Abstract
Chromite mining activities are indispensable for production of goods and services. Sukinda is a major mining site of Odisha, India, polluted by chromium, which is highly toxic in its hexavalent form. The Sukinda valley is a rich source of chromites, amounting to almost 95% of Cr available in India, and is the fourth most polluted site worldwide. Immediate solutions are needed to protect the health of biotic species of this region and their surroundings. Here we review chromite mining in India, impact of chromite pollution on plants and the environment, and phytoremediation of Cr-polluted soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The economic stability and advancement of a country are highly dependent on its rich mineral resources. The mining sector which involves the utilization of the minerals therefore can be assumed to play a crucial role in the economic growth of a nation (Groves et al. 2007). Mining comes under the primary sector of the Indian economy and is a bulky sector as far as employment and job creation is concerned. Mining activities although contribute 10–12% of GDP of the total industrial sector in India but are also quite infamous for pollution of the environment. In about 3100 mines (both public and private) operating in India, approximately 5,60,000 people are employed daily. The complete detail on the Indian mining sector synthesized from the reports from the Indian Bureau of Mines (IBM 2000, 2004) is listed in Table 1.
The vulnerability of the mine workers to various toxic pollutants present in the mining environment may lead to deleterious health impacts. Exposure of toxic heavy metals in and around the mining sites during the mining process contributes largely to pollution of the air, water, and soil. Heavy metals being persistent in the environment are not only a major concern to public health but also affect other living organisms (Abdu et al. 2017) and the food chain (Malik et al. 2019; Sevgi et al. 2009).
Chromium (Cr) is an important mineral having worldwide importance and widely used in industrial processes. However, it expresses its toxicity on the environment and human health (Peng and Guo 2020). It is unique than other metals in a way that its toxicity is dependent on the available oxidation states—Cr(III) and Cr(VI), unlike other metals whose toxicities are based on their total available concentrations in the environment (Kimbrough et al. 1999). The trivalent state or Cr(III) is considered to be benign and an essential micronutrient (Nieboer and Jusys 1988; Kahlon et al. 2018), whereas the hexavalent form, also denoted as Cr(VI), is listed as a Group-I human carcinogen (IARC 1990). The USEPA (United States Environmental Protection Agency) classifies materials as hazardous if they contain leachable Cr(VI). Chromium occurs naturally in the environment as Cr(III), whereas the Cr(VI) form arises from various industrial activities (Mudhoo et al. 2012). The first step in the industrial use of chromium begins with the chromite mining process. Chromium is generally found to be associated with iron (ferrous chromite) in the mining sites (Westbrook 1983; Hartford 1983). The problem with chromium lies in its two most stable states—Cr(III) and Cr(VI), which are inter-convertible among themselves in the environment. This inter-conversion mainly depends on the types of compounds present, pH of the environment, moisture, temperature, presence or absence of oxidizing and reducing agents, and many more. Chromium though is naturally found in the rocks in the benign Cr(III) form, but mining activities could expose the same to the environment and create a chance of inter-conversion to the highly toxic Cr(VI) form (Gunkel-Grillon et al. 2014). Thus, chromite mining is considered to be a serious threat to the environment as well as public health. The paper aims to discuss the negative impact of chromite mining on the Indian environment, with special emphasis on the Sukinda chromite mines of Odisha.
The Indian chromite situation
The worldwide shipping grade chromite resources account for over 12 billion tonnes among which the majority of the resources (95%) are located in South Africa (84%), Zimbawe (6%), Kazakhstan (5%). India (2%) lies 4th in the list followed by other countries like Brazil, USA, Canada, Russia, Finland, and others which collectively account for the remaining 3% of the share (Fig. 1). However, as per the 2009 statistics, India is the 2nd highest chromite ore producing country in the world (Das and Singh 2011).
Indian chromite deposits account for 2% of the total resources of the world. Approximately 98.6% of chromite resources of the country are located in Odisha out of which 95% are found in the Sukinda valley, situated in Cuttack and Jajpur districts of the state. The remaining percentage of the resources are found in Jharkhand, Karnataka, Goa, Maharashtra, Tamil Nadu, and Andhra Pradesh, while very few in Manipur, Nagaland, Jammu and Kashmir, and the Andaman and Nicobar islands. Deposits of chromite in various states of India (Fig. 2) are found to be scattered over certain regions also referred to as ‘belts’. The major chromite belts are the Sukinda, Bhalukasuni—Nilgiri and Ramagiri in Odisha, Jojohatu—Roroburu in Jharkhand, Bhandara—Nagpur, Chandrapur and Sindhudurg in Maharashtra, Janaram block, Konayyapalem block, Linganapetta block, Sriramgiri block and Kondapalli block in Andhra Pradesh, Nuggihali and Sindhuvalli—Talur in Karnataka, Karunglapatti, Sitampundi, and Solavanur-Mallanayakkanpalaiyam–Karapaddi in Tamil Nadu (Table 2).
The large exploitation and utilization of chromium resources in India, although have benefitted the nation economically, in the process have also dealt with heavy damage to the environment and the people. Most of the chromium-related pollution occurring in India mainly arises from mining sites and certain specific industries (especially tanneries, dye, and other chemical manufacturing industries and steel manufacturing plants). Tata Environmental Research Institute reported that out of the 7.2 million tons of hazardous wastes generated from Indian industries each year, approximately around 72% are disposed of in an improper fashion (TERI 2003). We infer that the chromite mining industry is largely responsible for maximum contamination of the environment, due to improper disposal of wastes containing Cr(VI) as the major contaminant.
Chromite mining at Sukinda Valley
The most dreaded example of hexavalent chromium (Cr(VI)) pollution in India lies in the Sukinda valley located in the Jajpur district of Odisha. It accounts for approximately 95% of the total chromite reserves of the country. The south Kaliapani mining area situated in the Sukinda valley accounts for approximately 97% of the chromite reserves of the state (IBM 2010). The valley contains around 183 million tons of deposits (Tiwary et al. 2005) and produces around 3.8 million tons of chromite ore per year (Das and Mishra 2010; Dhal et al. 2011). Widespread contamination of the valley due to excessive mining of chromite ores has made it to be considered as the fourth most polluted place in the world (Blacksmith Institute report 2007). The chromite mine of TISCO in the Sukinda valley is the largest open cast mine in India. Some of the other open cast mines in the region are operated by companies like Orissa Mining Corporation Ltd (OMC), M/s Indian Metals & Ferro Alloys Ltd (IMFAL), FACOR, and Balasore alloys (IBM 2013). A huge amount of wastes (in million tons) are generated by the mines operating in the valley and thus spread to nearby water bodies and leach into the groundwater, contaminating the water used for drinking purposes in the locality. The contamination mainly occurs due to Cr(VI), which is considered a major environmental carcinogen and is highly toxic (Mishra and Sahu 2013). Lack of adequate space for disposal of generated wastes is also a major problem and needs to be looked upon seriously. The Bhimtanagar chromite mine operated by M/S TISCO consists of dumps reaching to an abnormal height of 80 m due to the same problem of space adequacy. High concentrations of Cr(VI) and Cr(III) have been detected in the waters of the Damsala nallah that drains the Sukinda valley and also in the downstream of the river Brahmani and the Dhamra estuary, though in lesser quantity. Water samples collected from the wells falling within the chromite belt region of Sukinda have also been found to be contaminated (OPCB 2000). Analysis of surface water samples used for drinking purposes in the area revealed a much higher concentration of Cr(VI) than the maximum permissible limit of 0.1 mg/l. At one of the sampling points in the effluent channel of the chromite beneficiation plant of TISCO, the Cr(VI) concentration was found to be as high as 52 mg/l (Dubey et al. 2001).
Indian Bureau of Mines in collaboration with BRGM, France, carried out a project titled ‘Development of Application Techniques in relation to Environmental Management of Mines and Waste Recoveries’ and prepared a Regional Environmental Impact Assessment (REA) report. The monitoring report generated by REA observed that the surface water quality exceeded the drinking water standards in the Damsala nallah. Most of the groundwater samples investigated were found to have water quality exceeding the quality standards for drinking purposes. The fauna and flora of the region were also found to be highly contaminated. In all the sampling points, Cr(VI) was found to be present at a higher concentration (IBM 2013) (Table 3). We attribute the release of high loads of Cr(VI) to intensive mining activities in the region. It leads to environmental deformation.
Impacts of chromite mining on the environment
Reports portray the metal mining process as one of the major contributors to the pollution of the natural environment, especially the water bodies (Moncur et al. 2005). The fine heavy metal particles have all the chances of being adsorbed to the ground surface, flow off to nearby lakes and rivers, and as well as leach into the groundwater (Mulligan et al. 2001). Chromium is regarded as a very important metal having uses in various industrial processes, thus leading to its increased demand. Excessive demand for the metal has also led to an increase in the mining activities of chromite ores. Though chromium is present in the non-toxic trivalent form in the earth’s surface, due to open cast mining activities the trivalent Cr(III) comes in contact with atmospheric air and water and has maximum chances of oxidizing to the toxic hexavalent(Cr(VI)) form. Separation of chromite ores from hard rocky surfaces involves techniques like blasting and drilling followed by crushing. A huge amount of fine ore particles and dust generated get blown away through the air to nearby localities, thus posing chances of causing Cr(VI) contamination through atmospheric deposition (Rosas et al. 1989). Mines also use water to control dust during drilling and other mining operations. These mine wash water can seep into the groundwater or flow to the nearby water bodies thereby contaminating them (Das 2018). Accumulation of rainwater in the open mining pits and drainage from such pits also bear the same consequences. Dewatering of open pits during the rainy season to enable continuous mining activities may also heavily contaminate nearby places or water bodies into which the excess water is discarded. The chromite mining process is largely related to the generation of a huge amount of wastes and rocks in the form of overburden (Dhakate and Singh 2008). Drainage from such overburdens may also bear the possibility of leaching out a large number of heavy metals into the soil and nearby water systems. High levels of Cr(VI) in the soil and water bodies may result in the toxic heavy metal being accumulated in the plants and crops, thus easily getting transferred through the food chain. Some of the beneficial microflora of the soil may get affected due to the presence of Cr(VI), thus leading to loss of soil fertility. Humans are also equally or even to a longer extent victimized upon exposure to the Cr(VI) via various media. Humans are exposed to Cr(VI) via inhalation, oral intake as well as dermal contact (Das and Singh 2011). Inhalation of air dispersed with Cr(VI) particulates that arise from mining activities is a serious threat to human health and may lead to several ailments of the lungs and the respiratory tract. Consumption of contaminated water or food crops also possesses the chances of various diseases of the stomach and the alimentary canal. Several other human activities like the use of the Cr(VI)-contaminated water of rivers and ponds present nearby the mining localities for purposes like bathing, cleaning, and washing may also lead to various skin diseases.
Chromite mining activities expose the heavy metal Cr(VI) to the surrounding environment. Cr(VI) being a proven environmental carcinogen, expresses its toxicity on the biological entities. Several pieces of research have been carried out to study the toxicological impact of this heavy metal on plants, animals, microbes, and humans. In the following section, we discuss in brief about the toxicological implications of Cr(VI) on the living biota residing in and near the mining sites.
Hexavalent chromium (Cr(VI)) has been reported to impart its toxicity on various parameters related to plant growth and development (Table 4) like germination (Lopez-Luna et al. 2009; Dey et al. 2009), radical growth (Corradi et al. 1995), growth of roots (Sundaramoorthy et al. 2010; Rout et al. 1997; Samantary 2002; Barcelo et al. 1985), shoot length (Mallick et al. 2010; Lopez-Luna et al.2009), leaf growth (Dube et al. 2003) and yield (Sundaramoorthy et al. 2010). Cr(VI) has also been reported to impact various physiological processes in plants like photosynthesis (Panda and Choudhury 2005; Paiva et al. 2009; Liu et al. 2008), mineral uptake (Singh et al. 2013; Sundaramoorthy et al. 2010; Liu et al. 2008; Gopal et al. 2009; Gupta et al. 2000), enzymatic activities (Samantary 2002; Zaimoglu et al. 2011), protein activities (Vajpayee et al. 1999, 2000), electron transport (Dixit et al. 2002; Vranova et al. 2002). The toxicity of Cr(VI) affects the overall health of the plants, thereby leading to plant death. Loss of plants due to Cr(VI) toxicity at industrial and mining sites largely contributes toward biodiversity loss and may bring about a perturbance in the environmental homeostasis. High levels of Cr(VI) concentration in the soil also lead to poor crop growth and reduced crop yield which may affect overall crop production of a place. Crop loss may further prove to be a deterrent toward the financial and economic growth of farmers of the region.
Besides causing severe plant toxicity, Cr(VI) is found to have an impounding effect on humans (Fig. 3) either through inhalation, oral intake, or dermal contact (Das and Singh 2011).
Exposure routes, mode of intake and associated health issues of Cr(VI) in humans. Cr(VI) or hexavalent chromium released from the chromite mines is exposed to the aerial, water, and soil environment by leaching or as dust particles. Humans come in contact with the toxic heavy metal either through breathing, consumption of polluted water, or by penetration through skin. Unintended intake of the toxic heavy metal in humans leads to occurrence of several health issues
Laborers working in the chromite mines are always at a high risk of being affected by Cr(VI) contamination and its toxic effects. Cr(VI) is a proven sensitizer of the skin as well as the respiratory tract. It induces nasal irritation and upon continuous exposure may lead to the formation of nasal perforations (Menezes et al. 2004; Holland and Avery 2009). Cr(VI) also leads to skin ulcers only when there is an existing cut or abrasion on the skin and may even further lead to the formation of ‘chromium holes’ (Beyersmann and Hartwig 2008; Zhitkovich 2005). Cr(VI) has also been reported to cause cancer of the lungs in humans and animals. The probable mechanism behind the carcinogenesis has been discussed herein.
The rapid uptake of Cr(VI) has been reported to occur in human and animal cells via the sulfate carriers (Sugiyama 1992). The Cr(VI) further gets reduced to Cr(III) by the action of several cellular reductants, thus causing genotoxicity (Bianchi et al. 1983). The reduction process of Cr(VI) to Cr(III) generates several intermediates like Cr(V), Cr(IV), and reactive oxygen species (ROS). The intermediates, especially Cr(V), lead to the formation of bulky DNA adducts in the p53 gene of human lung cells. The ROS formed also induce oxidative damage to the DNA of the p53 gene (Arakawa et al. 2012). Being a tumor suppressor gene, p53 works to regulate the expression of several target genes under various cellular stress conditions and helps in DNA repair. Any damage to this particular gene implies a failure of the DNA repair mechanism, leading to mutations and uncontrolled cell divisions, thus leading to lung cancer. Inhalation of Cr(VI) concentration exceeding 0.001 mg/m3 through the mouth may also cause stomach ulcers (Lindberg and Hestidania 1983). Cr(VI) negatively affects myocardial activity. This may occur either by directly affecting the blood vessels or indirectly by reducing the pulmonary functions (Kleiner et al. 1970). Shmitova reported that transfer of Cr(VI) can occur to the fetus from the placenta of a pregnant woman being exposed occupationally to the toxic heavy metal (Shmitova 1980). We therefore conclude that upon regular and prolonged exposure, Cr(VI) may impart toxic effects and even at worse prove lethal to humans.
Chromium in small quantities is also required by the microorganisms as a nutrient. However, an excessive amount of chromium in the environment has been found to pose detrimental effect on these microorganisms (Silver et al. 2001). Cr(VI) has been found to impart toxic and mutagenic effects on most of the bacteria. Cr(VI) has been found to inhibit bacterial cell growth by causing elongation and enlargement of the bacterial cell as well as by inhibiting the cell division (Coleman 1988). Exposure to Cr(VI) has also been found to bring about morphological changes (Bondarenko and Ctarodoobova 1981) and a reduction in pathogenicity (Yamini et al. 2004) among the bacteria. Cr(VI) may bring about alterations in the cytoskeleton of algae, thus leading to loss of motility (Bassi and Donini 1984). Green algae have been found to be affected by Cr(VI), which inhibits the photosynthesis process (Corradi et al. 1995). The toxicity of chromium in microbes is also highly dependent on the oxidation states. Cr(VI) being highly soluble and mobile can easily penetrate through the bacterial cell membrane and into the cytoplasm, thus exerting toxic effects (Katz and Salem 1993). Microbes being an essential part of the biotic environment, we raise the concern that Cr(VI) pollution may disturb the microbial biodiversity that will further create environmental imbalance.
Phytoremediation for decontamination of Cr(VI) mining sites
The rise in population coupled with large-scale urbanization and industrialization has led to severe pollution of the soil. Improper disposal of wastes generated from various industries and mines enriched with heavy metals is the main factor behind soil pollution (Ye et al. 2017; Kumar et al. 2019; Bali et al. 2020). Soil is a very precious natural resource and forms the base for several agricultural activities. Hence, its contamination by heavy metals like hexavalent chromium (Cr(VI)) must be prevented. Taking into consideration the several serious consequences posed by heavy metals like Cr(VI) on the soil, several remediation techniques have been developed and applied (Liu et al. 2018). These include various physicochemical methods and some biological methods like bioremediation and phytoremediation. The physicochemical methods are although sometimes effective, but in the process affect several soil parameters like fertility and biodiversity (Khalid et al. 2017). Biological techniques are advantageous over other techniques in being cost-effective, and eco-friendly in their approach (Megharaj and Naidu 2017). The use of microorganisms (bioremediation) to clean-up contaminated soil is although a quick method, but it is still a daunting task as far as its feasibility is concerned when applied on a large scale. Hence, the current paper argues in favor of using plants (phytoremediation) for the decontamination of Cr(VI) polluted soils at mining areas. Phytoremediation is a widely accepted technique for its feasibility and eco-friendly nature (Muthusaravanan et al. 2018). In the process of phytoremediation, plants are used to remove pollutants from the environment or to contain them within their system, thus reducing those pollutants (Jensen and Gujarathi 2016; Vamerali et al. 2010). Plants having the ability to withstand the toxicity and grow in high concentrations of Cr(VI)-polluted mining sites should be selected for the remediation (Das et al. 2017). Therefore, we highly suggest proper selection of plant species for successful remediation of Cr(VI) contaminated soils.
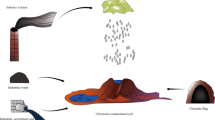
The most interesting and important thing about phytoremediation is that it not only uses plants but also takes into account other parameters like soil characteristics, nature of the heavy metal, and microorganisms present in the soil (Das et al. 2018) (Fig. 4).
Factors responsible for increasing phytoextraction of Cr(VI) from the soil. Organic amendments lead to release of organic acids that donates proton (H+) to the soil. The released proton helps in intake of anionic inorganic phosphate (Pi) through an energy driven active process leading to alkalinization of soil. This possibly increases the accumulation of Cr(VI), a structural analogue of Pi, in hyperaccumulators. Microbes referred to as plant growth promoting rhizobacteria have been found to enhance the phytoremediation process by several means like methylation, alteration of soil pH, favoring redox reactions, and by secreting siderophores, biosurfactants, and several organic acids. The size of soil particles (fine particles) also positively influences the phytoavailability of Cr(VI) and its translocation into plants
Hyperaccumulators in the translocation of Cr(VI)
To reduce the availability of toxic hexavalent chromium (Cr(VI)) at off-site environmental components is one of the emerging fields of soil remediation. It includes the stabilization of this toxic element in the soil rhizosphere and/or its accumulation in foliar tissues of plants employed for remediation purposes. In this context, the role played by translocation is gaining importance. Translocation is a vital physiological process for plants to collect macro- and micronutrients from the soil along with water. The used xylem tissues for this purpose have the potential to extract Cr(VI) from the contaminated soils of industrial and mining belts. The functioning of these xylem tissues in conducting strands ultimately loads Cr(VI) in the intracellular vacuoles of foliar regions in addition to its deposit in the root and stem cells.
Plants uptake hexavalent chromium (Cr(VI)) from the soil via the root system and translocate to the aerial parts. Cr(VI) uptake by plant roots can either occur by apoplastic transport system or the symplastic transport system. In the case of apoplastic transportation, Cr(VI) is carried through the intercellular spaces of the roots. Symplastic transportation involves specific ion channels or carriers like sulfate or phosphate for the uptake (Chaudhary et al. 2018). Unlike non-hyperaccumulating plants that store metals in the vacuoles, the hyperaccumulator plants efficiently translocate these metals from the roots to shoots through xylem using the symplastic pathway. Transfer through the xylem is facilitated by several membrane proteins like P-ATPase (P-type ATPase), HMT-ATPase (Helminthosporium maydis T ATPase), MATE (Multi-antimicrobial extrusion), and oligopeptide transport proteins (Chandra et al. 2018). Upon translocation, the hyperaccumulator plants sequester the toxic metals in the vacuoles of the leaf cells (Sharma et al. 2016). In this mechanism, the toxic metals are chelated with specific ligands and these metal–ligand complexes are then sequestered in the vacuoles by various families of transporter proteins like CAX (cation exchangers), HMA (heavy metal ATPases), ABC (ATP-binding cassette), NRAMP (natural resistance-associated macrophage protein), CDF (cation diffusion facilitator), ZAT (zinc transporters) and many more (Chandra et al. 2018). We hereby underline the importance of different pathways and the involvement of various proteins and ligands for efficient translocation of Cr(VI) into plants.
The hexavalent chromium (Cr(VI)) toxicity in the soil is reduced with the possible transformation of highly toxic Cr(VI) to trivalent chromium (Cr(III)) using the metabolism of selective groups of hyperaccumulators. The response of chromium to membrane transporters of plants used for transport of inorganic phosphates from the soil depends upon its chemical speciation and expressed through genetic or non-genetic alterations. The acceptance of the toxic Cr(VI) by those membrane transporters is possibly decided by the anionic structures of orthophosphate and chromate. After the intake, it may be channelized inside the cells, utilizing the biochemical machinery responsible for phosphate metabolism. The mobility of Cr species is variable and alters with the variation in the redox state. The high mobility of Cr(VI) is possibly linked with its chemical structure at the oxidation state. Contrary to it, the structural changes occur at the reduced state, Cr(III) changes its docking pattern, and mostly impermeable to move across the cell membrane. The quick transformation of Cr(VI) to Cr(III) is pH dependent. We have noticed the importance of chemical speciation and its behavior during sequestration of the toxic elements during phytoremediation.
Rhizospheric soil chemistry
The chemistry of rhizospheric soil also plays an important role in the phytoavailability of heavy metals like hexavalent chromium(Cr(VI)). Soil properties like pH, organic content, and texture greatly influence the availability of metals for phytoremediation (Shah and Daverey 2020). Cr(VI) mostly occurs as an oxyanion (CrO −24 ) and at low soil pH (< 5.0) remains adsorbed in the soil, thereby reducing its uptake in plants. However, at high pH (> 5.0), Cr(VI) remains highly mobile and thereby demonstrates high phytoavailability. The reaction of metals with organic matter to form an organometallic complex in the soil is also a pH-dependent process. At alkaline pH, the free metal ions in the soil form hydroxyl products that intensify organometallic complex formation, thereby reducing their toxicity. The low soil pH limits the availability of anionic inorganic phosphates in the soil. Its intake from the soil by the hyperaccumulators is influenced by proton mediated co-transport mechanism (Ullrich and Novacky 1990). The Pht1 phosphate transporters present in the cellular membranes of hyperaccumulators are possibly guiding this movement of inorganic phosphate from the soil to plants parts through the soil–root interface (Rausch and Bucher 2002; Bucher 2007). The intake of inorganic phosphates from the soil is a proton-dependent active process (Ullrich and Novacky 1990). It leads to the alkalinization of the soil. Possibly, a similar mechanism is utilized by the hyperaccumulating plants to intake structural analog Cr(VI) from the contaminated mining and industrial sites of Sukinda. The release of organic acids from the supplemented materials can be used as a source of proton streaming to boost up the remediation of Cr(VI) from the contaminated sites.
The texture of the soil also plays an important role in the phytoavailability of heavy metals, thereby directly relating to the phytoextraction efficiency of the plants (Złoch et al. 2017). Fine soil particles having a size of less than 100 µm have higher reactivity and surface area as compared to coarse soil particles. Therefore, finer particles tend to have higher concentrations of heavy metals like Cr(VI) in them. Lotfy and Mostafa (2014) observed that fine-textured soils exhibited high bioavailability of heavy metals like Cr and enhance its translocation in plants. Hence, we consider that the physical and chemical properties of soil also have a major role to play in enhancing the translocation of Cr(VI) from soil to different plant tissues.
The plant–microbes interaction
Plant–microbes interaction plays a crucial role in the phytoremediation of heavy metals. These microorganisms are referred to as plant growth promoting rhizobacteria (Khan et al. 2009) and can efficiently enhance the phytoremediation process by several means like methylation, alteration of soil pH, favoring redox reactions, by secreting siderophores, biosurfactants, and several organic acids (Table 5). Microorganisms can produce several acids that increase the availability of metals for phytoextraction by bringing about a change in the soil pH (Yang et al. 2018). A study demonstrated the role of citric and oxalic acids in enhancing the chromium phytoremedial ability of the Suaedavera plant (Gómez-Garrido et al. 2018). Microbes also oxidize or reduce metals directly or via the action of oxidizing or reducing agents produced by them. The redox reactions bring about a reduction in heavy metal phytotoxicity by stabilizing these metals in the soil and also by transforming them into non-toxic forms (Ma et al. 2016). A study on Cellulosimicrobium cellulans revealed its ability to reduce hexavalent chromium (Cr(VI)) to less mobile and comparatively non-toxic trivalent chromium (Cr(III)) in the soil (Chatterjee et al. 2009). Nayak et al. (2018) reported a Bacillus cereus strain that was found to be highly efficient in the reduction of Cr(VI) and a few other metals. The strain efficiently improved the phytoremedial ability of Vetiveria zizanioides. The adept ability of microorganisms in biosorption of heavy metals also assists the plants to a greater extent in the process of phytoremediation. The sorption of metals by microorganisms can take place by either the passive or active mechanism. Passive sorption occurs via an interaction between the metals and the functional groups present on the surface of dead microbial cells (Fomina and Gadd 2014). The active sorption process involves the uptake of metals from the soil by living microbial cells employing different mechanisms. The metallothioneins present inside the microbial cells bind to the metals and facilitate their sequestration in particular intracellular organelles. Certain microorganisms have been found to produce amphiphilic substances known as ‘biosurfactants’. The biosurfactants can desorp metals from the soil and in turn increase their solubility and mobility, making the metals bioavailable for plant uptake (Lal et al. 2018). Bacillus subtilis SHB13, isolated from the marine source, was found to produce the biosurfactant ‘surfactin’, which efficiently reduced 98% of 100 ppm chromium and 74% of Cr(VI) within 72 h of action (Swapna et al. 2016). Microorganisms present in the plant rhizosphere produce several organic acids reportedly enhancing the phytoremedial ability of plants by increasing heavy metals and nutrients uptake (Yang et al. 2018). Gómez-Garrido et al.(2018) reported the active involvement of citric and oxalic acid toward enhanced phytoremediation of chromium in Suaedavera plant. Rhizospheric microorganisms especially bacteria secrete low molecular weight compounds called siderophores that act as an iron chelator and supply iron to plants under metal stress soil conditions. The supply of a sufficient quantity of iron ensures in alleviating chlorophyll biosynthesis that remains suppressed in plants due to heavy metal-induced iron deficiency. Siderophores via chelation bring about a reduction in the formation of free radicals around the zone of plant roots and shields the microbial phytohormones from oxidative damage induced by the heavy metals. This not only ensures enhanced phytoextraction efficiency in plants but also protects the plant from pathogenic microorganisms in the soil (Ahemad 2015). We the authors acknowledge the importance of microbes and their involvement as a facilitator in the Cr(VI) remediation process. Besides, we hereby profoundly state that the microbial populations act as a first line of defense for the plant against various pathogens and stress as reviewed from several literature sources.
Conclusion
Chromium is an important metal that finds wide applicability in several industrial sectors. Chromite mines provide raw materials for several industrial establishments and are important as far as the economic growth of a country like India is concerned. However, economic growth should not be at the cost of the environment. Cr(VI), the toxic form of the element and a proven carcinogen, imparts adverse effects on the environment and its living entities. The widespread contamination of areas like Sukinda valley in India due to excessive chromite mining activities in the region has drawn the attention of several researchers toward possible clean-up efforts. In this context, phytoremediation appeals to be the most sustainable measure for cleaning the contaminated sites. Proper selection of plants along with physicochemical soil parameters and the study of plant–microbe interaction could help in devising efficient remediation strategies for the mining areas.
References
Abdu N, Abdullahi AA, Abdulkadir A (2017) Heavy metals and soil microbes. Environ Chem Lett 15:65–84. https://doi.org/10.1007/s10311-016-0587-x
Ahemad M (2015) Enhancing phytoremediation of chromium-stressed soils through plant-growth-promoting bacteria. Genet Eng Biotechnol N13(1):51–58. https://doi.org/10.1016/j.jgeb.2015.02.001
Arakawa H, Weng M, Chen W, Tang M (2012) Chromium(VI) induces both bulky DNA adducts and oxidative DNA damage at adenines and guanines in the p53 gene of human lung cells. Carcinogenesis 33(10):1993–2000
Bahadur A, Ahmad R, Afzal A, Feng H, Suthar V, Batool A, Khan A, Mahmood-ul-Hassan M (2017) The influences of Cr-tolerant rhizobacteria in phytoremediation and attenuation of Cr(VI) stress in agronomic sunflower (Helianthus annuus L.). Chemosphere 179:112–119. https://doi.org/10.1016/j.chemosphere.2017.03.102
Bali AS, Sidhu GPS, Kumar V (2020) Root exudates ameliorate cadmium tolerance in plants: a review. Environ Chem Lett 18:1243–1275. https://doi.org/10.1007/s10311-020-01012-x
Barcelo J, Poschenrieder C, Gunse J (1985) Effect of chromium(VI) on mineral element composition of bush beans. J Plant Nutr 8:211–217
Bassi M, Donini A (1984) Phallotoxin visualization of F-actin in normal and chromium poisoned Euglena cells. Cell Biol Int Rep 8:867–871
Beyersmann D, Hartwig A (2008) Carcinogenic metal compounds: recent insight into molecular and cellular mechanisms. Arch Toxicol 82(8):493
Bianchi V, Celotti L, Lanfreanchi G, Majone F, Marin G, Di Montal A, Sponza G, Tamino G, Venier P, Zantideschi A, Levis AG (1983) Genetic effects of chromium compounds. Mutat Res 117:279–300
Black Smith Institute Report (2007) The world’s worst polluted places. A project of Blacksmith Institute, pp 16–17
Bondarenko BM, Ctarodoobova AT (1981) Morphological and cultural changes in bacteria under the effect of chromium salts. J Microbiol Epidemiol Immunobiol 4:99–100
Bucher M (2007) Functional biology of plant phosphate uptake at root and mycorrhiza interfaces. New Phytol 173:11–26
Chandra R, Kumar V, Singh K (2018) Hyperaccumulator versus nonhyperaccumulator plants environment waste management. In: Chandra R, Dubey NK, Kumar V (eds) Phytoremediation of environmental pollutants. CRC Press, Boca Raton, pp 14–35
Chatterjee S, Sau GB, Mukherjee SK (2009) Plant growth promotion by a hexavalent chromium reducing bacterial strain, Cellulosimicrobiumcellulans KUCr3. World J MicrobBiot 25:1829–1836. https://doi.org/10.1007/s11274-009-0084-5
Chaudhary K, Agarwal S, Khan S (2018) Role of phytochelatins (PCs), metallothioneins (MTs), and heavy metal ATPase (HMA) genes in heavy metal tolerance. In: Prasad R (ed) Mycoremediation and environmental sustainability. Springer, Cham, pp 39–60. https://doi.org/10.1007/978-3-319-77386-5
Chitraprabha K, Sathyavathi S (2018) Phytoextraction of chromium from electroplating effluent by Tageteserecta (L.). Sustain Environ Res 28:128–134. https://doi.org/10.1016/j.serj.2018.01.002
Coleman RN (1988) Chromium toxicity: effects on microorganisms with special reference to the soil matrix. In: Nriagu JO, Nieboer E (eds) Chromium in the natural and human environments. Wiley, New York, pp 335–368
Corradi MG, Gorbi G, Ricci A, Torelli A, Bassi AM (1995) Chromium-induced sexual reproduction gives rise to a Cr-tolerant progeny in Scenedesmusacutus. Ecotoxicol Environ Saf 32:12–18
Das PK (2018) Phytoremediation and nanoremediation: emerging techniques for treatment of acid mine drainage water. Def Life Sci J 3(2):190–196. https://doi.org/10.14429/dlsj.3.11346
Das AP, Mishra S (2010) Biodegradation of the metallic carcinogen Hexavalent chromium Cr(VI) by an indigenously isolated bacterial strain. J Carcinog 9:6
Das AP, Singh S (2011) Occupational health assessment of chromite toxicity among Indian miners. Indian J Occup Environ Med 15:6–13. https://doi.org/10.4103/0019-5278.82998
Das PK, Das BP, Dash P (2017) Hexavalent chromium induced toxicity and its remediation using macrophytes. Pollut Res 36(1):92–98
Das PK, Das BP, Dash P (2018) Role of plant species as hyper-accumulators in the decontamination of hexavalent chromium contaminated soil. Indian J Environ Health 38(12):1016–1024
Dey SK, Jena PP, Kundu S (2009) Antioxidative efficiency of Triticum aestivum L. exposed to chromium stress. J Environ Biol 30(4):539–544
Dhakate R, Singh VS (2008) Heavy metal contamination in groundwater due to mining activities in Sukinda valley, Orissa—a case study. J Geogr Reg Plan 1(4):058–067
Dhal B, Thatoi HN, Das NN, Pandey BD (2011) Environmental quality of the Boula-Nuasahi chromite mine area in India. Mine Water Environ 30:191–196
Din BU, Amna, Rafique M, Javed MT, Kamran MA, Mehmood S, Khan M, Sultan T, Hussain Munis MF, Chaudhary HJ (2020) Assisted phytoremediation of chromium spiked soils by Sesbania Sesban in association with Bacillus xiamenensis PM14: a biochemical analysis. Plant Physiol Biochem 146:249–258
Dixit V, Pandey V, Shyam R (2002) Chromium ions inactivate electron transport and enhance superoxide generation in vivo in pea (Pisumsativum L. cv: Azad) root mitochondria. Plant Cell Environ 25:687–693
Dube BK, Tewari K, Chatterjee J, Chatterjee C (2003) Excess chromium alters uptake and translocation of certain nutrients in citrullus. Chemosphere 53(9):1147–1153
Dubey CS, Sahoo BK, Nayak NR (2001) Chromium(VI) in waters in parts of Sukinda chromite valley and health hazards, Orissa, India. Bull Environ Contam Toxicol 67:541–548. https://doi.org/10.1007/s00128-001-0157-0
Fomina M, Gadd GM (2014) Biosorption: current perspectives on concept, definition and application. Bioresour Technol 160:3–14. https://doi.org/10.1016/j.biortech.2013.12.102
Gómez-Garrido M, Mora Navarro J, Murcia Navarro FJ, Faz Cano Á (2018) The chelating effect of citric acid, oxalic acid, amino acids and Pseudomonas fluorescens bacteria on phytoremediation of Cu, Zn, and Cr from soil using Suaedavera. Int J Phytoremediat 20(10):1033–1042
Gopal R, Rizvi AH, Nautiyal N (2009) Chromium alters iron nutrition and water relations of spinach. J Plant Nutr 32(9):1551–1559
Groves WA, Kecojevic VJ, Komljenovic D (2007) Analysis of fatalities and injuries involving mining equipment. J Saf Res 38:461–470
Gunkel-Grillon P, Laporte-Magoni C, Lemestre M et al (2014) Toxic chromium release from nickel mining sediments in surface waters, New Caledonia. Environ Chem Lett 12:511–516. https://doi.org/10.1007/s10311-014-0475-1
Gupta R, Mehta R, Kumar N, Dahiya DS (2000) Effect of chromium(VI) on phosphorus fractions in developing sunflower seeds. Crop Res 20:46–51
Hartford W (1983) Chromium chemicals. In: Grayson M (ed) Kirk-Othmer encyclopedia of chemical technology, vol 6, 3rd edn. Wiley-Interscience, New York, pp 83–120
Holland SL, Avery SV (2009) Actin-mediated endocytosis limits intracellular Cr accumulation and Cr toxicity during chromate stress. Toxicol Sci 111(2):437–446
IARC (1990) Chromium, nickel, and welding, Monogr on the evaluation of carcinogenic risks to humans, vol 49. International Agency for Research on Cancer, Lyons
Indian bureau of mines (2000) National Mineral Inventory—an overview. Nagpur, pp 1–48
Indian bureau of mines (2004) Indian mineral year book. Nagpur, pp 276–476
Indian bureau of mines (2010) Indian minerals yearbook. pp 18–51
Indian bureau of mines (2013) Monograph on chromite. pp 1–153
Jensen CD, Gujarathi NP (2016) Methyl jasmonate improves radical generation in macrophyte phytoremediation. Environ Chem Lett 14:549–558. https://doi.org/10.1007/s10311-016-0591-1
Joshi PM, Juwarkar AA (2009) In vivo studies to elucidate the role of extracellular polymeric substances from azotobacter in immobilization of heavy metals. Environ Sci Technol 43:5884–5889
Juarez AB, Barsanti L, Passarelli V et al (2008) In vivo microspectroscopy monitoring of chromium effects on the photosynthetic and photoreceptive apparatus of Eudorinaunicocca and Chlorella Kessleri. J Environ Monit 10(11):1313–1318
Kahlon SK, Sharma G, Julka JM et al (2018) Impact of heavy metals and nanoparticles on aquatic biota. Environ Chem Lett 16:919–946. https://doi.org/10.1007/s10311-018-0737-4
Katz SA, Salem H (1993) The toxicology of chromium with respect to its chemical speciation: a review. J Appl Toxicol 13:217–224
Khalid S, Shahid M, Niazi NK, Murtaza B, Bibi I, Dumat C (2017) A comparison of technologies for remediation of heavy metal contaminated soils. J Geochem Explor 182:247–268. https://doi.org/10.1016/j.gexplo.2016.11.021
Khan MS, Zaidi A, Wani PA et al (2009) Role of plant growth promoting rhizobacteria in the remediation of metal contaminated soils. Environ Chem Lett 7:1–19. https://doi.org/10.1007/s10311-008-0155-0
Kimbrough DE, Cohen Y, Winer AM, Creelman L, Mabuni C (1999) A critical assessment of chromium in the environment. Crit Rev Environ Sci Technol 29(1):1–46. https://doi.org/10.1080/10643389991259164
Kleiner AM, Stolbun BM, Likhacheva YI (1970) Indices of the functional status of the myocardium and hemodynamics in chromium occupational poisoning with chromium compounds. Gig Tr Prof Zabol (Russian) 14:7–10
Kumar V, Sharma A, Kaur P, Sidhu GPS, Bali AS, Bhardwaj R, Thukral AK, Cerda A (2019) Pollution assessment of heavy metals in soils of India and ecological risk assessment: a state-of-the-art. Chemosphere 216:449–462. https://doi.org/10.1016/j.chemosphere.2018.10.066
Lal S, Ratna S, Said OB, Kumar R (2018) Biosurfactant and exopolysaccharide-assisted rhizobacterial technique for the remediation of heavy metal contaminated soil: an advancement in metal phytoremediation technology. Environ Technol Innov 10:243–263. https://doi.org/10.1016/j.eti.2018.02.011
Lindberg E, Hestidania G (1983) Chrome plating symptoms findings in the upper airways and effects on lung function. Arch Environ Health 38(6):367–374
Liu D, Zou J, Wang M, Jiang W (2008) Hexavalent chromium uptake and its effects on mineral uptake, antioxidant defence system and photosynthesis in Amaranthus viridis L. Bioresour Technol 99(7):2628–2636
Liu L, Li W, Song W, Guo M (2018) Remediation techniques for heavy metal-contaminated soils: principles and applicability. Sci Total Environ 633:206–219. https://doi.org/10.1016/j.scitotenv.2018.03.161
Lopez-Luna J, Gonzalez-Chavez MC, Esparza-Garcıa FJ, Rodrıguez-Vazquez R (2009) Toxicity assessment of soil amended with tannery sludge, trivalent chromium and hexavalent chromium, using wheat, oat and sorghum plants. J Hazard Mater 163(2–3):829–834
Lotfy SM, Mostafa AZ (2014) Phytoremediation of contaminated soil with cobalt and chromium. J Geochem Explor 144:367–373
Ma Y, Rajkumar M, Zhang C, Freitas H (2016) Beneficial role of bacterial endophytes in heavy metal phytoremediation. J Environ Manag 174:14–25. https://doi.org/10.1016/j.jenvman.2016.02.047
Malik LA, Bashir A, Qureashi A et al (2019) Detection and removal of heavy metal ions: a review. Environ Chem Lett 17:1495–1521. https://doi.org/10.1007/s10311-019-00891-z
Mallick S, Sinam G, Mishra RK, Sinha S (2010) Interactive effects of Cr and Fe treatments on plants growth, nutrition and oxidative status in Zea mays L. Ecotoxicol Environ Saf 73(5):987–995
Megharaj M, Naidu R (2017) Soil and brownfield bioremediation. Microb Biotechnol 10:1244–1249. https://doi.org/10.1111/1751-7915.12840
Menezes MB, Sabino CD, Franco MB, Maia EC, Albinati CC (2004) Assessment of workers’ contamination caused by air pollution exposure in industry using biomonitors. J Atmos Chem 49(1–3):403–414
Mishra H, Sahu HB (2013) Environmental scenario of chromite mining at Sukinda valley—a review. Environ Eng Manag J4(4):287–292
Moncur MC, Ptacek CJ, Blowes DW, Jambor JL (2005) Release, transport, and attenuation of metals from an old tailings impoundment. Appl Geochem 20:639–659
Mudhoo A, Garg VK, Wang S (2012) Removal of heavy metals by biosorption. Environ Chem Lett 10:109–117. https://doi.org/10.1007/s10311-011-0342-2
Mulligan CN, Yong RN, Gibbs BF (2001) Remediation technologies for metal-contaminated soils and groundwater: an evaluation. Eng Geol 60:193–207
Muthusaravanan S, Sivarajasekar N, Vivek JS et al (2018) Phytoremediation of heavy metals: mechanisms, methods and enhancements. Environ Chem Lett 16:1339–1359. https://doi.org/10.1007/s10311-018-0762-3
Nayak AK, Panda SS, Basu A, Dhal NK (2018) Enhancement of toxic Cr(VI), Fe, and other heavy metals phytoremediation by the synergistic combination of native Bacillus cereus strain and Vetiveria zizanioides L. Int J Phytoremediat 20:682–691. https://doi.org/10.1080/15226514.2017.1413332
Nieboer E, Jusys AA (1988) Biologic chemistry of chromium. In: Nriagu JO, Nierboor E (eds) Chromium in the natural and human environments. Wiley, New York, pp 21–79
Nieman RH (1965) Expansion of bean leaves and its suppression by salinity. J Plant Physiol 40:156–161
Oliveira H (2012) Chromium as an environmental pollutant: insights on induced plant toxicity. J Bot 2012:1–8. https://doi.org/10.1155/2012/375843
Paiva LB, de Oliveira JG, Azevedo RA, Ribeiro M, da Silva G, Vitoria AP (2009) Ecophysiological responses of water hyacinth exposed to Cr3+ and Cr6+. Environ Exp Bot 65(2–3):403–409
Panda SK, Choudhury S (2005) Chromium stress in plants. Braz J Plant Physiol 17(1):95–102
Peng H, Guo J (2020) Removal of chromium from wastewater by membrane filtration, chemical precipitation, ion exchange, adsorption electrocoagulation, electrochemical reduction, electrodialysis, electrodeionization, photocatalysis and nanotechnology: a review. Environ Chem Lett. https://doi.org/10.1007/s10311-020-01058-x
Ramírez V, Baez A, López P, Bustillos MDR, Villalobos MA, Carreño R, Contreras JL, Muñoz Rojas J, Fuentes-Ramírez LE, Martínez J, Munive JA (2019) Chromium hyper-tolerant Bacillus sp. MH778713 assists phytoremediation of heavy metals by mesquite trees (Prosopislaevigata). Front Microbiol 10:1833. https://doi.org/10.3389/fmicb.2019.01833
Rausch C, Bucher M (2002) Molecular mechanisms of phosphate transport in plants. Planta 216:23–37
Rosas I, Belmont R, Baez A, Villalobos-Pietrini R (1989) Some aspects of the environmental exposure to chromium residues in Mexico. Water Air Soil Pollut 48(3–4):463–475
Rout GR, Samantaray S, Das P (1997) Differential chromium tolerance among eight mungbean cultivars grown in nutrient culture. J Plant Nutr 20(4–5):473–483
Samantary S (2002) Biochemical responses of Cr-tolerant and Cr-sensitive mung bean cultivars grown on varying levels of chromium. Chemosphere 47(10):1065–1072
Sevgi E, Coral G, Gizir AM, Sagun MK (2009) Investigation of heavy metal resistance in some bacterial strains isolated from industrial soils. Turk J Biol 34:423–431
Shah V, Daverey A (2020) Phytoremediation: a multidisciplinary approach to clean up heavy metal contaminated soil. Environ Technol Innov 18:100774. https://doi.org/10.1016/j.eti.2020.100774
Shmitova LA (1980) Content of hexavalent chromium in the biological substrates of pregnant women and women in the immediate post-nasal period engaged in the manufacture of chromium compounds. Gig Tr Prof Zabol 2(2):33–35
Silver S, Schottel J, Weiss A (2001) Bacterial resistance to toxic metals determined by extrachromosomal R factors. Int Biodeterior Biodegrad 48:263–281
Singh HP, Mahajan P, Kaur S et al (2013) Chromium toxicity and tolerance in plants. Environ Chem Lett 11:229–254. https://doi.org/10.1007/s10311-013-0407-5
Sugiyama M (1992) Role of physiological antioxidants in Cr(VI)-induced cellular injury. Free Radic Biol Med 12:397–407
Sundaramoorthy P, Chidambaram A, Ganesh KS, Unnikannan P, Baskaran L (2010) Chromium stress in paddy: (i) nutrient status of paddy under chromium stress; (ii) phytoremediation of chromium by aquatic and terrestrial weeds. CR Biol 333(8):597–607
Swapna TH, Papathoti NK, Khan MY, Reddy G, Hameeda B (2016) Bioreduction of Cr(VI) by biosurfactant producing marine bacterium Bacillus subtilis SHB 13. J Sci Ind Res India 75:432–438
TERI (2003) Hazardous waste management in India. A policy discussion forum base paper. Tata Energy Research Institute, New Delhi, India
Tiwary RK, Dhakate R, Rao VA, Singh VS (2005) Assessment and prediction of contaminant migration in ground water from chromite waste dump. J Environ Geol 48:420–429
Ullrich CI, Novacky AJ (1990) Extra and intracellular pH and membrane potential changes induced by K, Cl, H(2)PO(4), and NO(3) uptake and fusicoccin in root hairs of Limnobium stoloniferum. Plant Physiol 94:1561s–1567s
Vajpayee P, Sharma SC, Rai UN, Tripati RD, Yunus M (1999) Bioaccumulation of chromium and toxicity to photosynthetic pigments, nitrate reductase activity and protein content of Nelumbo nucifera Gaetrn. Chemosphere 39:2159–2169
Vajpayee P, Tripati RD, Rai UN, Ali MB, Singh SN (2000) Chromium accumulation reduces chlorophyll biosynthesis, nitrate reductase activity and protein content of Nymphaea alba. Chemosphere 41:1075–1082
Vamerali T, Bandiera M, Mosca G (2010) Field crops for phytoremediation of metal-contaminated land. A review. Environ Chem Lett 8:1–17. https://doi.org/10.1007/s10311-009-0268-0
Vernay P, Gauthier-Moussard C, Hitmi A (2007) Interaction of bioaccumulation of heavy metal chromium with water relation, mineral nutrition and photosynthesis in developed leaves of Lolium perenne L. Chemosphere 68(8):1563–1575
Vranova E, Inze D, Van Breusegem F (2002) Signal transduction during oxidative stress. J Exp Bot 53:1227–1236
Westbrook J (1983) Chromium and chromium alloys. In: Grayson M (ed) Kirk-Othmer encyclopedia of chemical technology, vol 6, 3rd edn. Wiley-Interscience, New York, pp 54–82
Yamini H, Devaraj N, Balachandran UN (2004) A Schiff base complex of chromium(III): an efficient inhibitor for the pathogenic and invasive potential of Shigella dysenteriae. J Inorg Biochem 98:387–392
Yang P, Zhou XF, Wang LL, Li QS, Zhou T, Chen YK, Zhao ZY, He BY (2018) Effect of phosphate-solubilizing bacteria on the mobility of insoluble cadmium and metabolic analysis. Int J Environ Res Public Health 15(7):1330
Ye S, Zeng G, Wu H, Zhang Chang Dai J, Liang J, Yu J, Ren X, Yi H, Cheng M, Chen Zhang (2017) Biological technologies for the remediation of co-contaminated soil. Crit Rev Biotechnol. https://doi.org/10.1080/07388551.2017.1304357
Zaimoglu Z, Koksal N, Basci N, Kesici M, Gulen H, Budak F (2011) Antioxidative enzyme activities in Brassica juncea L. and Brassica oleracea L. plants under chromium stress. J Food Agric Environ 9(1):676–679
Zhitkovich A (2005) Importance of chromium− DNA adducts in mutagenicity and toxicity of chromium (VI). Chem Res Toxicol 18(1):3–11
Złoch M, Kowalkowski T, Tyburski J, Hrynkiewicz K (2017) Modeling of phytoextraction efficiency of microbially stimulated Salix dasyclados L. in the soils with different speciation of heavy metals. Int J Phytoremedia 19:1150–1164. https://doi.org/10.1080/15226514.2017.1328396
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Das, P.K., Das, B.P. & Dash, P. Chromite mining pollution, environmental impact, toxicity and phytoremediation: a review. Environ Chem Lett 19, 1369–1381 (2021). https://doi.org/10.1007/s10311-020-01102-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-020-01102-w