Abstract
Objective
The extraction of the hemicellulose fraction of sugarcane bagasse (SCB) by acid hydrolysis was evaluated in an autoclave and a Parr reactor aiming the application of the hydrolysate as a carbon source for lipid production by Lipomyces starkeyi.
Results
The hydrolysis that resulted in the highest sugar concentration was obtained by treatment in the Parr reactor (HHR) at 1.5% (m/v) H2SO4 and 120 °C for 20 min, reaching a hemicellulose conversion of approximately 82%. The adaptation of the yeast to the hydrolysate provided good fermentability and no lag phase. The fermentation of hemicellulose-derived sugars (HHR) by L. starkeyi resulted in a 27.8% (w/w) lipid content and YP/S of 0.16 g/l.h. Increasing the inoculum size increased the lipid content by approximately 61%, reaching 44.8% (w/w).
Conclusion
The hemicellulose hydrolysate from SCB is a potential substrate for L. starkeyi to produce lipids for biodiesel synthesis based on the biorefinery concept.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fluctuations in the price of fossil fuels coupled with concerns about energy security and an increasing awareness of the environmental impact associated with CO2 emissions, have caused the development of alternative energy solutions to one with become a global priority (Santamauro et al. 2014). Thus, changing the global energy mixture to renewable and sustainable sources is necessary (Furlan et al. 2013) and biofuels are one of the best options to initiated the transition from short-term petroleum-based fuels (Balan 2014). Biodiesel is an important renewable fuel and a possible candidate to replace fossil fuels and become a source of primary energy for the world (Feofilova et al. 2010). This biofuel is produced by transesterification of triacylglycerols (TAGs), obtaining glycerol as a byproduct (Lopes et al. 2020). However, the application of edible vegetable oils (soybean, palm, rapeseed, peanut, sunflower) (Mishra and Goswami 2017; Thangaraj et al. 2019), as raw material in the production of biodiesel leads to restrictions on economic production and competitive commercialization of this biofuel since the use of these feedstocks can represent approximately 70–95% of the production costs (Ma et al. 2018) and competes with food crops for agricultural land and environmental impact (Santamauro et al. 2014). Microbial oils, termed single cell oils (SCOs), have many interesting characteristics such as adding nutritional quality to biomass for animal feed and possibly replacing vegetable oil and fats such as cocoa butter, palm oil and specific fatty acids, i.e. PUFAs (polyunsaturated fatty acids), for human nutrition and food application (Galán et al. 2019; Bharathiraja et al. 2017; Ochsenreither et al. 2016). In addition, SCOs are considered promising candidates for the production of biodiesel, as they present advantages such as similar fatty acid composition to that of vegetable oils, no seasonality to their production, no dependence on arable lands and production by cultivation in bioreactors (Lopes et al. 2020; Xavier et al. 2017; Santamauro et al. 2014).
Oleaginous organisms, such as microalgae, bacteria, fungi and yeast have the ability to store lipids at more than 20% (w/w) of their dry weight (Christophe et al. 2012). Lipomyces starkeyi is an excellent lipid producer since is capable of accumulating triacylglycerols at levels over 70% of its dry cell weight and assimilating different carbon sources (Takaku et al. 2020; Sutanto et al. 2018). The high cost of raw materials, such as sucrose and glucose, is the major obstacle to SCO becoming an ecomonically feasible raw material for biodiesel production, and using low-cost feedstock could decrease the cultivation costs (Matsakas et al. 2014; Lopes et al. 2020). Thus, finding inexpensive substrates and selecting suitable microorganisms able to efficiently assimilate these substrates for SCO production and suitable solvents for downstream processes are important aspects to be considered for cost-effective production of biofuels (Subramaniam et al. 2010; Zhao et al. 2012; Xiong et al. 2015).
Hemicellulose, a constituent of lignocellulosic biomass, is an inexpensive alternative carbon source that can be used after sugar extraction as hemicellulosic hydrolysate (HH). Hemicellulose extraction can be carried out by the pretreatment of lignocellulosic biomass such as sugarcane bagasse (SCB) using physical, chemical, physico-chemical or biological methods (Canilha et al. 2013; Bonturi et al. 2017; Tsegaye et al. 2019). These pretreatment techniques promote the breakdown of the hemicellulose-lignin-cellulose complex, hydrolyzing hemicellulose and releasing fermentable sugars such as xylose, arabinose and glucose (Canilha et al. 2013). Diluted acid pretreatment is one of the most commonly applied methods for hemicellulose depolymerization and has been reported in many studies (Brandenburg et al. 2016; Bonturi et al. 2017; Galbe and Walberg 2019; Xavier et al. 2017; Tsegaye et al. 2019; Aguilar et al. 2002). Undesired compounds that inhibit microbial growth such as furfural, 5-hydroxymethylfurfural and acetic acid are produced as byproducts during the hydrolysis of hemicellulose in addition to sugars (Galbe and Walberg 2019; Brandenburg et al. 2016; Canilha et al. 2013). To overcome the inhibitory effect the development of strains tolerant to toxic compounds through the adaptation of cells or high cell density cultivation can be an alternative to the detoxification of lignocellulosic hydrolysates to be used in obtaining sustainable products and value-added biomolecules (Bonturi et al. 2017; Westman and Franzén 2015).
Since hemicellulose hydrolysate is a secondary stream in 2G ethanol plants due to the difficulty in fermenting xylose with ethanol-producing microorganisms and since the hemicellulosic fraction is the second most abundant polysaccharide in nature, hemicellulose hydrolysate can be considered a promising renewable and sustainable feedstock for industrial applications (Lopes et al. 2020; Xavier et al. 2017). Therefore, this work aimed to extract hemicellulose from sugarcane bagasse by dilute acid pretreatment using different equipment and evaluate the use of hemicellulosic hydrolysates in the production of microbial oil.
Material and methods
Raw material
Sugarcane bagasse (SCB) samples were kindly provided by CTBE (National Laboratory of Science and Technology of Bioethanol) from Usina da Pedra, Serrana-SP, Brazil. These samples were labeled A and R for the treatments using an autoclave and a Parr reactor, respectively. The sugarcane bagasse moisture content was approximately 9%. The chemical composition of the pretreated sugarcane bagasse was determined using the method reported by Gouveia et al. (2009), Rocha et al. (2011), Canilha et al. (2011).
Pretreatment of sugarcane bagasse
The sugarcane bagasse was pretreated by acid hydrolysis with dilute sulfuric acid to obtain the hemicellulosic hydrolysate for lipid production. The pretreatment adapted from Aguilar et al. (2002) was performed in an autoclave or a 7.5 L Parr reactor model 4554 (Parr Instrument Company, Illnois- USA) with 1.5% (w/v) H2SO4 at 120 °C for 20 min (Fig. 1). Pressures in the autoclave and Parr reactor were approximately 14.2 psi and 30 psi, respectively. The hemicellulosic hydrolysate (liquid phase) was named HH.
The extent of hemicellulose conversion by acid hydrolysis was calculated by relating the amount of hemicellulose obtained (g) to the amount of bagasse (dry basis) used in the pretreatment, according to the Eq. 1.
where CH: Hemicellulose conversion (%). F: Hemicellulose percentage of sugarcane bagasse. B: Bagasse mass (g). \(Mh\): Mass of hemicellulose after the pretreatment* (g). *Hemicellulose = pentoses (xylose + arabinose)*0.88 + furfural*1.38 + acetic acid*0,72 (Canilha et al. 2011).
Strain and medium
The yeast utilized was L. starkeyi DSM 70,296, maintained in YPD agar medium at 4 °C in a refrigerator. Pre-inoculation was carried out in YPX medium composed of 20 g/l xylose, 10 g/l yeast extract and 10 g/l peptone. The composition of the inoculum and fermentation medium was as follows (per liter): 20 g of xylose (synthetic medium), 0.66 g of yeast extract, 0.45 g of (NH4)2SO4, 1 g of Na2HPO4, 3.5 g of KH2PO4, 0.4 g of Mg2SO4.7H2O, 0.04 g of CaCl2.2H2O, 0.08 g of ZnSO4.7H2O, 0.001 g of CuSO4.5H2O, 0.001 g of CoCl2.6H2O and 0.005 g of (NH4)2Mo2O7 (pH 5.5). For all other studies, HHA and HHR were used as carbon sources, supplemented with the salts of synthetic medium at the same concentrations; pH 5.5, and a carbon-to-nitrogen (C/N) ratio of 50; and sterilized at 120 °C for 15 min (Xavier et al. 2017).
Culture conditions
Adaptation of L. starkeyi to hemicellulosic hydrolysate (HH)
L. starkeyi was adapted to HH to reduce the lag phase during HH cultivation due to the potential inhibitors present in hemicellulosic hydrolysate and lead to improved fermentation efficiency. The adaptation was performed by transferring 30% (v/v) of pre-inoculum cultivated in YPX to 100% HH medium and during the exponential-growth phase of yeast it was transferred successively to 100% of HH medium, similar to the adaptation performed by Aristizabal (2013). The exponential-growth phase was considered as the phase in which the specific growth rate was maximum and constant. Adaptation 1 (AD-1) represents the first transference from the pre-inoculum to 100% HH medium (inoculum) and adaptation 2 (AD-2) refers to the second transference to HH and so on until 7 steps of adaptation (AD-7). Experiments were carried out in 250 mL flasks in an orbital shaker incubator at 28 °C, 200 rpm, pH 5.5 and a working volume of 100 mL. The maximum specific growth rate (µmax) was determined by the Eq. 2.
where X: concentration of cells (g L−1). t: fermentation time (h).
Fermentations
For fermentations of synthetic medium (xylose as carbon source) and HH medium the pre-inoculum was prepared by propagation of L. starkeyi in synthetic liquid medium (YPX) for 48 h at 28 °C, 200 rpm, pH 5.5 in an orbital shaker (Xavier et al. 2017). The inoculum was incubated for approximately of 30 h in liquid medium (HH or synthetic medium) and the concentration of approximately 1.0 g/l of cells was used for the experiments. Fermentations were conducted in 250 mL shaking flasks with a working volume of 100 mL under the same inoculum preparation conditions. Aliquots were collected at various intervals and stored at − 20 °C until analysis of the substrate concentration, dry cell weight (DCW) and lipid content.
Analytical methods
The concentrations of xylose, arabinose, glucose and acetic acid in the hydrolysates were determined by ion chromatography (Metrohm, Switzerland). For sugar determinations, a Metrohm system (polystyrene/divinylbenzene copolymer column, particle size of 5 mm, dimensions of 150 × 4.0 mm, 871 Advanced Bioscan detector), NaOH (0.1 mM/L) at 1.0 mL/min as the eluent, column and detector temperatures of 30 °C were used. The Somogy-Nelson colorimetric method was used to monitor on-time the sugar concentration on-line during fermentation (Tapia et al., 2012).
Acetic acid was determined using a Metrosep organic acid column (250 × 7.8 mm Metrohm AG CH 9101), mobile phase consisting of 0.5 mM H2SO4, an injection volume of 196 μL and a conductivity detector (Xavier et al. 2017). For the determination of furfural and hydroxymethylfurfural (HMF), high-performance liquid chromatography (HPLC Waters) was used with the following setup and conditions: a Delta-Pak C18 column (150 × 3.9 mm, 5 µm, 300 Å) at 25 °C, a UV detector (486) at 280 nm, a mobile phase consisting of acetonitrile (2.5%): H3PO4 2 mM (1:1) at a flow rate of 0.5 ml/min and an injection volume of 10 µl. Before injection samples for determination of sugars and inhibitors were filtered through polyvinylidene fluoride (PVDF) and polytetrafluorethylene (PTFE) syringe filters each with pore sizes of 0.45 μm and diameters 13 mm (Xavier et al. 2017).
The cell concentration was determined by the cell dry weight (Xavier et al. 2017). During fermentation the cell biomass was monitored by turbidimetry and calculated from a standard curve made by plotting the dry weight of cells vs the OD600.
The inorganic nitrogen content was determined by the Berthelot reaction as described by Srienc et al. (1984).
The lipid content was quantified by Bligh-Dyer’s method (Bligh and Dyer 1959; Manirakiza et al. 2001). Prior to lipid extraction, the lyophilized cells were pretreated with 2 M HCl at 80 °C for 1 h (Tapia et al. 2012; Xavier et al. 2017).
Results and discussion
Hemicellulosic hydrolysate (HH) from sugarcane bagasse (SCB)
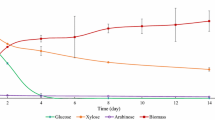
Two types of equipment were used to perform SCB hydrolysis to obtain hydrolysates with high a concentration of xylose and a low content of inhibitors. The HHs obtained by the autoclave and Parr reactor were named HHA and HHR, respectively. The acid hydrolysis of SCB using an autoclave or Parr reactor released sugars such as xylose, which was the major hemicellulosic sugar produced and reached concentrations of approximately 13.2 and 18.5 g/l, respectively, and glucose and arabinose, produced at lower concentrations (Fig. 2). It may be noted that HHR had an approximately 40% higher xylose concentration than did HHA possibly due to the pressure difference in the systems. Glucose can originate from the cellulosic fraction or heteropolymers of the hemicellulose fraction (Aguilar et al. 2002). It is possible to observe a low glucose concentration because the hydrolysis of hemicellulose hardly damages the cellulosic fraction, as hemicellulose bonds are weaker than cellulose bonds and require milder hydrolysis conditions than to produce hexoses (Aguilar et al. 2002; Tsoutsos et al. 2011). This was verified through the mass balance applied to the bagasse pretreatment process in autoclave and Parr reactor where only 3.32% and 4.8%, respectively, of the cellulosic fraction fed (A = 14.85 g and R = 127.2 g) was detected in the hydrolyzate, as a consequence of the low glucose concentration.
In addition to sugars, acetic acid (Fig. 2), furfural and 5-hydroxymethilfurfural (HMF) were produced during the pretreatment of SCB (Fig. 3). These compounds are generated by the degradation of the hemicellulose and cellulose fractions of lignocellulosic biomass and are considered potential inhibitors of microbial metabolism, hindering the bioconversion of sugars into desired products (Canilha et al. 2010). In both hydrolysates, the furfural concentration was higher than the HMF concentration due to the increased degradation of pentoses during acid hydrolysis (Fig. 3). Weak acids, furans (5-HMF, furfural) and 5-HMF are released when hemicellulose and cellulose are broken down, respectively (Chandel et al. 2011). The furfural and HMF concentrations remained below 300 ppm and 200 ppm, respectively, in both hydrolysates. Acetic acid is formed by hydrolysis of the acetyl groups in hemicellulose as a result of the deacetylation of acetylated pentosans (Chandel et al. 2010). The acetic acid concentration was 3.6 g/l in HHR and 2.6 g/l in HHA (Fig. 2). Chandel et al. (2007) obtained a similar composition of sugarcane bagasse hydrolyzed with 1.5% HCl at 140 °C for 30 min resulting in 17.2 g/l xylose, 3.8 g/l glucose, 2.56 g/l arabinose, and 3.5 g/l acetic acid.
Under the same operating conditions the sugar and inhibitor compositions were higher in HHR than in HHA, however, the hemicellulose conversion reached approximately 82% compared to 65% for HHA. The improvement in hemicellulose yield may have been due to the better operating conditions in the reactor than in the autoclave, which could have improved the efficiency of hydrolysis as well as the recovery of sugars from the hydrolysates. However, it may also have prevented the reduction in volatile inhibitor concentrations by evaporation (Lenihan et al. 2010; Chandel et al. 2011) generating higher levels of inhibitors in the HHA. The extents of hemicellulose conversions found in this work can be considered a good result, even that obtained in the autoclave (~ 65%), since only 1 step was needed to extract of hemicellulose. Comparatively, Lopes et al. (2020) carried out a hydrothermal treatment for the solubilization of Eucalyptus uograndis hemicellulose followed by hydrolysis at 121 °C for 60 min with 0.5% (v/v) H2SO4, and the total sugar reached 96% of the hydrolysate.
The high conversion of hemicellulose was also evidenced by the chemical composition of sugarcane bagasse before and after pretreatment (Table 1). The hemicellulose fraction showed a reduction greater than 75% reaching a hemicellulose content of 5% for both pretreated SCBs. In addition, the pretreatment resulted in an enrichment of the cellulose content by at least approximately 17%.
Adaptation of L. starkeyi to HH
Some microorganisms have the ability to degrade inhibitors and this natural strategy only needs to be exploited or enhance to overcome the inhibitors in lignocellulose biomass. In some cases, this is done through adaptation and genetic engineering. It is desirable to develop adapted lipid-producing yeasts that requires minimal or no detoxification treatment, as it not only reduces the cost of detoxification, but also prevents the loss of fermentable sugars from hydrolysates (Parawira and Tekere 2011). One alternative to circumvent inhibitor problems is to improve microorganisms by evolutionary engineering (Koppram et al. 2012). This strategy is based on a systematic selection procedure in which the long term adaptation of cells under selective pressure favors a desired phenotype (Hacisalihoğlu et al. 2018; Koppram et al. 2012). This phenomenon produces variants of the cell population with a selective advantage that exponential take over the initially dominating cells (Koppram et al. 2012).
Many evolutionary engineering studies have attempted to adapt microorganisms to hydrolysates and increase their tolerance to inhibitory compounds, such as HMF and furfural to improve the fermentation process to obtain new products (Kootstra et al. 2009; Zhu et al., 2009; Helmberger et al. 2011; Silva et al. 2014; Bonturi et al. 2017). Bonturi et al. (2017) reported the successful adaptation of Rhodosporidium toruloides to sugarcane bagasse hydrolysate increasing its tolerance to inhibitors and its lipid production compared to those of the parental strain. However, due to inhibition and stress by toxic substances most studies have carried out the adaptation of microorganisms by successive cultivations in different proportions of hydrolysate. L. starkeyi showed an increase in cell growth and a reduced lag phase when cultivated in 100% hydrolysate, that is, when the inoculum was prepared directly in HH medium (Fig. 4). The maximum specific growth rate (µmax) considerably increased from 0.04 h−1 to 0.07 h−1 at AD-2 phase. The highest µmax was achieved in the AD-4 phase for both hydrolysates, reaching µmax similar to that reported by Garzón (2009) for the xylose fermentation. After AD-4 phase the µmax was reduced reaching the lowest rate at AD-7 phase (Table 2). The adapted yeast from AD-4 phase was stored at -80 °C into respective HH medium with 10% (v/v) glycerol to be used for later fermentations. However, this adapted yeast did not showed good fermentability when trying to reactivate it in the hydrolysate showing long lag phase. Thus, this yeast from AD-4 phase was not considered for the further experiments.
When microorganisms are exposed to inhibitors during cultivation, short- or long-term adaptations can be promoted (Bonturi et al. 2017). Therefore, for subsequent experiments the adaptation AD-1 was of applied to hydrolysates due to the significant improvement in fermentability when the inoculum was prepared with hydrolysate (Fig. 4). Furthermore, the adaptation of yeasts to the lignocellulosic hydrolysate prior to fermentation is suggested as an alternative approach to detoxification (Parawira and Tekere 2011; Bonturi et al. 2017).
The sugar consumption and inhibitor profile were monitored only during AD-7 (Figs. 5 and 6). No lag phase was observed, but immediate growth and a reduction in inhibitor concentration where observed during fermentation.
The significant improvement in fermentability from AD-1 to AD-2 strongly indicates that this yeast has great tolerance to inhibitors, such as furfural, HMF and acetic acid, at the concentrations found in this study. Good results have been presented from adapted yeast strains, but additional investigations need to be carried out to determine how long the improved characteristics derived from adaptation remain after the application of selective pressures.
Fermentations
L. starkeyi can consume xylose efficiently and produce lipids. In synthetic culture medium, the yeast was able to produce 10.4 g/l of cells, a lipid accumulation of 29.1% (w/w) and a lipid yield of 0.14 g/g (g lipid/g xylose) (Fig. 7, Table 3). Xavier et al. (2017) reported lipid contents and yields of 36.8% (w/w) and 0.16 g/g, respectively, when L. starkeyi was cultivated on xylose using approximately 3.0 g/l inoculum. The inoculum size has a considerable effect on lipid biosynthesis and cell mass concentration. Anschau et al. (2014) found an improvement in the lipid content of 25.3 to 31.7% (w/w) and a yield of biomass and lipids three times higher than those reached when 1.0 g/l inoculum was applied for culture in a glucose and xylose mixture. Liu et al. (2012) reported that microbial oil production was closely related to inoculation concentration when L. starkeyi was cultivated in monosodium glutamate wastewater at different inoculum concentrations. The nitrogen content was depleted after 12 h of cultivation and since storage lipid synthesis occurs under nitrogen-limited conditions (Ratledge 2013) the remaining sugars were diverted to SCO production.
The main sugars obtained from hydrolysates, such as xylose and glucose, but not arabinose, were fully assimilated by L. starkeyi without appreciable inhibition, suggesting the importance of carrying out the previous adaptation to HH (Figs. 8a and 9a). L. starkeyi showed diauxic growth consuming xylose and glucose sequentially during cultivations of HH due to glucose repression, as found by Zhao et al. (2008). Additionally, the consumption of acetic acid, furfural and HMF (Fig. 8b, 9b) during fermentation of hydrolysates was observed. Xavier et al. (2017) demonstrated that L. starkeyi can metabolize acetic acid, a lignocellulose hydrolysis byproduct, for growth and lipid production. Some works have shown the application of acetic acid as the sole carbon source in feedstock or as a co-substrate for oleaginous yeast cultivation (Masri et al. 2019; Xavier et al. 2017; Huang et al. 2016; Liu et al. 2015a, b; Gong et al. 2015). The tolerance to aldehyde compounds is most likely due to the ability of microorganisms to convert these compounds to the corresponding less inhibitory alcohols (Nilsson et al. 2005). First, the yeast ferments the sugars; it reduces toxic furfural to noninhibitory furfuryl alcohol and HMF to 5-hydroxymethylfurfuryl alcohol in a prolonged lag phase (Li et al. 2011). Bioreduction of furfural and HMF may shorten the lag phase, highlighting the importance of prior adaptation of yeast to the hydrolysate. In general, the effects of furans can be explained by a redirection in yeast energy to fix the damage caused by furans and by reduced intracellular ATP and NAD(P)H levels, either by enzymatic inhibition or consumption/regeneration of cofactors (Almeida et al. 2007). L. starkeyi has key genes related to the metabolism of inhibitors that indicate a natural ability to process these compounds (Xavier et al. 2017).
Kinetic profile of L. starkeyi during fermentation of HHA. a Cell growth and sugars consumption: (fileed diamond) Xylose, (open circle) Glucose, (filled square) Arabinose, (open triangle) DCW; b Inhibitors: (filled circle) Acetic acid, (filled triangle) Furfural, (open square) HMF. All analyzes were performed in triplicate and error bars denote the standard deviation
Kinetic profile of L. starkeyi during fermentation of HHR. a Cell growth and sugars consumption: (filled diamond) Xylose, (open circle) Glucose, (filled square) Arabinose, (open triangle) DCW; b Inhibitors: (filled circle) Acetic acid, (filled traingle) Furfural, (open square) HMF. All analyzes were performed in triplicate and error bars denote the standard deviation
The cultivation of L. starkeyi in hydrolysates showed kinetic profiles similar to those for cultivation in xylose (Figs. 7, 8, 9). However, lipid accumulations of 20% and 27.8% for HHA and HHR, respectively, were reached, which were lower than the 29.1% for xylose (Table 3). The lipid yield (YP/S) obtained from the hydrolysates was slightly different from that for the xylose culture, although the highest yield of 0.16 g/g was achieved for HHR. The lower production of microbial oil from HHA may have been due to the lower substrate content, since similar kinetic parameters were observed for the cultivations. A culture of HHR using approximately 3.0 g/l inoculum under the same operating conditions showed a kinetic behavior similar to that presented with the use of 1.0 g/l inoculum. Nevertheless, increasing the inoculum concentration substantially improved the lipid content to 44.8% (w/w), representing an increase of 61.2%. The lipid yield for this cultivation was 0.15 g/g. Xavier et al. (2017) found lipid accumulation of 36.8% (w/w) and 26.9% (w/w) for xylose and HH cultivations, respectively, using approximately 3.0 g/l inoculum. Juanssilfero et al. (2018) investigated the effect of different sizes of an L. starkeyi NBRC10381 inoculum on lipid production using glucose and/or xylose as the carbon source. The authors reported reaching a lipid content of more than 80% (w/w) for with high inoculum sizes. The microbial oil accumulated with xylose was 86.6% (w/w) using approximately 6.0 g/l inoculum. Liu et al. (2015a) reported an increase in lipid accumulation from 36.4% (w/w) to 47.2% (w/w) when undetoxified corncob hydrolysate was used in high cell density culture with a two-stage nitrogen feeding strategy. The lipid productivity from HH and xylose was nearly constant at 0.04 g/l.h (PL) (Table 3).
Nitrogen was exhausted after 24 and 36 h for HHA and HHR, respectively, with no remaining sugars, besides arabinose, detected at the end of fermentation. Many microorganisms such as Yarrowia lipolytica (Gao et al. 2020), Rhodotorula glutinis (Maza et al. 2020), Cryptococcus curvatus (Park et al. 2017), Rhodotorula toruloides (Lopes et al. 2020), and Trichosporon cutaneum (Gao et al. 2014) are able to grow on many types of substrates and produce high amounts of lipids. Lipomyces starkeyi can assimilate a broad range of substrates including glucose, xylose, a mixture of sugars, molasses, glycerol, the hemicellulosic fraction of birch wood, sap from felled old oil palm trunks, hydrolysate form oil palm empty fruit bunch, and lignocellulosic hydrolysates, to grow and produce high contents of lipids (Dien et al. 2016; Wild et al. 2010; Bonturi et al. 2015; Juanssilfero et al. 2018, 2019; Anschau et al. 2014; Gong et al. 2012; Vieira et al. 2014; Liu et al. 2017; Brandenburg et al. 2016; Thanapimmetha et al. 2019; Xavier et al. 2017). In addition, new carbon sources can be explored for this yeast by investigating carbohydrates obtained from unconventional sources such as moss Rhodobryum ontariense (Kindb.) Kindb. (Pejin et al. 2012). Thus, L. starkeyi has a great biotechnological advantage due to its ability to grow on inexpensive substrates, such as hemicellulosic hydrolysate, for the production of lipids and to metabolize potential fermentation inhibitors.
Microbial oil produced by L. starkeyi has a composition similar to that of some vegetable oils, such as palm oil (Juanssilfero et al. 2019; Xavier et al. 2017) that are used for biodiesel production. The major fatty acids of L. starkeyi-derived oil are oleic acid (C18:1) and palmitic acid (C16:0) (Juanssilfero et al. 2019; BonturI et al. 2015; Liu et al. 2017; Xavier et al. 2017; Sutanto et al. 2018; Brandenburg et al. 2016), comprising approximately 80% of the total fatty acids. The fatty acid profile of yeast lipids is dependent on the type of substrate used for growth as well as the fermentation conditions, and thus, the proportions of oleic and palmitic acids can be affected (Sutanto et al. 2018). L. starkeyi-derived lipids from hydrolysates of agricultural or industrial residues present long-chained unsaturated fatty acids containing 16 and 18 carbons, which dominate the fatty acid composition (Tchakouteu et al. 2015; Wang et al. 2014; Juanssilfero et al. 2019; Xavier et al. 2017; Liu et al. 2017; Vieira et al. 2014) indicating that it is a potential feedstock for the production of biodiesel. Based on the compositional data on the lipids produced by L. starkeyi reported in many studies and the remarkable similarity of this oil to palm oil, it is suggested that biodiesel produced from oil obtained from this yeast meets the standard specifications for biodiesel (Juanssilfero et al. 2019; Xavier et al. 2017).
Conclusion
The pretreatment of sugarcane bagasse with dilute acid was able to solubilize at least approximately 65% of hemicellulose, generating fermentable sugars, especially xylose, and this solubilization efficiency reached 82% when the hydrolysis was carried out in a Parr reactor under the same conditions, resulting in a higher concentration of sugars.
The adaptation of L. starkeyi showed high µmax when used directly in the hydrolysate in successive transfers but the yeast was unable to maintain this performance when preserved and reactivated for a new fermentation of HH. However, preparing the inoculum directly in 100% hydrolysate significantly improved the fermentability of the hemicellulosic hydrolysate without an appreciable lag phase. L. starkeyi was able to grow and produce SCO efficiently when cultivated using hemicellulose hydrolysate from sugarcane bagasse, presenting a yield and lipid content similar to those when pure xylose was used. Moreover, this yeast has a tolerance to furfural, HMF and acetic acid and could consume these potential inhibitors during fermentation. Increasing the inoculum size from 1.0 to 3.0 g/l increased the lipid content 1.6 times over that from HHR culture. Therefore, the hemicellulosic hydrolysate from sugarcane bagasse has the potential to be a source of low-cost raw material for the biodiesel production chain, and L. starkeyi has been shown to be a promising oleaginous yeast for fermentation processes that employ pentoses as carbon sources.
References
Aguilar R, Ramirez JA, Garrote G, Vázquez M (2002) Kinetic study of the acid hydrolysis of sugar cane bagasse. J Food Eng 55:309–318
Almeida JRM, Modig T, Petersson A, Hähn-Hägerdal B, Lidén G, Gorwa-Grauslund MF (2007) Increased tolerance and conversion of inhibitors in lignocellulosic hydrolysates by Saccharomyces cerevisiae. J Chem Technol Biot 4(82):340–349
Anschau A, Xavier MCA, Hernalsteens S, Franco TT (2014) Effect of feeding strategies on lipid production by Lipomyces starkeyi. Bioresour Technol 157:214–222
Aristizabal RVS (2013) Produção de leveduras oleaginosas em meio de cultura contendo hidrolisado de bagaço de cana-de-açúcar. Dissertation, State University of Campinas.
Balan V (2014) Current challenges in commercially producing biofuels from lignocellulosic biomass. ISRN Biotechnol. https://doi.org/10.1155/2014/463074
Bharathiraja B, Sridharan S, Sowmya V, Yuvaraj D, Praveenkumar R (2017) Microbial Oil - A Plausible Alternate Resource for Food and Fuel Application. Bioresour Technol 233:423–432. https://doi.org/10.1016/j.biortech.2017.03.006
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Bonturi N, Matsakas L, Nilsson R, Christakopoulos P, Miranda E, Berglund K, Rova U (2015) Single Cell Oil Producing Yeasts Lipomyces starkeyi and Rhodosporidium toruloides: Selection of Extraction Strategies and Biodiesel Property Prediction. Energies 8(6):5040–5052
Bonturi N, Crucello A, Viana AJC, Miranda EA (2017) Microbial oil production in sugarcane bagasse hemicellulosic hydrolysate without nutrient supplementation by a Rhodosporidium toruloides adapted strain. Process Biochem 57:16–25
Brandenburg J, Blomqbist J, Pickova J, Bonturi N, Sandgren M, Passoth V (2016) Lipid production from hemicellulose with Lipomyces starkeyi in a pH regulated fed-batch cultivation. Yeast 33:451–462
Canilha L, Carvalho W, Felipe M, Silva JB, Giulietti M (2010) Ethanol production from sugarcane bagasse hydrolysate using Pichia stipitis. Appl Biochem Biotechnol 161:84–92
Canilha L, Santos VT, Rocha GJ, Silva JB, Giulietti M, Silva SS, Felipe MG, Ferraz A, Milagres AM, Carvalho W (2011) A study on the pretreatment of a sugarcane bagasse sample with dilute sulfuric acid. J Ind Microbiol Biotechnol 38(9):1467–1475
Canilha L, Rodrigues RDCLB, Antunes FAF, Chandel AK, Milessi TSDS, Felipe MDGA, Silva SSD (2013) Bioconversion of Hemicellulose from Sugarcane Biomass Into Sustainable Products. In: Ekinci D (ed) Biochemistry, Genetics and Molecular Biology. InTech, London, pp 15–44
Chandel AK, Kapoor RK, Singh A, Kuhad RC (2007) Detoxification of sugarcane bagasse hydrolysate improves ethanol production by Candida shehatae NCIM 3501. Bioresour Technol 98(10):1947–1950
Chandel AK, Singh OV, Rao LV (2010) Biotechnological Applications of Hemicellulosic derived sugars:state-of-the-art. Sustain Biotechnol. https://doi.org/10.1007/978-90-481-3295-9_4
Chandel AK, Silva SS, Singh OV (2011) Detoxification of Lignocellulosic Hydrolysates for improved bioethanol production. InTech, London, pp 225–246
Christophe G, Kumar V, Nouaille R, Gaudet G, Fontanille P, Pandey A, Soccol CR, Larroche C (2012) Recent Developments in Microbial Oils Production: a Possible Alternative to Vegetable Oils for Biodiesel Without Competition with Human Food? Braz Arch Biol Technol 55(1):29–46
Dien BS, Slininger PJ, Kurtzman CP, Moser BR, O’bryan PJ, (2016) Identification of superior lipid producing Lipomyces and Myxozyma yeasts. AIMS Environ Sci 3(1):1–20
Feofilova EP, Sergeeva YE, Ivashechkin AA (2010) Biodiesel-fuel: Content, production, producers, contemporary biotechnology (Review). Appl Biochem Microbiol 46(4):369–378
Furlan FF, Filho RT, Pinto FHPB, Costa CBB, Cruz AJG, Giordano RLC, Giordano RC (2013) Bioelectricity versus bioethanol from sugarcane bagasse. Biotechnol Biofuels 6:142
Galán B, Santos-Merino M, Nogales J, de la Cruz F, García JL (2019) Microbial Oils as Nutraceuticals and Animal Feeds. In: Goldfine H (ed) Health Consequences of Microbial Interactions with Hydrocarbons, Oils, and Lipids. Handbook of Hydrocarbon and Lipid Microbiology. Springer, Cham
Galbe M, Wallberg O (2019) Pretreatment for biorefneries: a review of common methods for eficient utilisation of lignocellulosic materials. Biotechnol Biofuels 12:294
Gao Q, Cui Z, Zhang J, Bao J (2014) Lipid fermentation of corncob residues hydrolysate by oleaginous yeast Trichosporon cutaneum. Bioresour Technol 152:552–556
Gao R, Li Z, Zhou X, Bao W, Cheng S, Zheng L (2020) Enhanced lipid production by Yarrowia lipolytica cultured with synthetic and waste-derived high-content volatile fatty acids under alkaline conditions. Biotechnol Biofuels 13:3
Garzón, C SL (2009) Produção microbiana de lipídios. Dissertation, State University of Campinas.
Gong Z, Wang Q, Shen H, Hu C, Jin G, Zhao ZK (2012) Co-fermentation of cellobiose and xylose by Lipomyces starkeyi for lipid production. Bioresour Technol 117:20–24
Gong Z, Shen H, Zhou W, Wang Y, Yang X, Zhao ZK (2015) Efficient conversion of acetate into lipids by the oleaginous yeast Cryptococcus curvatus. Biotechnol Biofuels 8:189
Gouveia ERN, Souto-Maior AM (2009) Validação de metodologia para a caracterização química de bagaço de cana-de-açúcar. Quim Nova 32(6):1500–1503
Hacisalihoğlu B, Turanli-Yildiz B, Çakar ZP (2018) Evolutionary Engineering Applications in Microbial Ethanol Production. JSM Biotechnol Biomed Eng 5(1):1082
Helmberger S, Kahr H, Jäger AG (2011) Yeast adaptation on the substrate straw. Bioenergy Technology (BE), in: World Renewable Energy Congress, Linkoping, Sweden 492–499. https://www.researchgate.net/publication/269131598_Yeast_Adaptation_on_the_Substrate_Straw Acessed January 2016.
Huang XF, Liu JN, Lu LJ, Peng KM, Yang GX, Liu J (2016) Culture strategies for lipid production using acetic acid as sole carbon source by Rhodosporidium toruloides. Bioresour Technol 206:141–149
Juanssilfero AB, Kahar P, Amza RL, Miyamoto N, Otsuka H, Matsumoto H, Kihira C, Thontowi A, Yopi OC, Prasetya B, Kondo A (2018) Effect of inoculum size on single-cell oil production from glucose and xylose using oleaginous yeast Lipomyces starkeyi. J Biosci Bioeng 125:695–702
Juanssilfero AB, Kahar P, Amza RL, Yopi SK, Ogino C, Prasetya B, Kondo A (2019) Lipid production by Lipomyces starkeyi using sap squeezed from felled old oil palm Trunks. J Biosci Bioeng 127(6):726–731
Kootstra AMJ, Beeftink HH, Scott EL, Sanders JPM (2009) Comparison of dilute mineral and organic acid pretreatment for enzymatic hydrolysis of wheat straw. Biochem Eng J 46(2):126–131
Koppram R, Albers E, Olsson L (2012) Evolutionary engineering strategies to enhance tolerance of xylose utilizing recombinant yeast to inhibitors derived from spruce biomass. Biotechnol Biofuels 5:32
Lenihan P, Orozco A, O’neill E, Ahmad MNM, Rooney DW, Walker GM (2010) Dilute acid hydrolysis of lignocellulosic biomass. Chem Eng J 156(2):395–403
Li Q, Metthew Lam LK, Xun L (2011) Biochemical characterization of ethanol-dependent reduction of furfural by alcohol dehydrogenases. Biodegradation 22(6):1227–1237
Liu JX, Yue QY, Gao BY, Ma ZH, Zhang PD (2012) Microbial treatment of the monosodium glutamate wastewater by Lipomyces starkeyi to produce microbial lipid. Bioresour Technol 106:69–73
Liu Y, Wang Y, Liu H, Zhang JA (2015a) Enhanced lipid production with undetoxified corncob hydrolysate by Rhodotorula glutinis using a high cell density culture strategy. Bioresour Technol 180:32–39
Liu ZJ, Liu LP, Wen P, Li N, Zong MH, Wu H (2015b) Effects of acetic acid and pH on growth and lipid accumulation of the oleaginous yeast Trichosporon fermentans. BioResources 10:4152–4166
Liu L, Zong M, Hu Y, Li N, Lou W, Wu H (2017) Efficient microbial oil production on crude glycerol by Lipomyces starkeyi AS 2.1560 and its kinetics. Process Biochem 58:230–238
Lopes HJS, Bonturi N, Miranda EA (2020) Rhodotorula toruloides Single Cell Oil Production Using Eucalyptus urograndis Hemicellulose Hydrolysate as a Carbon Source. Energies 13:795
Ma Y, Gao Z, Wang Q, Liu Y (2018) Biodiesels from microbial oils: Opportunity and challenges. Bioresour Technol 263:631–641
Manirakiza P, Covaci A, Schepens P (2001) Comparative study on total lipid determination using Soxhlet, Roese-Gottlieb, Bligh & Dyer and modified Bligh & Dyer extraction methods. J Food Compos Anal 100(14):93
Masri MA, Garbe D, Mehlmer N, Brück TB (2019) A sustainable, high-performance process for the economic production of waste-free microbial oils that can replace plant-based equivalents. Energy Environ Sci. https://doi.org/10.1039/C9EE00210C
Matsakas L, Sterioti AA, Rova U, Christakopoulos P (2014) Use of dried sweet sorghum for the efficient production of lipids by yeast Lipomyces starkeyi CBS 1807. Ind Crops Prod 62:367–372
Maza DD, Vinartab SC, Suc Y, Guillamonc JM, Aybara MJ (2020) Growth and lipid production of Rhodotorula glutinis R4, in comparison to other oleaginous yeasts. J Biotechnol 310:21–31
Mishra VK, Goswami R (2017) A review of production, properties and advantages of biodiesel. Biofuels. https://doi.org/10.1080/17597269.2017.1336350
Nilsson A, Gorwa-Grauslund MF, Hahn-Hagerdal B, Liden G (2005) Cofactor dependence in furan reduction by Saccharomyces cerevisiae in fermentation of acid-hydrolyzed lignocellulose. Appl Environ Microbiol 71(12):7866–7871
Ochsenreither K, Glück C, Stressler T, Fischer L, Syldatk C (2016) Production Strategies and Applications of Microbial Single Cell Oils. Front Microbiol 7:1539. https://doi.org/10.3389/fmicb.2016.01539
Parawira W, Tekere M (2011) Biotechnological strategies to overcome inhibitors in lignocellulose hydrolysates for ethanol production: review. Crit Rev Biotechnol 31(1):20–31
Park GW, Chang HN, Jung K, Seob C, Kim YC, Choi JH, Woo HC, Hwang IJ (2017) Production of microbial lipid by Cryptococcus curvatus on rice strawhydrolysates. Process Biochem 56:147–153
Pejin B, Iodice C, Tommonaro G, Sabovljevic M, Bianco A, Tesevic V, Vajs V, De Rosa S (2012) Sugar composition of the moss Rhodobryum ontariense (Kindb.) Kindb. Nat Prod Res. https://doi.org/10.1080/14786419.2010.535163
Ratledge C (2013) Microbial oils: an introductory overview of current status and future prospects. Oilseeds & fats Crops and Lipids (OCL). https://doi.org/10.1051/ocl/2013029
Rocha GJM, Martin C, Soares IB, Maior AMS, Baudel HM, Abreu CAM (2011) Dilute mixed-acid pretreatment of sugarcane bagasse for ethanol production. Biomass Bioenerg 35(1):663–670
Santamauro F, Whiffin FM, Scott RJ, Chuck CJ (2014) Low-cost lipid production by an oleaginous yeast cultured in non-sterile conditions using model waste resources. Biotechonol Biofuels 7:34–34
Silva DDV, Arruda PV, Dussán KJ, Felipe MGA (2014) Adaptation of Scheffersomyces stipitis Cells as a Strategy to the Improvement of Ethanol Production from Sugarcane Bagasse Hemicellulosic Hydrolysate. Chem Eng Trans 38:427–432
Srienc F, Arnold B, Bailey JE (1984) Characterization of intracellular accumulation of poly-beta-hydroxybutyrate (PHB) in individual cells of Alcaligenes eutrophus H16 by flow cytometry. Biotechnol Bioeng 26:982
Subramaniam R, Dufreche S, Zappi M, Bajpai R (2010) Microbial lipids from renewable resources: production and characterization. J Ind Microbiol Biotechnol 37(12):1271–1287
Sutanto S, Zullaikah S, Tran-Nguyen PL, Ismadji S, Jua YH (2018) Lipomyces starkeyi: Its current status as a potential oil producer. Fuel Process Technol 177:39–55
Takaku H, Matsuzawa T, Yaoi K, Yamazaki H (2020) Lipid metabolism of the oleaginous yeast Lipomyces starkeyi. Appl Microbiol Biotechnol. https://doi.org/10.1007/s00253-020-10695-9
Tapia EV, Anschau A, Coradini ALV, Franco TT, Deckmann AC (2012) Optimization of lipid production by the oleaginous yeast Lipomyces starkeyi by random mutagenesis coupled to cerulenin screening. AMB Express 2(64):1–8
Tchakouteu SS, Kalantzi O, Gardeli C, Koutinas AA, Aggelis G, Papanikolaou S (2015) Lipid production by yeasts growing on biodiesel-derived crude glycerol: strain selection and impact of substrate concentration on the fermentation efficiency. J Appl Microbiol 118(4):911–927
Thagaraj B, Solomon PR, Muniyandi B, Ranganathan S, Lin L (2019) Catalysis in biodiesel production—a review. Clean Energy 3(1):2–23
Thanapimmetha A, Peawsuphon N, Chisti Y, Saisriyoot M, Srinophakun P (2019) Lipid production by the yeast Lipomyces starkeyi grown on sugars and oil palm empty fruit bunch hydrolysate. Biomass Convers Bior. https://doi.org/10.1007/s13399-019-00532-z
Tsegaye B, Balomajumder C, Roy P (2019) Microbial delignification and hydrolysis of lignocellulosic biomass to enhance biofuel production: an overview and future prospect. Bull Natl Res Cent 43:5
Tsoutsos T, Bethanis D (2011) Optimization of the Dilute Acid Hydrolyzator for Cellulose-to-Bioethanol Saccharification. Energies 4(12):1601–1623
Vieira JPF, Ienczak JL, Rossell CEV, Pradella JGC, Franco TT (2014) Microbial lipid production: screening with yeasts grown on Brazilian molasses. Biotechnol Lett 36(12):2433–2442
Wang R, Wang J, Xu R, Fang Z, Liu A (2014) Oil production by the oleaginous yeast Lipomyces starkeyi using diverse carbon sources. BioResources 9(4):7027–7040
Westman J, Franzén CJ (2015) Current progress in high cell density yeast bioprocesses for bioethanol production. Biotechnol J 10:1185–1195
Wild R, Patil S, Popovic M, Zappi M, Dufreche S, Baipai R (2010) Lipids from Lipomyces starkeyi. Food Technol Biotechnol 48:329–335
Xavier MCA, Coradini ALV, DeckmannAC FTT (2017) Lipid production from hemicellulose hydrolysate and acetic acid by Lipomyces starkeyi and the ability of yeast to metabolize inhibitors. Biochem Eng J 118:11–19
Xiong L, Huang C, Yang XY, Lin XQ, Chen XF, Wang C, Wang B, Zeng XA, Chen XD (2015) Beneficial effect of corncob acid hydrolysate on the lipid production by oleaginous yeast Trichosporon dermatis. Prep Biochem Biotechnol 45(5):421–429
Zhao X, Kong X, Hua Y, Feng B, Zhao Z (2008) Medium optimization for lipid production through co-fermentation of glucose and xylose by the oleaginous yeast Lipomyces starkeyi. Eur J Lipid Sci Technol 110(5):405–412
Zhao X, Peng F, Du W, Liu C, Liu D (2012) Effects of some inhibitors on the growth and lipid accumulation of oleaginous yeast Rhodosporidium toruloides and preparation of biodiesel by enzymatic transesterification of the lipid. Bioprocess Biosyst Eng 35(6):993–1004
Zhu JJ, Yong Q, Xu Y, Chen SX, Yu SY (2009) Adaptation fermentation of Pichia stipitis and combination detoxification on steam exploded lignocellulosic prehydrolysate. Natural Sci 1(1):47–54
Acknowledgments
We would like to thank FAPESP (Foundation for Research Support of the State of São Paulo), CAPES (Coordination for the Improvement of Higher Education Personnel) and CNPq (National Council for Scientific and Technological Development) for their support of this research. We would like to thank LNBR (Brazilian Biorenewables National Laboratory) and Dr. Carlos Vaz Rossel for providing the sugarcane bagasse and the Parr reactor.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
da Cunha Abreu Xavier, M., Teixeira Franco, T. Obtaining hemicellulosic hydrolysate from sugarcane bagasse for microbial oil production by Lipomyces starkeyi. Biotechnol Lett 43, 967–979 (2021). https://doi.org/10.1007/s10529-021-03080-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-021-03080-7