Abstract
To make the process of producing sophorolipids by Candida bombicola truly sustainable, we investigated production of these biosurfactants on biomass hydrolysates. This study revealed: (1) yield of sophorolipds on bagasse hydrolysate decreased from 0.56 to 0.54 and to 0.37 g/g carbon source when yellow grease was dosed at 10, 40 and 60 g/L, respectively. In the same order, concentration of sophorolipids was 35.9, 41.9, and 39.3 g/L; (2) under similar conditions, sophorolipid yield was 0.12, 0.05 and 0.04 g/g carbon source when corn stover hydrolysate was mixed with soybean oil at 10, 20 and 40 g/L. Sophorolipid concentration was 11.6, 4.9, and 3.9 g/L for the three oil doses from low to high; and (3) when corn stover hydrolysate and yellow grease served as the substrates for cultivating the yeast in a fermentor, sophorolipid concentration reached 52.1 g/L. Upon further optimization, sophorolipids production from ligocellulose will be indeed sustainable.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biosurfactants, synthesized naturally by microorganisms, have many advantages over those that are chemically synthesized from petrochemical or oleo-chemical sources [7]. These advantages include low or no toxicity and biodegradability. In addition, if biosurfactants are produced from renewable resources, then these bioproducts are sustainable and environmentally friendly. Sophorolipids are a group of compounds that comprise two components: a sophorose head (a dimeric sugar residue) and a hydroxylated fatty acid [3] and are synthesized by several non-pathogenic yeast strains, Candida bombicola, C. apicola, C. batistae, Wickerhamiella domericqiae and Rhodotorula bogoriensis [23]. As surface active biocompounds, sophorolipids have replaced part of their chemical counterparts in household/laundry detergents and are consumed at around 10 million tons per year [30]. In addition, due to their structural diversity and related physicochemical variability, sophorolipids have been used in the fields of cosmetic, hygienic, medical and pharmaco-dermatological [8] [20] [12]. Furthermore, through chemical reactions, such as acid hydrolysis, sophorolipids can be converted to inducers of cellulases [10, 11] which have broad applications in producing biofuels from renewable biomass feedstocks.
Conventionally, sophorolipids are produced by yeast strains grown on both sugar and oil feedstocks. Sugars, such as glucose [9], sucrose [13], galactose, lactose [32, 33] and soy molasses [29] have been tested. Hydrophobic substrates, such as oils, fatty acids, and their corresponding esters, alkanes have been investigated [30]. The highest yield was reported to be 422 g/L when deproteinized whey and rapeseed oil were used as substrates for C. bombicola [4]. The second highest concentration of 400 g/L was obtained when the same yeast strain was cultivated on honey and corn oil in a fed-batch mode [25]. To make sophorolipids’ production process even greener and more sustainable, we have investigated the feasibility of producing these biosurfactants from hydrolysates developed from sweet sorghum bagasse (SSB) and corn fiber [27]. Both hydrolysates, although contain a wide variety of chemicals that are considered to be inhibitory to microbial fermentation, supported growth of C. bombicola. Significant production of sophorolipids by this yeast strain was observed when soybean oil was provided.
For the purpose of lowering the production cost, in this study, we further studied the yield of sophorolipids when waste restaurant oil or yellow grease was supplemented as a source of oil molecules. Based on what we know so far, this is the first study on evaluating sophorolipid production from cellulosic hydrolysates with the addition of yellow grease. The rationale for selecting yellow grease is twofold: (1) yellow grease, compared to soybean oil or other oil sources, is much cheaper. The current market price is around $1/gallon which is one third of that of soybean oil; and (2) according to US EPA’s estimate, US hotels and restaurants generate 3 billion gallons (11.4 billion L) of waste oil per year (www.epa.gov/region9/waste/biodiesel/questions.html). Thus, the volume of yellow grease is great enough for producing sophorolipids through yeast fermentation. Certainly, yellow grease can be used to generate biodiesel or other kinds of biofuels. But since sophorolipids have much higher market values than those of biofuels, use of yellow grease for producing sophorolipids would be much more attractive from the perspectives of investment.
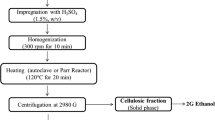
Besides evaluating sophorolipids yield on hydrolysates of SSB and yellow grease, we also aimed to study sophorolipids production from hydrolysates derived from corn stover. This is to consider the enormous abundance of corn stover as a potential biomass feedstock. More importantly, the corn stover hydrolysates used in this study were developed from a novel deacetylation and disc refining (DDR) pretreatment process. As described below, the DDR process followed by enzymatic hydrolysis led to hydrolysates with high concentrations of sugars and basically no presence of potential fermentation inhibitors [1]. To the best of our knowledge, no studies have been performed on using these hydrolysates supplemented with either soybean oil or yellow grease for sophorolipid production.
Materials and methods
Microorganism and inoculum preparation
Candida bombicola (ATCC 22214) was routinely maintained on agar plates at 4 °C. The plates contained (per liter): glucose, 100 g; yeast extract, 10 g; urea, 1 g; and agar, 20 g. Yeast colonies were transferred to fresh plates every 6 weeks. To start an inoculum, colonies were introduced to an autoclaved medium with the same composition as the agar plates but without the presence of agar. The inoculum culture was incubated at 25 °C in a shaker set at 120 rpm for 2 days before use.
Pretreatment of sorghum bagasse
Bagasse of sweet sorghum which was studied in our previous works [2, 15, 17, 18, 31] was used in this study. But instead of using a 10% solid loading during acid pretreatment, a bagasse loading of 20% was adopted for the purpose of increasing total sugar concentrations in the derived hydrolysates. Briefly, pretreatment of sorghum bagasse was conducted by mixing 20 g bagasse (dry weight) with 100 mL of 0.5% sulfuric acid followed by autoclaving at 121 °C for 1 h. Following pretreatment, the slurry was centrifuged at 4000×g for 10 min. The acidic supernatant thereafter was adjusted to a pH of 6.0 using NaOH [14] and was used in the following studies without further treatment.
Pretreatment of corn stover
Deacetylated disc refined (DDR) corn stover hydrolysate was provided by the national renewable research laboratory (NREL) in the United States (US). In short, corn stover harvested at Hurley County (South Dakota, the US) was first knife milled to pass through a 19 mm (0.75 in.) round screen [1]. The screened corn stover was then added to a 1900-L paddle mixer along with a dilute 0.1 M sodium hydroxide solution to achieve a solid slurry of 8% (w/w). After the slurry was heated to 80 °C and held for 2 h, the liquor was allowed to drain and the solid was rinsed and pumped to a continuous screw press (Vincent Corp. Model CP10, Tampa, Florida, the US) for dewatering to between 45 and 50% (w/w) of total solids. The deacetylated corn stover was then mechanically refined in a commercial scale disc refiner (Sprout model 401 36-inch). Corn stover particles after the deacetylation and disc refining processes were hydrolyzed by Novozyme (Franklinton, North Carolina, US) cellulase (12–15 FPU CTec3) and hemicellulase (HTec3) at a ratio of 4:1 at 48 °C for 96 h. The total enzyme loading was 20 mg protein/g cellulose. After enzymatic hydrolysis, the liquid portion was used in yeast fermentation as detailed in the following. Similar to the hydrolysates obtained from sorghum bagasse, the corn stover hydrolysates were used as is.
Fermentation of C. bombicola on Cellulosic Hydrolysates
Regarding hydrolysates derived from sorghum bagasse, yellow grease at 10 g/L, 40 g/L or 60 g/L was supplemented. The yellow grease used was from the cafeteria at Southern Illinois University Carbondale, where soybean oil was used for frying. For each yellow grease concentration tested, three replicate cultures were established. Each culture included a certain volume of yellow grease, 10% of yeast inoculum and a different volume of bagasse hydrolysates to ensure that the total volume in each 250-mL Erlenmeyer flask was 50 mL. Three control cultures containing (per liter): glucose, 100 g; yeast extract, 10 g; urea, 1 g; and yellow grease, 40 g were also set up at the same time. In terms of flasks containing corn stover hydrolysates, soybean oil at 10 g/L, 20 g/L or 40 g/L was added. The control cultures comprised glucose at 100 g/L, yeast extract at 10 g/L, urea at 1 g/L and soybean oil at 40 g/L. All flasks were cultivated in a shaking incubator set at 25 °C and 120 rpm. At different time points, a 1-mL sample was withdrawn from each culture for checking possible contamination and for measuring cell dry weight and sophorolipid concentrations.
Fermentation of corn stover hydrolysates in a 3-liter fermentor
To understand whether better process control can benefit sophorolipid production from corn stover hydrolysates, the same hydrolysates were used in a 3-liter fermentor (Eppendorf, Hauppauge, NY, USA). The testing condition was: temperature, 25 °C; mixing speed, 500 rpm; DO: 25–30% of DOsat; initial pH = 5.0; air flow rate, 0.5 vvm (volume of air/volume of liquid per minute). DO was controlled by air flow rate. The fermentation was started by adding 711 mL of corn stover hydrolysates together with 9.7 mL of yellow grease and 80 mL of C. bombicola inoculum. The initial yellow grease concentration was 10 g/L. On a daily basis, samples (3 mL) were withdrawn from the fermentor for testing cell biomass, sugar concentrations and residual yellow grease concentration. Between days 1 and 1.5, a volume of 97 mL of yellow grease was supplemented. The same was done again between days 4 and 5. At the end of day 7, the fermentation was terminated and the final culture was analyzed as described above.
Analysis
Sophorolipids and residual oil extraction
Two different volumes, the 1-mL samples and the remaining volume of the final day cultures, were used for extractions of sophorolipids and residual oil through liquid–liquid extraction as reported by others [28, 29]. In addition, considering the possible presence of organic solvent extractable compounds in the hydrolysates studied here, the two hydrolysates were processed for solvent extraction, too. For all samples, equal volume of ethyl acetate as that of the sample was used twice for sophorolipids extraction. Ethyl acetate layer at the top was then subjected to extraction by using two volumes of hexane for residual oil. The remaining ethyl acetate fraction and the hexane soluble were proceeded with solvent evaporation. The dry weights of sophorolipids and residual oil were determined gravimetrically after subtracting ethyl acetate and hexane extractable from the corresponding hydrolysates, respectively. Subsequently after ethyl acetate extraction, the water phase in the original samples was centrifuged at 10,000g for 5 min. The resulting liquid layer was used for HPLC analysis. The pellet was used for determining cell dry weight through drying in an oven at 105 °C overnight.
Yellow grease characterization
Compared to soybean oil, the yellow grease looked fairly turbid. To determine the true oil content, yellow grease was extracted with equal volume of hexane for three times. After phase separation, the hexane layer was evaporated to dryness in a Rotavap. Weight of the residue was used to calculate oil content. The remaining non-hexane soluble was centrifuged at 5000g for 10 min. The liquid portion was weighed and dried to measure moisture content while the solid portion was weighed to obtain solid content. In addition, to understand the oil composition, representative samples of the yellow grease and soybean oil were reacted with methanol and sulfuric acid for transesterification following procedures reported previously [21]. The resulting fatty acid methyl esters (FAMEs) were analyzed by a gas chromatography (GC) following a procedure we published previously [16].
High-performance liquid chromatography (HPLC)
All samples were filtered through hydrophilic 0.2-μm filters beforehand to eliminate any potential particles. The HPLC (Shimadzu Scientific Instrument, Inc. Columbia, MD, USA) with a refractive index detector was used to determine the concentrations of monomeric sugars (glucose, xylose, and arabinose) and non-sugar compounds (formic acid, acetic acid, levulinic acid, and 5-hydroxymethylfurfural (5-HMF), furfural) in samples. Chemical separation was carried out using an Aminex HPX 87H column (5 μm, 30 cm × 4.6 mm, Bio-Rad, CA, USA) set at 50 °C. Freshly prepared 0.005 M sulfuric acid was used as the mobile phase with a flow rate of 0.6 mL/min. A 20-μL sample injection volume was used. All concentrations of the chemicals under investigation were calculated based on calibration curves established for each compound using external standards.
Results and discussion
Yellow grease and soybean oil
The yellow grease used in this study had a density of 914.7 g/L. Through hexane extraction, it was found that the yellow grease contained 99.4% of hexane soluble, 0.13% of solid and 0.46% of moisture. This yellow grease sample had basically the same fatty acid profile as that of the soybean oil used (Table 1). For both oil samples, C18:1n7 (cis-vaccenic acid), C16:0 (palmitic acid), C18:3n4 (isomer of linolenic acid) and C18:0 (stearic acid) were the dominant fatty acids. Thus, apparently, even though after repeated use and the resulting yellow grease looked cloudy, the process of frying did not change the fatty acid composition of the oil. The composition of yellow grease used in this study is different from those reported [28]. This could be due to the fact that different oil was used for frying.
Fermentation using C. bombicola on sorghum bagasse hydrolysates with yellow grease supplementation in shaking flasks
As described above, the sorghum bagasse hydrolysate used in this study was derived from pretreating bagasse using 0.5% sulfuric acid at 20% solid loading for 1 h. The resulting hydrolysate contained 36.4 g/L of glucose, 37.9 g/L of xylose and 1.7 g/L of arabinose with a total sugar concentration of 76.1 g/L. These concentrations were almost twice of those obtained when pretreating SSB at a 10% solid loading with other conditions remaining the same [27]. Following ethyl acetate and hexane extraction, the hydrolysate was found to contain 5.9 ± 0.8 and 5.0 ± 0.3 g/L of compounds that were ethyl acetate and hexane soluble, respectively.
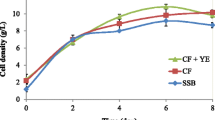
When yellow grease was supplemented at three concentrations, 10, 40 or 60 g/L, C. bombicola grew well (Figs. 1, 2). The exponential growth phase typically lasted for two days and was followed by a long stationary phase. For all three yellow grease doses tested, glucose was consumed rapidly in four days. Xylose and arabinose were both utilized by the cells, but at much slower rates compared with that of glucose. As we reported before, all monosaccharides were consumed simultaneously.
With 10, 40 and 60 g/L of yellow grease, the final day cell density was 5.7 ± 0.8, 6.4 ± 0.8 and 7.8 ± 0.4 g/L, respectively, which was similar to 6.9 ± 1.35 g/L observed for the controls with pure glucose and yellow grease at 40 g/L (Fig. 2). Among those with the hydrolysates, higher yellow grease concentration resulted in an increase of cell growth, but the difference was not statistically significant. At day 14, content of pure sophorolipids was 35.9 ± 1.7, 41.9 ± 9.1, and 39.3 ± 16.5 g/L for yellow grease at 10, 40, and 60 g/L, respectively. These sophorolipids concentrations were much higher than 7.7 g/L detected from the controls and higher than 34 g/L reported when C. bombicola was cultivated on a standard medium with yellow grease added in a step-wise fashion to a fermentor [28]. Thus, compared with the standard medium which comprised 100 g/L glucose, 10 g/L of yeast extract, and 1 g/L of urea, sorghum bagasse hydrolysate appeared to be a better substrate, which is consistent with what we reported previously [27]. In addition, based on other publications on C. bombicola [6, 23, 29], yield of sophorolipids on bagasse hydrolysates could be improved substantially if a fed-batch instead of batch feeding scheme and/or better control of pH and oxygen level are adopted. It needs to be noted that the sophorolipid concentrations reported here were after subtracting ethyl acetate extractable in the hydrolysates used assuming those extractable were not utilized by the yeast during fermentation. This analysis was also applied when calculating yield of pure sophorolipids from corn stover hydrolysates as detailed below.
With increasing yellow grease concentration from 10 to 60 g/L, the residual oil left after 14 days was 0.0, 7.9 ± 6.0 and 14.0 ± 3.9 g/L. Again, this was after subtracting hexane extractable in the original hydrolysates. Yield of sophorolipids (g sophorolipids/g carbon source) was calculated by dividing sophorolipids produced with total consumption of sugars and oils. As shown in Fig. 3, with increasing yellow grease concentrations, the yield decreased from 0.56 ± 0.05 to 0.54 ± 0.13 and to 0.37 ± 0.18 g sophorolipids/g carbon source (sugars plus oil). The yield difference between cultures with 10 and 40 g/L of yellow grease was not significant. However, when yellow grease concentration was 60 g/L, sophorolipid yield was significantly lowered. This could be due to the impurities in yellow grease. During frying, at least three kinds of reactions take place: hydrolytic, oxidative, and thermolytic [22]. As a result, various chemicals, such as alkanes, alkenes, aldehydes, hydrocarbons, and semialdehydes can be present in yellow grease, which might have affected sophorolipid production and secretion.
The large standard deviations for some results reported here were due to the fact that the presence of yellow grease led to non-homogenous cultures, especially at higher concentrations. Thus, sample withdrawal at different time points might contribute to inconsistent loss of yellow grease and eventually resulted in variations in the final day results. But even with some large difference between replicate samples, it is obvious that C. bombicola utilized sugars in SSB hydrolysates and yellow grease efficiently and had the highest sophorolipid yield of 0.56 g/g carbon source at the lowest concentration of yellow grease. This yield is comparable with those reported. For example, an overall consumption of 140 g/L rapeseed oil and 300 g/L of glucose led to a yield coefficient of 0.68 [26]. A similar yield of 0.65 was demonstrated when rapeseed ethyl ester of 184 g/L and glucose of 304 g/L was consumed [5]. For these two cases, a fed-batch cultivation was employed for producing large quantity of sophorolipids.
Fermentation using C. bombicola on corn stover hydrolysates with soybean oil supplementation in shaking flasks
Corn stover hydrolysate obtained through the DDR process contained 87.3 g/L of glucose, 59.4 g/L xylose and 3.1 g/L of arabinose. Furfural and 5-HMF were not detected by HPLC. The total concentration of monomeric sugars was 149.8 g/L. With these high sugar concentrations and for all three tested doses of soybean oil, C. bombicola utilized all three sugars simultaneously during the first two days when exponential growth took place (Fig. 4). After two days when cells enter stationary phases, however, consumption of xylose and arabinose was extremely slow. Same phenomenon was observed for controls where only glucose and xylose at concentrations similar to those in corn stover hydrolysates and 40 g/L of soybean oil were supplemented. These observations were different from those when bagasse hydrolysates served as the sugar sources. In the case of bagasse hydrolysates, the total initial sugar concentrations were around 65 g/L considering diluting the hydrolysates by the added inoculum. As shown in Fig. 1, all monosaccharides, glucose, xylose and arabinose were consumed simultaneously. Thus, considering all of these reasons, the slow utilization of pentose sugars should not be owing to the complexity of chemicals in the corn stover hydrolysate. Rather, high sugar concentration or substrate inhibition might be the explanation. Currently, we are conducting experiments to understand this observation.
For this part of the study, we chose to use soybean oil instead of yellow grease considering: (1) use of corn fiber hydrolysate for sophorolipid production resulted in less than one-fifth of that from sorghum bagasse hydrolysates under similar experimental conditions; and (2) unlike bagasse hydrolysate which was tested on soybean oil already [27], this is the first study to investigate sophorolipid production on corn stover hydrolysates. We sought to study three lower oil doses between 10 and 40 g/L since the initial high concentration of an oil feedstock did not lead to high yield of sophorolipids as demonstrated above for bagasse hydrolysate,
As shown in Figs. 4 and 5, C. bombicola grew rapidly under four tested conditions. By the end of day 14, the biomass concentration was 6.1 ± 0.2, 5.9 ± 0.9, 5.4 ± 0.1 and 5.8 ± 0.1 g/L for soybean oil concentration at 10, 20, 40 g/L and the control where glucose (80 g/L), xylose (55 g/L) and soybean oil (40 g/L) were added, respectively. Statistically, no difference in terms of cell growth could be detected. In the same order, concentration of pure sophorolipids were 11.6 ± 1.5, 4.9 ± 0.8, 3.9 ± 0.4 and 16.1 ± 0.7 g/L. Thus, with increasing soybean oil concentration, a decreasing yield of sophorolipids was observed, which was similar to what was observed when bagasse hydrolysates and yellow grease served as the substrates. These phenomena may indicate that additional oil did not enhance the fermentation but limited the cell productivity. Comparing the two conditions where content of soybean oil of 40 g/L was the same and total sugar concentrations were similar, corn stover hydrolysates yielded less sophorolipids than those with pure glucose and xylose. Hence, unknown compounds in corn stover hydrolysate might contribute to this lower sophorolipid yield. A similar yield of 15.6 g/L of sophorolipids were observed when corn fiber hydrolysates derived from dilute acid pretreatment was used as the sugar sources together with 100 g/L of soybean oil [27]. Even though corn fiber and corn stover have different compositions and the resulting hydrolysates are very different, too, these two feedstocks appeared to be not as good as sorghum bagasse on serving as the carbon substrates. The exact explanations deserve further investigations.
Similar to cells grown on bagasse hydrolysates and yellow grease as described above, C. bombicola did not consume all soybean oil during the 2-week cultivation period. Residual oil left was 2.3 ± 1.9, 5.5 ± 4.0, and 20.7 ± 1.5 g/L for oil content of 10, 20, and 40 g/L, respectively. For the controls with pure glucose and xylose, the residual oil was 24.0 ± 5.6 g/L. Considering all carbons (sugars and oil) utilized, yield of sophorolipids was 0.12 ± 0.01, 0.05 ± 0.01, 0.04 ± 0.0, and 0.15 ± 0.0 (Fig. 3). Compared to those observed when C. bombicola grew on sorghum bagasse hydrolysates and yellow grease, these yield were much lower.
Fermentation using C. bombicola on corn stover hydrolysates with yellow grease supplementation in a fermentor
Considering the extremely low yield of sophorolipids on corn stover hydrolysates and soybean oil, we set out to investigate whether the yield can be improved when the cultivation conditions were better controlled. As shown in Fig. 6, C. bombicola grew well and reached the end of exponential growth phase by day 2 with a cell dry weight of 8.5 g/L. Throughout the 7-day experimental period, while all sugar concentrations decreased with time (Fig. 6), concentration of sophorolipids steadily increased to 52.1 g/L at the end (Fig. 6). The final yield of sophorolipids was calculated as 0.34 g/g (sugars plus yellow grease). This computation considered sugars and yellow grease initially present, those lost during sampling and those remaining in the final cultures. This yield was much higher than 0.12 g/g when fermentation was performed in an uncontrolled batch mode. Thus, with much better mixing and aeration, well-controlled content of dissolved oxygen and a stable pH, significant amount of sophorolipids can be produced from corn stover hydrolysates. In addition, the sophorolipid production rate of 7.4 g/L-day was much faster than 3.0 g/L-day when sorghum bagasse hydrolysates were used in the batch cultivation mode.
It has been generally practiced that during the stationary phase when sophorolipids production takes off, a lipid substrate should be fed step-wise or continuously to enhance yield of sophorolipids [6, 29]. For example, to achieve a sophorolipid concentration of 422 g/L, rapeseed oil was supplemented at 100 g/L at four times throughout a 18-day fermentation [4]. In this fermentor study, since enough sugars remain in the culture all the time, corn stover hydrolysates was not fed during the experiment. Instead, yellow grease was supplemented twice to stimulate sophorolipids production. But it appeared that the cells did not need too much of yellow grease and only 27.5% of what was added was utilized for producing sophorolipids (Table 2). Thus, for future investigations, the lipid substrate should be added just enough to avoid wasting of this material.
Few studies have been conducted on producing sophorolipids from renewable feedstocks. The one with delignined corncob residue hydrolysate reported a sophorolipid concentration of 42.1 g/L when the hydrolysates were detoxified by passing through activated carbon and oleic acid was used as the oil substrate [19]. In this study, both sorghum bagasse hydrolysates and corn stover hydrolysates were used directly after either pretreatment or pretreatment/enzymatic hydrolysis. No further treatment or detoxification was performed on these two liquors and we have observed a sophorolipid concentration of 52.1 g/L when yellow grease served as the lipid source. It needs to be noted that sophorolipid yield from lignocellulosic hydrolysates cannot be compared with those from pure sugars considering that the hydrolysates are highly complex. Although fermentable sugars and some acids if there are any can be identified and quantified by HPLC, a great number of compounds in the hydrolysates are not quantifiable and may cause inhibition to fermentation. Detailed studies have revealed hundreds of chemicals in cellulosic hydrolysates as a result of the severe pretreatment steps [21, 24]. However, using lignocellulosic feedstocks for producing sophorolipids represents a potentially sustainable and renewable approach for generating these valuable compounds. Upon further process optimization, the cost effectiveness of this process will be improved significantly.
Conclusion
Both sorghum bagasse hydrolysates derived from a simple acid pretreatment scheme and corn stover hydrolysates developed from an extensive alkaline based pretreatment procedure supported cell growth and sophorolipid production by C. bombicola. Assisted by the presence of yellow grease at 10 g/L, a yield of sophorolipids of 0.56 g/g carbon source was achieved in a batch cultivation mode with sorghum bagasse hydrolysates. Much lower yield of 0.12 g/g was observed when corn stover hydrolysates and soybean oil were used as the substrates. However, when corn stover hydrolysates and yellow grease were added to a fully controlled fermentor, the sophorolipid yield was 0.34 g/g. Therefore, both biomass hydrolysates can potentially serve as sources of sugars for producing valuable sophorolipids. The yield of this target product can be further enhanced by optimizing the fermentation process.
References
Chen X, Shekiro J, Pschorn T, Sabourin M, Tao L, Elander R, Tucker MP (2014) A highly efficient dilute alkali deacetylation and mechanical (disc) refining process for the conversion of renewable biomass to lower cost sugars. Biotechnol Biofuels 7(1):98
Choudhary R, Umagiliyage AL, Liang Y, Siddaramu T, Haddock J, Markevicius G (2012) Microwave pretreatment for enzymatic saccharification of sweet sorghum bagasse. Biomass Bioenergy 39:218–226
Ciesielska K, Van Bogaert IN, Chevineau S, Li B, Groeneboer S, Soetaert W, Van de Peer Y, Devreese B (2014) Exoproteome analysis of Starmerella bombicola results in the discovery of an esterase required for lactonization of sophorolipids. J Proteom 98:159–174
Daniel H-J, Reuss M, Syldatk C (1998) Production of sophorolipids in high concentration from deproteinized whey and rapeseed oil in a two stage fed batch process using Candida bombicola ATCC 22214 and Cryptococcus curvatus ATCC 20509. Biotechnol Lett 20:1153–1156
Davila A-M, Marchal R, Vandecasteele J-P (1992) Kinetics and balance of a fermentation free from product inhibition: sophorose lipid production by Candida bombicola. Appl Microbiol Biotechnol 38:6–11
Davila A-M, Marchal R, Vandecasteele J-P (1997) Sophorose lipid fermentation with differentiated substrate supply for growth and production phases. Appl Microbiol Miotechnol 47:496–501
Desai JD, Banat IM (1997) Microbial production of surfactants and their commercial potential. Microbiol Mol Biol Rev 61(1):47–64
Hillion G, Marchal R, Stoltz C, Borzeix F (1998) Use of a sophorolipid to provide free radical formation inhibiting activity or elastase inhibiting activity. Google Patents, US5756471 A
Hommel R, Weber L, Weiss A, Himmelreich U, Rilke O, Kleber H-P (1994) Production of sophorose lipid by Candida (Torulopsis) apicola grown on glucose. J Biotechnol 33:147–155
Huang TT (2013) Chemically modified sophorolipids and uses thereof. Google Patents, WO2013003291 A3
Huang TT (2014) Carbohydrate esters as inducers for gene expression. Google Patents, WO2014015179 A1
Isoda H, Kitamoto D, Shinmoto H, Matsumura M, Nakahara T (1997) Microbial extracellular glycolipid induction of differentiation and inhibition of the protein kinase C activity of human promyelocytic leukemia cell line HL60. Biosci Biotechnol Biochem 61:609–614
Klekner V, Kosaric N, Zhou Q (1991) Sophorose lipids produced from sucrose. Biotechnol Lett 13:345–348
Liang Y, Jarosz K, Wardlow AT, Zhang J, Cui Y (2014) Lipid production by Cryptococcus curvatus on hydrolysates derived from corn fiber and sweet sorghum bagasse following dilute acid pretreatment. Appl Biochem Biotechnol 173:2086–2098
Liang Y, Perez I, Goetzelmann K, Trupia S (2014) Microbial lipid production from pretreated and hydrolyzed corn fiber. Biotechnol Prog 30:945–951
Liang Y, Sarkany N, Cui Y, Yesuf J, Trushenski J, Blackburn JW (2010) Use of sweet sorghum juice for lipid production by Schizochytrium limacinum SR21. Bioresource Technol 101:3623–3627
Liang Y, Tang T, Siddaramu T, Choudhary R, Umagiliyage AL (2012) Lipid production from sweet sorghum bagasse through yeast fermentation. Renew Energy 40:130–136
Liang Y, Tang T, Umagiliyage AL, Siddaramu T, McCarroll M, Choudhary R (2012) Utilization of sorghum bagasse hydrolysates for producing microbial lipids. Appl Energy 91:451–458
Ma XJ, Li H, Wang DX, Song X (2014) Sophorolipid production from delignined corncob residue by Wickerhamiella domercqiae var. sophorolipid CGMCC 1576 and Cryptococcus curvatus ATCC 96219. Appl Microbiol Biotechnol 98:475–483
Maingault M (1999) Utilization of sophorolipids as therapeutically active substances or cosmetic products, in particular for the treatment of the skin. Google Patents, US5981497 A
Mills TY, Sandoval NR, Gill RT (2009) Cellulosic hydrolysate toxicity and tolerance mechanisms in Escherichia coli. Biotechnol Biofuels 2:1
Mittelbach M, Enzelsberger H (1999) Transesterification of heated rapeseed oil for extending diesel fuel. J Am Oil Chem Soc 76:545–550
Morya VK, Park JH, Kim TJ, Jeon S, Kim EK (2013) Production and characterization of low molecular weight sophorolipid under fed-batch culture. Bioresour Technol 143:282–288
Palmqvist E, Hahn-Hägerdal B (2000) Fermentation of lignocellulosic hydrolysates. II: inhibitors and mechanisms of inhibition. Bioresour Technol 74:25–33
Pekin G, Vardar-Sukan F, Kosaric N (2005) Production of sophorolipids from Candida bombicola ATCC 22214 using Turkish corn oil and honey. Eng Life Sci 5:357–362
Rau U, Hammen S, Heckmann R, Wray V, Lang S (2001) Sophorolipids: a source for novel compounds. Ind Crops Prod 13:85–92
Samad A, Zhang J, Chen D, Liang Y (2015) Sophorolipid production from biomass hydrolysates. Appl Biochem Biotechnol 175:2246–2257
Shah V, Jurjevic M, Badia D (2007) Utilization of restaurant waste oil as a precursor for sophorolipid production. Biotechnol Prog 23:512–515
Solaiman DK, Ashby RD, Zerkowski JA, Foglia TA (2007) Simplified soy molasses-based medium for reduced-cost production of sophorolipids by Candida bombicola. Biotechnol Lett 29:1341–1347
Van Bogaert INA, Saerens K, De Muynck C, Develter D, Soetaert W, Vandamme EJ (2007) Microbial production and application of sophorolipids. Appl Microbiol Biotechnol 76:23–34
Yesuf JLY-N (2012) Optimization of sugar release from sweet sorghum bagasse following solvation of cellulose and enzymatic hydrolysis using response surface methodology. Biomass Bioenergy 30:367–375
Zhou Q-H, Kosaric N (1995) Utilization of canola oil and lactose to produce biosurfactant withCandida bombicola. J Am Oil Chem Soc 72:67–71
Zhou Q, Kosaric N (1993) Effect of lactose and olive oil on intra-and extracellular lipids of Torulopsis bombicola. Biotechnol Lett 15:477–482
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing and financial interests.
Rights and permissions
About this article
Cite this article
Samad, A., Zhang, J., Chen, D. et al. Sweet sorghum bagasse and corn stover serving as substrates for producing sophorolipids. J Ind Microbiol Biotechnol 44, 353–362 (2017). https://doi.org/10.1007/s10295-016-1891-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-016-1891-y