Abstract
Dairy wastewater is characterized by a high content of hardly biodegradable dissolved, colloidal, and suspended organic matter. This work firstly investigates the performance of two individual electrochemical treatments, namely electrocoagulation (EC) and electro-oxidation (EO), in order to finally assess the mineralization ability of a sequential EC/EO process. EC with an Al anode was employed as a primary pretreatment for the conditioning of 800 mL of wastewater. A complete reduction of turbidity, as well as 90 and 81 % of chemical oxygen demand (COD) and total organic carbon (TOC) removal, respectively, were achieved after 120 min of EC at 9.09 mA cm−2. For EO, two kinds of dimensionally stable anodes (DSA) electrodes (Ti/IrO2-Ta2O5 and Ti/IrO2-SnO2–Sb2O5) were prepared by the Pechini method, obtaining homogeneous coatings with uniform composition and high roughness. The ·OH formed at the DSA surface from H2O oxidation were not detected by electron spin resonance. However, their indirect determination by means of H2O2 measurements revealed that Ti/IrO2-SnO2–Sb2O5 is able to produce partially physisorbed radicals. Since the characterization of the wastewater revealed the presence of indole derivatives, preliminary bulk electrolyses were done in ultrapure water containing 1 mM indole in sulfate and/or chloride media. The performance of EO with the Ti/IrO2-Ta2O5 anode was evaluated from the TOC removal and the UV/Vis absorbance decay. The mineralization was very poor in 0.05 M Na2SO4, whereas it increased considerably at a greater Cl− content, meaning that the oxidation mediated by electrogenerated species such as Cl2, HClO, and/or ClO− competes and even predominates over the ·OH-mediated oxidation. The EO treatment of EC-pretreated dairy wastewater allowed obtaining a global 98 % TOC removal, decreasing from 1,062 to <30 mg L−1.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Wastewater from animal operations has a negative impact on environment. In particular, great concerns arise from dairy wastewater because it is characterized by high organic matter content that is hardly biodegradable, which can damage the natural water streams in case of uncontrolled discharge. Refractory alcohols, carboxylic acids, and indole derivatives, among others, are usually identified in such effluents (Laor et al. 2008). Furthermore, studies on the water footprint of animal products estimate that up to 1,000 L H2O are necessary to produce 1 L of milk (Hoekstra 2010), which entails dramatic effects for the living beings in cases of release of untreated wastewater. Due to the large volume of effluents with variable characteristics generated in dairies, the dairy industry is considered as the most polluting among the food industries (Vourch et al. 2008).
In Mexico, the National Water Commission (Conagua) has reported that only 20 % of contaminated wastewater is treated before discharge to lakes, lagoons, and coastal areas (Comisión Nacional del Agua (Conagua) 2010). A case of particular interest is Baja California, which is considered as one of the Mexican states with lower drinking water supply, being further aggravated by the large water consumption from the expansive industrial sector, including the milk-producing industry. Therefore, water reuse is rather interesting in dairy industry since it would allow the decrease of both the water needs for cleaning dairies and herds and the risks for infection that arise from lagooning.
Dairy wastewater is most typically treated by bioremediation and physicochemical methods such as coagulation/flocculation. Unfortunately, the former requires large spaces and long times, whereas the latter presents high reagent costs and poor removal of soluble chemical oxygen demand (COD). On the other hand, at present, electrochemical technologies such as electrocoagulation (EC) and the electrochemical advanced oxidation processes (EAOPs) are receiving great attention for the removal of organic pollutants from waters, since they allow significant decontamination percentages with high efficiencies in compact reactors that only require simple equipments for operation and can be carried out at moderate costs (Anglada et al. 2009; Brillas et al. 2009; Panizza and Cerisola 2009).
EC is a primary wastewater treatment for inducing the controlled electrogeneration of flocculants/coagulants on site, usually under the application of a constant current. It is a complex process involving several chemical and physical phenomena with the formation of iron or aluminum cations from the dissolution of the corresponding sacrificial anode(s) and the simultaneous production of OH− anions by cathodic reduction of water. The polymeric metal hydroxides formed act as excellent coagulating agents to favor the removal of dissolved, colloidal, or suspended matter, eventually yielding great percentages of removal of color and turbidity. Coagulation mainly occurs by destabilization, once the metal cations combine with the negatively charged particles moving towards the anode by electrophoretic motion (Mollah et al. 2001; Martínez-Huitle and Brillas 2009). EC has been successfully tested to treat wastewaters from several industries (Szpyrkowicz 2005; Zodi et al. 2010; Cotillas et al. 2013), but only since recently it has been applied to the treatment of simulated and real dairy wastewater using iron/steel (Ayhan Şengil and Özacar 2006; Kushwaha et al. 2010) or aluminum (Tchamango et al. 2010; Bensadok et al. 2011) anodes. The latter have led to 60–80 and 96–100 % of COD and turbidity removal, respectively.
EAOPs can be applied as an effective post-treatment for the controlled electrogeneration of oxidizing species on site. Among them, electro-Fenton process (Anotai et al. 2011; Randazzo et al. 2011; Dirany et al. 2012) and electro-oxidation (EO) (Comninellis 1994; Tahar and Savall 1998; Marselli et al. 2003; Martínez-Huitle et al. 2004; Panizza and Cerisola 2004; Polcaro et al. 2004; Panizza and Cerisola 2006; Butrón et al. 2007; Cañizares et al. 2007; Panizza and Cerisola 2007; Hammami et al. 2008; Özcan et al. 2008; Ribeiro et al. 2008; Beteta et al. 2009; Flox et al. 2009; Hamza et al. 2009; Liu et al. 2009; Dirany et al. 2010; Fierro et al. 2010; Sirés et al. 2010; Bezerra Rocha et al. 2011; Souza Duarte et al. 2011; Flox et al. 2012; Chaiyont et al. 2013; El-Ghenymy et al. 2013a, b) are the most widespread technologies for treating synthetic solutions and real waters. In particular, EO turns out to be very appealing because it allows the ·OH-mediated decontamination of polluted wastewaters in all the pH range. Boron-doped diamond (BDD) anodes exhibit the highest performance (Marselli et al. 2003; Polcaro et al. 2004; Butrón et al. 2007; Cañizares et al. 2007; Panizza and Cerisola 2007; Hammami et al. 2008; Özcan et al. 2008; Beteta et al. 2009; Hamza et al. 2009; Liu et al. 2009; Dirany et al. 2010; Bezerra Rocha et al. 2011; Flox et al. 2012; El-Ghenymy et al. 2013a, b), but less expensive alternatives with a high oxidation power such as PbO2 (Tahar and Savall 1998; Panizza and Cerisola 2004; Flox et al. 2009; Sirés et al. 2010) and SnO2 (Comninellis 1994; Panizza and Cerisola 2009) have been proposed. SnO2 electrodes doped with antimony are easy to produce, but they have a limited lifetime. Lately, special attention has been paid to the manufacture of dimensionally stable anodes (DSA)-type electrodes due to their stability and acceptable oxidation ability. SnO2-based DSA electrodes with an IrO2 interlayer (Ti/IrO2-SnO2-Sb2O5) are more stable than the raw ones and are able to produce physisorbed hydroxyl radicals that allow the oxidation of organic compounds (Chaiyont et al. 2013). Also the metal and mixed-metal oxide electrodes based on RuO2 (Panizza and Cerisola 2006; Ribeiro et al. 2008) and IrO2 (Comninellis 1994; Panizza and Cerisola 2009; Fierro et al. 2010) are widely used in environmental electrochemistry because of their outstanding mechanical stability, long service lifetime, reasonable electrocatalytic activity, low cost, easiness of preparation, and successful scale-up. They have long been applied in the chlor-alkali industry thanks to their catalytic efficiency. IrO2-based DSA is especially interesting because it exhibits high corrosion resistance, being only slightly inferior to RuO2 in terms of electrocatalysis. IrO2 is known to be a good electrocatalyst for the oxidation of organic compounds thanks to the formation of adsorbed hydroxyl radicals (Fierro et al. 2010):
IrO2(·OH) can be further oxidized to a covalently bound species (chemisorbed active oxygen). The most common coatings incorporate Ta2O5 as a stabilizing agent (Martínez-Huitle et al. 2004; Panizza and Cerisola 2009).
The purpose of this work is to assess the combination of two electrochemical water treatments, namely EC with Al anodes for the removal of solids in order to separate a large fraction of organic matter and EO with DSA anodes for the destruction of the dissolved organic matter remaining in the pretreated wastewater, aiming at its potential application in the conditioning of dairy wastewater. Most EO experiments were performed with purpose-made Ti/IrO2-Ta2O5 by using the polymer precursor method (PPM) known as the Pechini method (Pechini and Adams 1967), although Ti/IrO2-SnO2–Sb2O5 were prepared as well for comparison. Among the sol–gel methods, the PPM is advantageous because it is simple, not sensitive to water, and gives rise to smoother surfaces and a more uniform composition without a mud-cracked surface appearance (Bitencourt et al. 2010). In particular, the Pechini-derived electrodes exhibit a longer lifetime (Forti et al. 2001; Bitencourt et al. 2010). The morphological and electrocatalytic properties were assessed by surface (scanning electron microscopy equipped with energy dispersive spectroscopy (SEM-EDX) and atomic force microscopy (AFM)) and electrochemical (linear sweep voltammetry (LSV) and cyclic voltammetry (CV)) analysis. Their ability to generate hydroxyl radicals has also been surveyed by two different techniques: (1) using a radical scavenger followed by electron spin resonance (ESR) analysis and (2) by spectrophotometric UV/Vis analysis of the Ti(IV)–H2O2 complex. Preliminary bulk electrolyses were performed with the model compound indole (i.e., a major component of the actual dairy wastewater of interest, as experimentally verified) in ultrapure water using NaCl and/or Na2SO4 media. Finally, the performance of the sequential method was investigated by applying EO to previously EC-pretreated actual wastewater.
Materials and methods
Chemicals
Indole was of reagent grade purchased from Sigma-Aldrich (purity ≥99 %). 5,5-Dimethyl-1-pyrroline-N-oxide (DMPO) for ESR spectroscopy from Fluka (purity ≥99 %) was used as trapping agent for ·OH detection. Anhydrous sodium sulfate and sodium chloride used as background electrolytes were of analytical grade purchased from Acros Organics. Reagent grade sulfuric acid and sodium hydroxide from VWR International were used to adjust the initial pH either to 3.0 or 8.0 whenever required. All the other chemicals used were either of HPLC or analytical grade from Sigma-Aldrich, Fluka, and Acros Organics. All solutions, except those of wastewater, were prepared with high-purity water obtained from a Millipore Milli-Q system with resistivity >18 MΩ cm.
Physicochemical characterization of the dairy wastewater
The wastewater was obtained from a dairy situated in km 58 of the Rosarito-Ensenada highway, in the heart of an important Mexican dairy region. About 14,000 L of milk are daily produced. The wastewater mainly results from cleaning of either cattle before milking, milk-sucking instruments, and cowsheds, and is further conveyed to a lagoon. The characteristics of the wastewater used in this work are summarized in Table 1. Note that the BOD5/COD ratio is 0.39, which suggests that it is a hardly biodegradable effluent because of the presence of many toxic molecules. Partitioning with organic solvents was used to extract the organic compounds from the water, which were subsequently analyzed by infrared spectroscopy. Indole derivatives, as well as alcohols, esters, carboxylic acids, and N-containing compounds were present in the samples, which agrees with characterization tests reported by Laor et al. (2008).
Preparation of DSA electrodes
Ternary oxide electrodes (Ti/IrO2-SnO2–Sb2O5) were obtained by PPM deposition onto pretreated Ti mesh as explained by Chaiyont et al. (2013). On the other hand, the Ti/IrO2-Ta2O5 electrodes were also prepared by PPM using a pretreated Ti mesh as the substrate. In this case, the precursor aqueous solution contained H2IrCl6 and TaCl5. The mixture was then applied to the support with a brush. Afterwards, the electrodes were heated at 100 °C for 10 min in a furnace in order to induce the polymerization of the precursor. This procedure was repeated three times. After the final coating, the electrodes were maintained at 550 °C for 30 min in order to ensure the calcination of the polymer and formation of the metal oxides that confer enough stability to the pieces.
Electrolytic system for electrocoagulation
All the EC trials were conducted in an open, undivided rectangular glass cell of 1-L capacity. A 55-cm2 (26.7 g) aluminum plate and a large surface iron mesh were employed as monopolar anode and cathode, respectively. All the experiments were performed in batch, operating galvanostatically by using direct current density (j) of 3.63 or 9.09 mA cm−2 (values referred to the anodic surface area).
Solutions of 800 mL containing the wastewater and 0.4 g NaCl as supporting electrolyte were electrolyzed for 120 min in 30-min intervals allowing sedimentation for 1 h between every two consecutive electrolyses. The addition of chloride intended to inhibit or slow down the anode passivation, which has been typically observed for Al due to the formation of an isolating, compact oxide interlayer.
Electrolytic system for electro-oxidation
All the EO trials were conducted in an open, undivided cylindrical glass cell of 150-mL capacity with a double jacket for circulation of external thermostated water to regulate the solution temperature at 25 °C. The anode was usually a 2.5 cm2 Ti/IrO2-Ta2O5 electrode, although Ti/IrO2-SnO2–Sb2O5 was used for comparison in some trials. The cathode was a Pt wire. The interelectrode gap was about 1 cm. All the experiments were performed at constant current, with vigorous stirring of the solution by means of a magnetic bar at 800 rpm to ensure mixing and the transport of reactants towards/from the electrodes.
Electrolyses were firstly carried out in ultrapure water. Solutions of 100 mL containing 100 mg L−1 total organic carbon (TOC) of indole and Na2SO4 and/or NaCl as supporting electrolyte were treated at different currents at pH 3.0 or 8.0. The ability of the DSA electrodes to produce ·OH from H2O oxidation was assessed in ultrapure water as well by spin-trapping and UV spectrophotometry. The former was conducted by using DMPO as the trapping agent (Marselli et al. 2003). Low concentrations of ·OH can be detected by firstly forming a spin adduct that exhibits a much longer lifetime than the radical alone and then obtaining the ESR spectrum of samples withdrawn at different electrolysis times. Solutions of 10 mM DMPO in 0.05 M Na2SO4 at pH 3.0 or 8.0 were electrolyzed galvanostatically at 0.1, 1, 10, and 59 mA cm−2 for 10 and 60 min using a system analogous to that described for EO but with a lower volume (10 mL) and smaller DSA area (0.2 cm2). In the second method, the H2O2 production was assessed at different times from electrolyses of 0.05 M Na2SO4 performed potentiostatically using Ag|AgCl (3.5 M KCl) and a Pt wire as the reference electrode and counter electrodes, respectively.
Solutions of 100 mL of EC-pretreated dairy wastewater were treated using the same setup described for indole solutions, but employing a 6.6 cm2 DSA anode instead.
Instruments and analysis procedures
EC was performed using a 6552A DC power supply from Agilent, whereas EO was conducted with an Amel 2063 potentiostat-galvanostat. All the electrolyses were performed at room temperature by means of thermostated cells or water baths. The solution pH was measured with a Crison 2000 pH meter. Conductimetric measurements were carried out with a Cole-Parmer instrument. An Imhoff tank was used to analyze the sedimentable solids (SS). A Hach DRB200 Digital Reactor Block and a Hach DR/890 portable colorimeter were used for measuring COD. The latter equipment was also used for analyzing the total suspended solids (TSS), free and total chlorine, and turbidity. BOD5 was assessed from the measurement of the dissolved oxygen based on the Winkler test. The inorganic anions contained in the wastewater were determined by ion chromatography upon injection into a Kontron HPLC model 465 coupled with a Waters 432 conductometric detector. A Waters IC-PAK Anions 150 mm × 4.6 mm (i.d), column with 10-nm particle size, at 40 °C was used. The mobile phase containing boric acid, sodium gluconate, sodium tetraborate, acetonitrile, butanol, and glycerine was eluted at 2.0 mL min−1. The analyses were carried out according to EPA 9056 method using PC Integration Pack from Kontron. The NH4 + content was determined by flow injection analysis technique based on colorimetry with blue of indole-phenol employing an ALPKEM model Flow Solution IV.
The DSA electrodes were extensively characterized by surface and electrochemical analyses. The morphology was examined by thermal field emission SEM using a JSM 6500F with an accelerating voltage of 15 kV equipped with an Oxford Inca 300 EDX analyzer. In some cases, a JSM 5400LV coupled to EDX analyzer was used instead. Data collection time was always 5 min. AFM was alternatively employed using a Nanosurf Easyscan 2 microscope working in the AC imaging mode (tapping mode) in air with silicon probes. For the electrochemical characterization, LSV and CV were carried out with an Autolab PGSTAT30 instrument, using a purpose-built, three-electrode undivided glass cell containing 50 mL of supporting electrolyte at pH 3.0 or 8.0 at room temperature, with a large area platinum gauze and Ag|AgCl (3.5 M KCl) mounted in a Luggin capillary as the counter and reference electrodes, respectively. The exposed area of the working DSA electrode was 1.28 cm2. All potentials in this work are referred to Ag|AgCl (3.5 M KCl).
The ability of the DSA electrodes to produce ·OH from H2O oxidation was analyzed by spin trapping and UV spectrophotometry. For the former, 400 μL of samples withdrawn upon electrolysis of DMPO were placed into a vial to be frozen with dry ice for ensuring preservation until analysis. In order to assess the presence of the DMPO spin adduct, the ESR spectra of samples were recorded at room temperature with a Bruker ESP300E spectrometer controlled by Win-EPR 2.3 SimFonia software. The conditions for the measurement were: X Band; 100 kHz modulation frequency with 1.5 G application; microwave power, 10 mW; central magnetic field, 3,350 G; and sweep width, 100 G. On the other hand, the concentration of H2O2 formed in electrolyzed solutions from ·OH recombination was determined from the light absorption of its Ti(IV) colored complex at λ = 408 nm using a Shimadzu 1800 UV/Vis spectrophotometer thermostated at 35 °C (Welcher 1975).
The mineralization of either indole solutions or wastewater was usually monitored from their TOC decay, measured with a Shimadzu VCSH TOC analyzer. Reproducible TOC values with an accuracy of ±1 % were found by injecting 50-μL aliquots into the analyzer. The changes in the UV/Vis spectrum of indole solutions upon EO were monitored from measurements between 200 and 800 nm with the previous spectrophotometer. For both kinds of analyses, samples were withdrawn at regular time intervals.
Results and discussion
Electrocoagulation of dairy wastewater
Based on the large content of solids in the wastewater under study, EC was considered as a suitable pretreatment method. Indeed, after 120 min of electrolysis, the initially turbid water became transparent, which can be confirmed by the turbidity measurements collected in Table 1 at two different current densities. A closer look to the measurements taken at different time periods (not shown) allowed concluding that the percentages of turbidity and COD removal already attained an almost constant value after 60 min. For example, at 3.63 mA cm−2, turbidity decreased by 82, 95, and 99.9 % at 30, 60, and 120 min, respectively. Similarly, at those times, COD was reduced by 65, 80, and 81 %. Figure 1a shows the progressive clarification of the wastewater samples at 3.63 mA cm−2, changing from initial brownish to final pale yellow. This transition can be basically related to the removal of suspended and sedimentable solids, which in turn allows justifying the progressive abatement of COD and TOC also reported in Table 1. Furthermore, EC also allowed a remarkable removal of aromatic pollutants. Figure 1b evidences that the UV/Vis spectrum of the raw wastewater was characterized by a high absorbance from 200 to 500 nm as expected from an effluent with a large content of toxic (i.e., benzenic) and/or colored pollutants. According to the aforementioned characterization of the wastewater (“Physicochemical characterization of the dairy wastewater” section) as well as the typical unpleasant odor arising from the presence of cow manure, the presence of indole derivatives can be certainly ensured. These are mainly formed during the decomposition of tryptophan and tryptophan-containing proteins. Figure 1b also shows that after the application of the EC process at 3.63 mA cm−2 for 120 min, a large percentage of these compounds had disappeared since the absorbance at 254–280 nm was much lower, and for λ > 400 nm it was almost negligible. The solution pre-eminently absorbed at 210 nm, which is related to the presence of aliphatic molecules.
The investigation at two current densities aimed at finding the most convenient condition for the removal of particulate matter. The results shown in Table 1 at the end of the EC process revealed that COD and TOC contents were about 50 and 25 % lower, respectively, at higher current density, whereas turbidity was almost the same in both cases. Electrolysis at 9.09 mA cm−2 was therefore more effective to induce the coagulation of solids and colloidal matter via charge neutralization, which directly involved a quicker decrease of turbidity and organic matter content. These results allowed concluding that EC is an optimal pretreatment method to further apply a secondary treatment such as EO with DSA electrodes.
Characterization of manufactured DSA electrodes
Figure 2 presents the SEM-EDX surface analyses carried out for studying the morphology and surface composition of DSA electrodes prepared by PPM as described in the “Preparation of DSA electrodes” section. A pure Ti mesh was used as the substrate as shown in Fig. 2a. The cleaning pretreatment yielded a rough surface that was ideal for the subsequent coatings. Figure 2b shows the SEM image of the Ti/IrO2-SnO2–Sb2O5 electrode. It was only used for carrying out some comparative EO experiments, but it is interesting to realize that high-quality anodes were obtained. As observed, the Ti surface was perfectly coated, unlike DSA prepared by thermal decomposition that typically exhibit broad cracks. The image depicts a slightly rough surface with particles of IrO2-SnO2-Sb2O5 between 1 and 5 μm in height, being uniformly distributed and giving rise to an average thickness of the coating of approximately 2 μm. Although impossible to distinguish, some small gaps are present between the particles, thus revealing the underlying Ti substrate, as confirmed by the EDX analysis. The distribution of the elements (Ir, Sn, and Sb) present on the surface was homogeneous, and results from EDX showed an atomic proportion of 18.1, 13.7, and 0.07 % for Ir, Sn, and Sb, respectively, while the Ti from substrate represented 43.1 %.
The appearance of the Ti/IrO2-Ta2O5 electrode can be observed in Fig. 2c. The electrocatalytic activity of these IrO2-based DSA electrodes is not only determined by the amount of Ir, but also by the morphology and crystalline structure. Again, the mud-cracked surface that is obtained upon thermal decomposition is avoided thanks to the milder manufacturing process. A good coverage results from PPM, with a uniform coating formed by ordered flakes with a grain size of about 5 μm. The presence of Ti, Ir, and Ta was confirmed by the EDX analysis.
The actual profile of the Ti/IrO2-Ta2O5 surface was further studied in detail by AFM. Figures 3a, b show the appearance of the bare, cleaned Ti and the DSA electrode, respectively. For the Ti substrate, the surface was smooth resulting in a very low roughness of 83 nm root mean square (rms). In contrast, a very rough surface with 612 nm rms can be seen for the DSA, which is related to the existence of evident oxide agglomerates exhibiting a certain diameter. The high surface roughness is very positive for upcoming electrochemical treatments since it is directly related to a larger surface area that automatically increases the number of electroactive sites and, therefore, enhances the electrocatalytic activity.
The ability of these rough DSA electrodes to produce ·OH was evaluated by two methods (see details in “Electrolytic system for electro-oxidation” and “Instruments and analysis procedures” sections). For spin trapping, 0.05 M Na2SO4 solutions in the absence (blank solution) or presence of 10 mM DMPO were treated by EO under different conditions of current and pH. Figure 4a depicts a representative ESR spectrum obtained using the Ti/IrO2-Ta2O5 electrode at 10 mA cm−2 and pH 3.0. All ESR spectra were similar to that of the blank solution, that is to say, without the characteristic bands of the ·OH-DMPO adduct. This agrees with findings on IrO2-based DSA using N,N-dimethyl-p-nitrosoaniline (RNO) as the spin trap (Panizza and Cerisola 2009). In contrast, Fig. 4b allows observing the spectrum obtained using Fenton’s reaction, where a 0.05-M Na2SO4 solution at pH 3.0 with H2SO4 and containing 10 mM DMPO, 1 mM H2O2, and 0.1 mM Fe2+ was allowed to react for 10 min. The four characteristic bands revealing the presence of the adduct can be clearly seen. Considering that ESR is able to detect concentrations as low as 1 × 10−8 M of hydroxyl radical, it can be hypothesized that either the amount of ·OH formed at the Ti/IrO2-Ta2O5 surface is below such threshold or this radical is quite chemisorbed on the surface. Another indirect technique was then employed in order to confirm or refute these results. EO of 0.05 M Na2SO4 solutions was carried out potentiostatically at various anodic potentials between +1.0 and +2.0 vs Ag|AgCl (3.5 M KCl). The presence or absence of the Ti(IV)–H2O2 complex was evaluated by UV/Vis spectrophotometry. No signal was detected at 408 nm, thus confirming that the ·OH concentration is so low that no measurable H2O2 was produced by dimerization. In contrast, H2O2 was detected in trials with the Ti/IrO2-SnO2–Sb2O5 anode at potentials between +1.5 and +1.9 V. This informs about the nature of the ·OH radicals formed on SnO2, which are partially physisorbed, i.e., more loosely adsorbed than those formed on IrO2-based DSA.
The electrochemical characterization of the Ti/IrO2-Ta2O5 electrode was performed in media made with 0.05 M Na2SO4, 0.05 M NaCl or mixtures of 0.025 M Na2SO4 + 0.025 M NaCl at pH 3.0 or 8.0 in order to simulate different typical conditions at the dairy, in the absence or presence of 5 mM indole as a representative of the organic pollutants contained in the wastewater of interest. The different background electrolytes did not exert great effects on the electrochemical responses and thus, only the CV results obtained in Na2SO4 are presented in Fig. 5a. Cyclic voltammograms were recorded between +0.2 and +1.2 V, corresponding to the stability region before hydrogen and oxygen evolution at 50 mV s−1. The appearance of redox peaks between 0.6 and 0.8 V can be usually related to the redox transition of oxygen iridium group species Ir(III)/Ir(IV) and/or Ir(V)/Ir(IV), which is typical for IrO2-based DSA (Xu et al. 2009). However, such response can be hidden for relatively low percentages of Ir (Li et al. 2006). This is the case for the present anode, as shown in Fig. 5a. A pseudocapacitative region arises due to charging of the electrical double layer, whereas the onset of the oxygen evolution reaction (OER) begins at more positive potentials, as explained below. The presence of indole simply led to a lower anodic and cathodic capacitance, probably due to the adsorption of the organic molecules on the surface. No faradaic processes were associated to it in this potential range, indicating that the molecule oxidation will only occur, if it turns out to be electrochemically active, at the same potentials as or higher than the OER. Next, linear sweep voltammograms were recorded between +0.6 and +1.6–1.8 V at 5 mV s−1 after cycling the potential forward and backward several times in order to ensure that the voltammogram was recorded under quasistationary conditions. As in the previous case, the behavior in Na2SO4 medium at pH 3.0 shown in Fig. 5b was representative for the other media as well. In the absence of indole, the O2 evolution started at about 1.32 V vs Ag|AgCl (3.5 M KCl), which agrees with tabulated values for IrO2-based DSA (Panizza and Cerisola 2009). This potential is +0.29 V more positive than the predicted thermodynamic potential of 1.23 V vs SHE in acid media, which allows classifying it as an anode with a low O2 evolution overpotential, but still favoring the ·OH-mediated oxidation of organic pollutants (Panizza and Cerisola 2009). As can be observed in Fig. 5b, the polarization curves shifted to less positive potentials in the presence of indole. The H2O discharge took place earlier and current intensity increased for a fixed value of the potential. This experimental evidence suggests the direct oxidation of the organic compound within the region of O2 evolution, which agrees with findings reported for the direct oxidation of oxalic acid at IrO2-Ta2O5 (Scialdone et al. 2009). Similarly, Keech et al. (2002) reported that indole undergoes direct electron transfer at Pt anodes.
Electrochemical characterization of the Ti/IrO2-Ta2O5 electrode in 0.05 M Na2SO4 in the absence (solid lines) or presence (dashed lines) of 5 mM indole at pH 3.0. a Cyclic voltammograms recorded at 50 mV s−1 between +0.2 and +1.2 V. b Linear sweep voltammograms obtained at 5 mV s−1 between +0.6 and +1.6–1.8 V
Electro-oxidation: testing of Ti/IrO2-Ta2O5 electrodes with solutions of indole
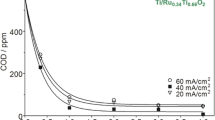
The performance of the Ti/IrO2-Ta2O5 anode in EO experiments was investigated with solutions of 100 mL containing 1 mM indole (100 mg L−1 TOC). The influence of the background electrolyte, applied current, and pH value is highlighted in Fig. 6 via the TOC evolution with electrolysis time. Figure 6a depicts the TOC decay for solutions containing 0.025 M Na2SO4 + 0.025 M NaCl. The effect of applied current was examined for 10, 100, and 200 mA at pH 8.0, which simulates the pH of the natural wastewater (Table 1). While TOC remained almost unchanged at 10 mA, the solution was slowly mineralized at 100 mA, with a maximum TOC removal of 30 % that was kept quite constant after 180 min. In contrast, the application of 200 mA led to a quicker, progressive TOC abatement, yielding the best results with 60 % mineralization at 360 min. This is partly due to the higher production of ·OH and the enhanced direct oxidation at the higher anode potential. Anyway, it is evident that a system based on EO alone using a Ti/IrO2–Ta2O5 electrode could not compete with alternative processes, which reinforces the proposal of a combined electrochemical treatment. The EO of oxalic acid has also been reported to proceed very slowly with this kind of DSA (Martínez-Huitle et al. 2004). As observed in Fig. 6a, EO at 200 mA and pH 3.0 instead of 8.0 almost yielded the same TOC removal rate. Thereafter, the electrolyses were then performed at pH 8.0, having in mind that this would prevent from pH adjustment when treating the wastewater.
a, b Normalized TOC removal with electrolysis time for the electro-oxidation of 100 mL of 1 mM indole using a Ti/IrO2-Ta2O5 anode. In a, the solution contained 0.025 M SO4 2− + 0.025 M Cl− and electrolyses were made at 10 mA (filled circle) , 100 mA (filled square), and 200 mA (filled diamond) at pH 8.0 and at 200 mA (white diamond) at pH 3.0. In plot b, the treatment was performed at pH 8.0 and 200 mA using 0.05 M SO4 2− (filled triangle) or 0.05 M Cl− (black down-pointing triangle). c Change in the UV/Vis spectra with time for the electro-oxidation of 1 mM indole in 0.05 M Cl− at pH 8.0 and 200 mA
Figure 6b depicts the effect of the reaction medium at 200 mA and pH 8.0. Compared to the mixed electrolyte, the mineralization was slower in 0.05 M Na2SO4, only reaching 25 % TOC abatement, whereas it was much quicker in 0.05 M NaCl, attaining almost 80 % at 360 min. The presence of Cl− was then essential, thereby highlighting the predominant role of oxidation mediated by chlorinated species over the ·OH-mediated oxidation. This is more clearly emphasized by the poor concentration of strongly adsorbed ·OH mentioned above at this IrO2-based DSA. In chloride media, the anodic discharge of anions plausibly led to the formation of Cl2 and HClO/ClO− (Comninellis and Nerini 1995; Martínez-Huitle and Brillas 2008), which was evidenced by their characteristic odor identified during the electrolyses. Such mixture of agents is able to partially oxidize some organic molecules like indole and/or its reaction by-products. Comninellis and Nerini (1995) also showed that the addition of 85 mM NaCl catalyzed the oxidation of phenol using Ti/IrO2 anodes due to the participation of ClO−. The solutions containing chloride turned white, then dark and pale coffee in color, with a certain amount of foam, and, finally, the solution became almost colorless. Note that, in the previous EC step, NaCl was added for increasing the solution conductivity; the positive contribution of Cl− now shown in EO opens the door to a beneficial sequential EC/EO treatment.
The time course of the solution absorbance was assessed for EO under the best conditions, i.e., in 0.05 M NaCl, as shown in Fig. 6c. At time 0, a clear absorption band appeared at about 280 nm, corresponding to the aromatic structure of indole. The band had already disappeared after 120 min of electrolysis, thanks to the action of ·OH and mainly to reactive chlorine species and the total remaining absorbance then derived from the formed by-products. Over time, the absorbance decreased because of the progressive removal of the intermediates, and a very low value was obtained at 480 min as expected from the low solution TOC achieved in this medium (Fig. 6b). On the contrary, the UV/Vis spectra in sulfate medium (not shown) remained practically unchanged, which agrees with the poor TOC removal shown in Fig. 6b.
Electro-oxidation: application to EC-pretreated dairy wastewater
Once the two individual processes were studied, the performance of the sequential treatment was assessed by using the best conditions previously established. Results of Table 1 gave evidence on the fact that the EC process applied at 9.09 mA cm−2 for 120 min allowed to decrease the TOC from 1,062 to 198 mg L−1. Samples of 100 mL taken from the resulting electrocoagulated water were then treated by EO with a Ti/IrO2-Ta2O5 anode. The area was 6.6 cm2 instead of 2.5 cm2 in order to favor the mass transport of Cl− (890 mg L−1, ca. 0.025 M) towards the anode, based on the important role of that mediated process. The electrolyses were performed at a constant current of 200 mA and at the natural pH of about 8.0. During the treatment, a large amount of foam was formed onto the liquid and an intense odor of Cl2 could be easily identified, becoming more intense with time. The trend of TOC over time is represented in Fig. 7. The mineralization rate was higher during the first 120 min with 72 % TOC decay. Afterwards, the decrease was less pronounced, but <30 mg L−1 TOC remained in the solution at 300 min. The good performance of the sequential electrochemical treatment has then been demonstrated and, therefore, a more detailed study will allow the optimization of all the parameters to propose the most convenient conditions for the in situ conditioning of the dairy wastewater. It is worth commenting that the integration of EC and EO has been recently reported for the treatment of carwash wastewaters, employing a BDD anode instead of much cheaper DSA (Panizza and Cerisola 2010).
Conclusions
This work has addressed several topics in order to assess the ability of a sequential electrochemical treatment for the remediation of a dairy wastewater:
-
1.
Based on the wastewater characterization, the effluent from this dairy, which is conveyed to a reservoir of 12.5 m3 had a very high organic matter content according to the Mexican legislation. Indole derivatives were important constituents of the wastewater.
-
2.
EC with Al anode works successfully as a primary electrochemical method for separating colloids and ionic species from wastewaters such as those generated in cow sheds and the dairy industry, as revealed by the large percentages of removal of turbidity, COD, and TOC determined. Since the coagulation and sedimentation kinetics controls the efficacy of the process, the incorporation of a conical section at the bottom of the cell and the reduction of the electrode separation would probably enhance the process.
-
3.
Two kinds of DSA anodes have been prepared by the Pechini method. They exhibited a high roughness, involving a large surface area for the OER. No signals corresponding to the ·OH-DMPO adduct from the Ti/IrO2-Ta2O5 anode were observed in the ESR spectra, which informs about the strong interaction of ·OH with the anode surface, being somewhat weaker in the Ti/IrO2-SnO2–Sb2O5 anode.
-
4.
The EO of indole solutions using the Ti/IrO2-Ta2O5 DSA showed that chloride medium favors the degradation of the organic matter, which can be explained by the mediation of Cl2, HClO, and/or ClO− formed on site. For this anode, this mechanism prevails over the action of ·OH.
-
5.
The EO of EC-pretreated wastewater allowed a decrease from 198 to <30 mg L−1 TOC. This means that up to 98 % of initial TOC was removed via the combined EC/EO treatment, being much more effective than the individual processes.
References
Anglada A, Urtiaga A, Ortiz I (2009) Contributions of electrochemical oxidation to waste-water treatment: fundamentals and review of applications. J Chem Technol Biotechnol 84:1747–1755
Anotai J, Sairiam S, Lu MC (2011) Enhancing treatment efficiency of wastewater containing aniline by electro-Fenton process. Sustain Environ Res 21:141–147
Ayhan Şengil I, Özacar M (2006) Treatment of dairy wastewaters by electrocoagulation using mild steel electrodes. J Hazard Mater 137:1197–1205
Bensadok K, El Hanafi N, Lapicque F (2011) Electrochemical treatment of dairy effluent using combined Al and Ti/Pt electrodes system. Desalination 280:244–251
Beteta A, Cañizares P, Rodrigo MA, Rodríguez L, Sáez C (2009) Treatment of door-manufacturing factories wastewaters using CDEO and other AOPs. A comparison. J Hazard Mater 168:358–363
Bezerra Rocha JH, Suely Fernandes N, Regina Souza K, da Silva DR, Quiroz MA, Martínez-Huitle CA (2011) Electrochemical decolourization process of textile dye in the presence of NaCl at boron doped diamond electrode. Sustain Environ Res 21:291–298
Bitencourt JFS, Ventieri A, Gonçalves KA, Pires EL, Mittani JC, Tamuti SH (2010) A comparison between neodymium doped alumina samples obtained by Pechini and sol–gel methods using thermo-stimulated luminescence and SEM. J NonCryst Solids 356:2956–2959
Brillas E, Sirés I, Oturan MA (2009) Electro-Fenton process and related electrochemical technologies based on Fenton’s reaction chemistry. Chem Rev 109:6570–6631
Butrón E, Juárez ME, Solis M, Teutli M, González I, Nava JL (2007) Electrochemical incineration of indigo textile dye in filter-press-type FM01-LC electrochemical cell using BDD electrodes. Electrochim Acta 52:6888–6894
Cañizares P, Lobato J, Paz R, Rodrigo MA, Sáez C (2007) Advanced oxidation processes for the treatment of olive-oil mills wastewater. Chemosphere 67:832–838
Chaiyont R, Badoe C, Ponce de León C, Nava JL, Recio FJ, Sirés I, Herrasti P, Walsh FC (2013) Decolorization of Methyl Orange dye at IrO2-SnO2-Sb2O5 coated titanium anodes. Chem Eng Technol 36:123–129
Comisión Nacional del Agua (Conagua) (2010). Estadísticas del agua en México. http://www.conagua.gob.mx/CONAGUA07/Publicaciones/Publicaciones/SGP-1-11-EAM2011.PDF. Accessed March 2011
Comninellis C (1994) Electrocatalysis in the electrochemical conversion/combustion of organic pollutants for waste water treatment. Electrochim Acta 39:1857–1862
Comninellis C, Nerini A (1995) Anodic oxidation of phenol in the presence of NaCl for wastewater treatment. J Appl Electrochem 25:23–28
Cotillas S, Llanos J, Cañizares P, Mateo S, Rodrigo MA (2013) Optimization of an integrated electrodisinfection/electrocoagulation process with Al bipolar electrodes for urban wastewater reclamation. Water Res 47:1741–1750
Dirany A, Sirés I, Oturan N, Oturan MA (2010) Electrochemical abatement of the antibiotic sulfamethoxazole from water. Chemosphere 81:594–602
Dirany A, Sirés I, Oturan N, Özcan A, Oturan MA (2012) Electrochemical treatment of the antibiotic sulfachloropyridazine: kinetics, reaction pathways, and toxicity evolution. Environ Sci Technol 46:4074–4082
El-Ghenymy A, Garrido JA, Rodríguez RM, Cabot PL, Centellas F, Arias C, Brillas E (2013a) Degradation of sulfanilamide in acidic medium by anodic oxidation with a boron-doped diamond anode. J Electroanal Chem 689:149–157
El-Ghenymy A, Cabot PL, Centellas F, Garrido JA, Rodríguez RM, Arias C, Brillas E (2013b) Electrochemical incineration of the antimicrobial sulfamethazine at a boron-doped diamond anode. Electrochim Acta 90:254–264
Fierro S, Kapalka A, Comninellis C (2010) Electrochemical comparison between IrO2 prepared by thermal treatment of iridium metal and IrO2 prepared by thermal decomposition of H2IrCl6 solution. Electrochem Commun 12:172–174
Flox C, Arias C, Brillas E, Savall A, Groenen-Serrano K (2009) Electrochemical incineration of cresols: a comparative study between PbO2 and boron-doped diamond anodes. Chemosphere 74:1340–1347
Flox C, Brillas E, Savall A, Groenen-Serrano K (2012) Kinetic study of the electrochemical mineralization of m-cresol on a boron-doped diamond anode. Curr Org Chem 16:1960–1966
Forti JC, Olivi P, De Andrade AR (2001) Characterisation of DSA®-type coatings with nominal composition Ti/Ru0.3Ti(0.7−x)SnxO2 prepared via a polymeric precursor. Electrochim Acta 47:913–920
Hammami S, Bellakhal N, Oturan N, Oturan MA, Dachraoui M (2008) Degradation of acid orange 7 by electrochemically generated ·OH radicals in acidic aqueous medium using a boron-doped diamond or platinum anode. A mechanistic study. Chemosphere 7:678–684
Hamza M, Abdelhedi R, Brillas E, Sirés I (2009) Comparative electrochemical degradation of the triphenylmethane dye Methyl Violet with boron-doped diamond and Pt anodes. J Electroanal Chem 627:41–50
Hoekstra AY (2010) The water footprint of animal products. In: D’Silva J, Webster J (eds) The meat crisis: developing more sustainable production and consumption. Earthscan, London, pp 22–33
Keech PG, Chartrand MMG, Bunce NJ (2002) Oxidation of simple indoles at a platinum anode. J Electroanal Chem 534:75–78
Kushwaha JP, Srivastava VC, Mall ID (2010) Organics removal from dairy wastewater by electrochemical treatment and residue disposal. Sep Purif Technol 76:198–205
Laor Y, Koziel JA, Cai L, Ravid U (2008) Chemical-sensory characterization of dairy manure odor using headspace solid-phase microextraction and multidimensional gas chromatography mass spectrometry-olfactometry. J Air Waste Manage Assoc 58:1187–1197
Li B-S, Lin A, Gan F-X (2006) Preparation and electrocatalytic properties of Ti/IrO2-Ta2O5 anodes for oxygen evolution. Trans Nonferrous Met Soc China 16:1193–1199
Liu L, Zhao G, Wu M, Lei Y, Geng R (2009) Electrochemical degradation of chlorobenzene on boron-doped diamond and platinum electrodes. J Hazard Mater 168:179–186
Marselli B, Garcia-Gomez J, Michaud P-A, Rodrigo MA, Comninellis C (2003) Electrogeneration of hydroxyl radicals on boron-doped diamond electrodes. J Electrochem Soc 150:D79–D83
Martínez-Huitle CA, Brillas E (2008) Electrochemical alternatives for drinking water disinfection. Angew Chem Int Ed 47:1998–2005
Martínez-Huitle CA, Brillas E (2009) Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods: a general review. Appl Catal B-Environ 87:105–145
Martínez-Huitle CA, Ferro S, De Battisti A (2004) Electrochemical incineration of oxalic acid. Role of electrode material. Electrochim Acta 49:4027–4034
Mollah MYA, Schennach R, Jose RP, David LC (2001) Electrocoagulation (EC)—science and applications. J Hazard Mater B84:29–41
Özcan A, Şahin Y, Koparal AS, Oturan MA (2008) Propham mineralization in aqueous medium by anodic oxidation using boron-doped diamond anode. Experimental parameters’ influence on degradation kinetics and mineralization efficiency. Water Res 42:2889–2898
Panizza M, Cerisola G (2004) Electrochemical oxidation as a final treatment of synthetic tannery wastewater. Environ Sci Technol 38:5470–5475
Panizza M, Cerisola G (2006) Olive mill wastewater treatment by anodic oxidation with parallel plate electrodes. Water Res 40:1179–1184
Panizza M, Cerisola G (2007) Electrocatalytic materials for the electrochemical oxidation of synthetic dyes. Appl Catal B-Environ 75:95–101
Panizza M, Cerisola G (2009) Direct and mediated anodic oxidation of organic pollutants. Chem Rev 109:6541–6569
Panizza M, Cerisola G (2010) Applicability of electrochemical methods to carwash wastewaters for reuse. Part 2: Electrocoagulation and anodic oxidation integrated process. J Electroanal Chem 638:236–240
Pechini MP, Adams N (1967) Method for preparing lead and alkaline earth titanates and niobates and coating method using the same to form a capacitor. U.S. Patent 3,330,697, 11 July 1967
Polcaro AM, Mascia M, Palmas S (2004) Electrochemical degradation of diuron and dichloroaniline at BDD electrode. Electrochim Acta 49:649–656
Randazzo S, Scialdone O, Brillas E, Sirés I (2011) Comparative electrochemical treatments of two chlorinated aliphatic hydrocarbons. Time course of the main reaction by-products. J Hazard Mater 192:1555–1564
Ribeiro J, Purgato FLS, Kokoh KB, Léger J-M, De Andrade AR (2008) Application of Ti/RuO2-Ta2O5 electrodes in the electrooxidation of ethanol and derivants: reactivity versus electrocatalytic efficiency. Electrochim Acta 53:7845–7851
Scialdone O, Randazzo S, Galia A, Filardo G (2009) Electrochemical oxidation of organics at metal oxide electrode: the incineration of oxalic acid at IrO2-Ta2O5 (DSA-O2) anode. Electrochim Acta 54:1210–1217
Sirés I, Low CTJ, Ponce-de-León C, Walsh FC (2010) The deposition of nanostructured β-PbO2 coatings from aqueous methanesulfonic acid for the electrochemical oxidation of organic pollutants. Electrochem Commun 12:70–74
Souza Duarte JP, Martínez-Huitle CA, da Silva DR (2011) Electrochemical treatment for removing petroleum polycyclic aromatic hydrocarbons (PAHs) from synthetic produced water using DSA-type anode: preliminary results. Sustain Environ Res 21:329–335
Szpyrkowicz L (2005) Hydrodynamic effects on the performance of electro-coagulation/electro-flotation for the removal of dyes from textile wastewater. Ind Eng Chem Res 44:7844–7853
Tahar NB, Savall A (1998) Mechanistics aspects of phenol electrochemical degradation by oxidation on a Ta/PbO2 anode. J Electrochem Soc 145:3427–3434
Tchamango S, Nanseu-Njiki CP, Ngmaeni E, Hadjiev D, Darchen A (2010) Treatment of dairy effluents by electrocoagulation using aluminium electrodes. Sci Total Environ 408:947–952
Vourch M, Balannec B, Chaufer B, Dorange G (2008) Treatment of dairy industry wastewater by reverse osmosis for water reuse. Desalination 219:190–202
Welcher FJ (1975) Standard methods of chemical analysis, vol. 2, 6th edn. Krieger, Huntington, p 1827, Part B
Xu L, Xin Y, Wang J (2009) A comparative study on IrO2-Ta2O5 coated titanium electrodes prepared with different methods. Electrochim Acta 54:1820–1825
Zodi S, Potier O, Lapicque F, Leclerc J-P (2010) Treatment of the industrial wastewaters by electrocoagulation: optimization of coupled electrochemical and sedimentation processes. Desalination 261:186–190
Acknowledgments
Support from CONACYT (Mexico) under program “Becas Mixtas 2012–2013 Movilidad en el extranjero”, as well as from MICINN (Spain) under project CTQ2010-16164/BQU, co-financed with FEDER funds, is acknowledged. The authors also thank the Centro de Graduados e Investigación en Química del Instituto Tecnológico de Tijuana.
Conflict of interest
The author(s) confirm that this article content has no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Borbón, B., Oropeza-Guzman, M.T., Brillas, E. et al. Sequential electrochemical treatment of dairy wastewater using aluminum and DSA-type anodes. Environ Sci Pollut Res 21, 8573–8584 (2014). https://doi.org/10.1007/s11356-014-2787-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-014-2787-x