizza M (2012) Applicability of diamond electrode/anode to the electrochemical treatment of a real textile effluent. Journal Electroanalytical Chemistry 674:103–107
Meireles, A.J.A., Marques, M., 2004. Estudos e leveantamentos ambientais, antropológicos e arqueológicos na Terra Indígena Tremembé de São José e Buriti, município de Itapipoca/CE. Parecer Técnico, Ministério Público Federal no Estado do Ceará MPF/CE, 98.
Meireles, A.J.A.; Vicente da Silva E. (2002) Abordagem geomorfológica para a realização de estudos integrados para o planejamento e gestão em ambientes flúvio marinhos. Scripta Nova - Geocrítica - Universidade de Barcelona - Espanha, v.7, n.118, p. 25
Mello, C.C.A., 2007. Assessment of environmental equity as an instrument of modernization and democratization of impact assessment procedures for development projects—case study: the licensing of shrimp farming in the states of Bahia and Ceará. PHASE - Project Sustainable and Democratic Brazil ETTERN-IPPUR-UFRJ. Rio de Janeiro. (Portuguese)
Neodo S, Rosestolato D, Ferro S, De Battisti A (2012) On the electrolysis of dilute chloride solutions: influence of the electrode material on faradaic efficiency for active chlorine, chlorate and perchlorate. Electrochim Acta 80:282–291
Ormond, J.G.P., Mello, G.A.T., Ferreira, P.R.P., Lima, C.A.O., 2004. A carcinicultura brasileira. BNDES Setorial, Rio de Janeiro (19):91–118
Paez-Ozuma F (2001) The environmental impact of shrimp aquaculture: causes, effects, and mitigating alternatives. Environ Manag 28(1):131–140
Panizza M, Cerisola G (2009) Direct and mediated anodic oxidation of organic pollutants. Chem Rev 109(12):6541–6569
Ramalho AMZ, Martínez-Huitle CA, Silva DR (2010) Application of electrochemical technology for removing petroleum hydrocarbons from produced water using a DSA-type anode at different flow rates. Fuel 89(2):531–534
Rocha JHB, Gomes MMS, Fernandes NS, Silva DR, Martínez-Huitle CA (2012) Application of electrochemical oxidation as alternative treatment of produced water generated by Brazilian petrochemical industry. Fuel Process Technol 96:80–87
Ronnback P, Troell M, Zetterstrom T, Babu DE (2003) Mangrove dependence and socio-economic concerns in shrimp hatcheries of Andhra Pradesh, India. Environ Conserv 30(4):344–352
Sampaio Y, Costa EF, Albuquerque E, Sampaio BR (2008) Impactos socioeconômicos do cultivo de camarão marinho em municípios selecionados do Nordeste brasileiro. Rev Econ Sociol Rural 46(4):1015–1042Piracicaba, SP
Santos ID, Afonso JC, Dutra AJB (2010) Behavior of a Ti/RuO2 anode in concentrated chloride medium for phenol and their chlorinated intermediates electrooxidation. Sep Purif Technol 76(2):151–157
Schroeder JP, Croot PL, Von Dewitz B, Waller U, Hanel R (2011) Potential and limitations of ozone for the removal of ammonia, nitrite, and yellow substances in marine recirculating aquaculture systems. Aquac Eng 45(1):35–41
Shanahan M (2003) Appetite for destruction. Ecologist 33:42–45
Virkutyte J, Jegatheesan V (2009) Electro-Fenton, hydrogenotrophic and Fe2+ ions mediated TOC and nitrate removal from aquaculture system: different experimental strategies. Bioresour Technol 100:2189–2197
Acknowledgments
The authors thank the financial support provided by the CAPES, CNPq, Shrimp Aquaculture Farm, ISMAR LTDA, and they also thank the Industry De Nora S.p.A. (Milan, Italy) for providing the Ti/Pt electrode.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
de Menezes, F.L.G., da Silva, A.J.C., Martínez-Huitle, C.A. et al. Electrochemical treatment of shrimp farming effluent: role of electrocatalytic material. Environ Sci Pollut Res 24, 6061–6070 (2017). https://doi.org/10.1007/s11356-016-7408-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-7408-4