Abstract
Delignined corncob residue hydrolysate (DCCRH) and detoxified DCCRH were used for single cell oil (SCO) and single cell protein (SCP) production of Cryptococcus curvatus ATCC 96219 and for sophorolipid (SL) production of Wickerhamiella domercqiae var. sophorolipid CGMCC 1576. Both C. curvatus and W. domercqiae could utilize glucose in DCCRH to grow and accumulate lipids or particle-shaped SLs. DCCRH detoxification by activated carbon adsorption not only improved cell growth and lipid accumulation of C. curvatus but also increased SL production and proportion of lactonic SL in total SL. A total biomass of 17.36 g/l with a lipid content of 44.36 % could be achieved after cultivation of C. curvatus on the detoxified DCCRH. The predominant fatty acids of the produced SCO were oleic, stearic, and palmitic acids (27.2, 20.5, and 15.7 %, respectively). When W. domercqiae cells were cultivated on DCCRH and SCO, total SL production of 39.08 g/l (DCCRH + SCO) and 42.06 g/l (detoxified DCCRH + SCO) were obtained. Furthermore, when cell lysate of C. curvatus, oleic acid, and DCCRH/detoxified DCCRH was used as nitrogen and carbon sources, total SL production reached 37.19 g/l and 48.97 g/l, respectively. These results demonstrated that renewable DCCRH can be utilized for the production of high-value SCO and SLs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Surfactants are now widely used in a variety of industries, such as pharmaceutical, cosmetics, food processing, detergent, petroleum, and environment industries and they are almost synthesized by chemical methods. Their use may lead to significant ecological problems, particularly in washing applications as these surfactants inevitably end up in the environment after use (Mann and Boddy 2000). Hence, biosurfactants, produced by microorganisms including bacteria, yeast, and mould, have attracted much more attention due to their good surface activity, biodegradability, biocompatibility, low toxicity, production under mild conditions, and from renewable materials (Lee et al. 2008). Among the biosurfactants, sophorolipids (SLs), which are mainly produced by Candida apicola, Candida bogoriensis, Candida bombicola, Wickerhamiella domercqiae, Pichia anomala, or Rhodotorula bogoriensis have attracted particular interests (Van Bogaert et al. 2010). All SLs have similar structures, that is, a hydrophilic moiety, one dimeric sugar sophorose molecule linked by a glycosidic bond to a hydroxyl group on a long-chain fatty acid, the lipophilic part (Fig. 1). SLs produced by the above yeasts were a mixture of different SL molecules, which vary in hydroxy fatty acid with different carbon chain lengths and saturation degrees, hydroxylation position (ω − 1 or terminal), and acetylation degree at the 6″ position of SLs (Ma et al. 2011; Otto et al. 1999).
Large-scale application of SLs was blocked by low product yield and high production cost. One way to lower production cost is to develop different processes to improve SL yield. The highest concentration of SLs was produced by cultivation of C. bombicola in a two-stage cultivation process (Daniel et al. 1998), fed-batch combined two-stage continuous process (Rau et al. 2001), and feeding rate-controlled fed-batch process (Kim et al. 2009). The cost of SL production is relatively high and raw materials account for 10–30 % of the overall cost (Wadekar et al. 2012). Hence, another way to reduce the cost of SL production is to develop other cheap fermentative feedstock. So far, biodiesel by-product streams, waste frying oil, industrial fatty acid residues, restaurant waste oil, and soybean dark oil have been exploited to replace commonly used hydrophobic carbon sources for SL production. Furthermore, to reduce hydrophilic carbon source cost, glucose can be replaced by glycerol, complex substrates, deproteinized whey concentrate, sugarcane, soy molasses, sweetwater, etc. In addition, expensive yeast extract and other nitrogen sources can be substituted by corn steep liquor, proteins, and other nitrogenous substances (Van Bogaert et al. 2011; Wadekar et al. 2012).
Lignocellulosic material is one of the most abundant renewable and underutilized resources in the world. Among all lignocellulosic materials, corncob is easier to be utilized as raw material for the production of xylitol, xylooligosaccharide, furfural, and related products. After xylose was consumed, lignin and cellulose were left in corncob residues and lignin can be further removed by alkali treatment to obtain delignined corncob residue (DCCR). DCCR, as an agriculture residue, could be converted into sugar by cellulase and the DCCR hydrolysate (DCCRH) could be used for production of high-value products such as ethanol (Liu et al. 2010).
Microbial oils, known as single cell oils (SCO), have long been considered as alternative oil sources of lipids found in the plant or animal kingdom (Fakas et al. 2009). A selected number of microorganisms are reported to accumulate intracellular lipids by utilizing various carbon substrates. Meanwhile, microbial proteins (single cell protein, SCP) could be developed when microorganisms ferment waste materials including wood, straw, cannery and food processing wastes, residues from alcohol production, hydrocarbon, or human and animal excreta (Vrati 1984). Among them, oleaginous microorganism of Cryptococcus curvatus is an effective strain in the process of converting various wastes into microbial biomass and lipid.
The approach of using SCO for SL production was previously demonstrated by Daniel et al. (1998). Daniel et al. (1999) later developed a two-stage process with C. curvatus ATCC 20509 and C. bombicola ATCC 22214 using deproteinized whey concentrates as substrates to produce SLs. When 10 g/l SCO and 80 g/l glucose were used, 12 g/l of SLs was produced. After cultivation process optimization, the highest SLs concentration of 422 g/l was obtained from deproteinized whey, 20 g/l SCO, and 400 g/l rapeseed oil in a two-stage fed-batch process using the above-mentioned two yeast strains (Daniel et al. 1998). In the present study, to develop new cheap substrates for SL production, conditions of SCO and SCP production by C. curvatus ATCC96219 from DCCRH were optimized first and then W. domercqiae var. sophorolipid CGMCC 1576 was cultivated on DCCRH and/or DCCRH-originated SCP and SCO for SL accumulation.
Materials and methods
DCCR, enzyme, and chemicals
DCCR was kindly supplied by Longlive Bio-Technology Co., Ltd., Yucheng, Shandong, China. Before enzymatic hydrolysis, samples were desiccated at room temperature and subsequently milled into powder. Water content was determined before the experiments. The composition of DCCR was as follows: glucan, 76.7 %; xylan, 3.1 %; lignin, 9.3 %; and ash, 9.1 %, when detected according to the method described in Technical report NREL (33–2008). KDN cellulase was a commercial liquid product purchased from KDN Biotech Co., Ltd. (Qingdao, Shandong, China) with a filter paper activity (FPA) of 70 FPU/ml. FPA was measured based on the method described by Ghose (1987), using a 1 × 6-cm strip of Whatman No. 1 filter paper, and expressed as international units (IU). One international unit of cellulase activity was defined as the amount of enzyme that liberates 1 μmol of glucose (reducing sugar as glucose) per minute under the assay conditions. Reagents used in high-performance liquid chromatography (HPLC) and gas chromatography (GC) analyses were of chromatographic grade and purchased from Tedia Company Inc. (USA). Other chemicals and reagents were of analytical grade and purchased from Tianjin Kaitong Chemical Reagent Co., Ltd. and Sinopharm Chemical Reagent Co., Ltd., China.
Enzymatic DCCR hydrolysis
DCCR was added to the Na acetate buffer (see details below) and treated in an autoclave at 115 °C for 30 min before it was hydrolyzed by cellulase. DCCRH was obtained by hydrolyzing 10 % DCCR with the enzyme dosage of 25 FPU/g of dry DCCR in 0.2 M Na acetate (pH 4.8) at 45 °C for 72 h on a rotary shaker. After hydrolysis of DCCR by KDN cellulase, the liquid fraction (DCCRH) was separated by vacuum filtration. The DCCRH was mixed with activated carbon (C) (1/100, w/v) and incubated at 80 °C for 30 min to remove residual acetic acid and other inhibitors. The mixture was filtered to collect the filtrate and recover the activated carbon. The filtrate from the above step was considered as detoxified hydrolysate. All DCCRHs were stored at 4 °C prior to use.
Microorganisms, media, and cultivation
Cultivation of C. curvatus
Yeast strain of C. curvatus ATCC96219 was a domesticated strain from Jerusalem artichoke and sweet potato hydrolysate, and originally from the American Type Culture Collection. It was maintained on yeast peptone dextrose (YPD) agar slants (glucose, 20 g/l; peptone, 20 g/l; yeast extract, 10 g/l; and agar, 20 g/l) at 4 °C. The preculture was performed on precultivation medium (in grams per liter: glucose, 20; yeast extract, 10; and peptone, 20) at 30 °C and 200 rpm for 30 h. Then, 8 % of seed culture was inoculated to cultivation medium. The culture medium contained (grams per liter): hydrolysate (nondetoxified or detoxified DCCRH); yeast extract, 4.0; KH2PO4, 2.0; Na2HPO4, 8.0; MgSO4·7H2O, 3.0; CaCl2·2H2O, 0.2; FeCl3·6H2O, 0.02; and ZnSO4·7H2O, 0.02. In synthetic medium, 60 g/l of glucose was used instead of the sugar in DCCRH. Cultivation was performed in a 2,000-ml conical flask containing 500 ml cultivation media on a rotary shaker at 30 °C and 200 rpm for 72 h.
After the sugar in DCCRH was used up (72 h later), cells were harvested and disrupted using a Constant Cell Disruption Systems Z plus 0.75 at 35 ksi (Constant System Ltd., UK). After autoclaving, the cell homogenate containing cell debris and lipids served as nitrogen source and a part of lipidic substrate for SL production by W. domercqiae.
Cultivation of W. domercqiae
W. domercqiae var. sophorolipid CGMCC 1576 was isolated by our laboratory and preserved at China General Microbiological Culture Collection Center (CGMCC). The strain was cultivated in seed medium (50 ml in a 300-ml flask) at 200 rpm at 30 °C for 16 h on a rotary shaker and then 2 % (v/v) of the seed culture was transferred to cultivation media and cultivated for 7 days at 200 rpm at 30 °C. The composition of the seed medium was as follows (grams per liter): glucose, 20; peptone, 20; and yeast extract, 10. In control group, cultivation medium contained the following ingredients (grams per liter): glucose, 80.0/60.0; yeast extract, 3.0; KH2PO4, 1.0; Na2HPO4·12H2O, 1.0; MgSO4·7H2O, 0.5; and oleic acid, 60.0 (v/v). The hydrolysate cultivation media are nondetoxified or detoxified DCCRH containing 60 ml/l hydrophobic substrate (oleic acid or SCO) and/or different nitrogen sources (1.5 g/l yeast extract or 40 ml/l cell homogenate containing cell debris and lipids). Sugar in DCCRH can substitute glucose as carbon source and the other ingredients were the same as that in the control group.
Acetic acid of different concentrations was added to the culture medium to investigate the effect of acetic acid in the hydrolysate on yeast growth and SL production of W. domercqiae, respectively. The experiments were carried out in triplicate for each selected acetic acid concentration and all results reported are the mean of three independent experimental results.
Analytical methods
Compositional analysis of DCCRH and residual glucose determination
The hydrolysate samples were analyzed by HPLC (Shimadzu, Japan) using an Aminex HPX-87H column (300 mm × 7.8 mm, Bio-Rad Laboratories, USA) at 60 °C and a refractive index (RI) detector. Contents of cellobiose, glucose, xylose, arabinose, and acetic acid were calculated according to standard curve interpolation. The mobile phase was H2O and the flow rate was 0.5 ml/min. Residual glucose concentration of the cultivation broth of C. curvatus and W. domercqiae was measured by SBA-40C biological sensor analyzer (Institute of Biology, Shandong Academy of Science, Shandong, China). The data are presented as the mean of three determinations by HPLC or SBA-40C.
Determination of biomass and lipid content of C. curvatus
C. curvatus cells were harvested by centrifugation, washed twice with distilled water, dried at 80 °C to constant weight, and the biomass was determined. Lipid content of the dried biomass was determined according to the procedure described by Li et al. (2001). That is, the cells were disrupted by 4 M HCl solution; extracted with ethanol, ether, and petroleum ether; centrifuged at 5,000 rpm and then clear supernatant containing extracted lipid (SCO) was obtained. After the solvent was removed by vacuum evaporation, the lipid was obtained. Lipid content was measured and expressed as a percentage of lipid to biomass (percent) and grams of lipid per liter of cultivation culture. For biomass and lipid content determination, 50 ml of fermentation broth was taken out in triplicate at the end of cultivation and the data were presented as the mean of three independent experimental results.
Determination of biomass and SL production of W. domercqiae
Biomass and SL production of W. domercqiae were determined according to the method described in our previous publication (Ma et al. 2011). Five milliliters of culture broth (in triplicate) of W. domercqiae was mixed with n-butanol/ethanol/chloroform (10/10/1) of equal volume and then centrifuged. After being washed twice with distilled water, the solid residue was dried and weighed. Residual glucose content was determined by the biosensor method mentioned above. The data of biomass and residual glucose were presented as the mean of three readings.
Lactonic SL production was measured by extraction of 0.5-ml cultivation broth with two volumes of ethyl acetate and, finally, using the anthrone method (Wodarczak and Buschmann 1995). For determination of total SL production, two volumes of acetonitrile were added to 0.5-ml broth to dissolve SLs followed by centrifugation. Residual glucose content in the supernatant was determined by the biosensor method mentioned above and total sugar content in the supernatant was quantified by the anthrone method. Total SL production was calculated according to the glucose standard curve with glucose content of total sugar content minus residual glucose content (Ma et al. 2011). For both lactonic and total SL production determinations, 10 μl of the samples was taken out in duplicate. After reacting with anthrone solution, 200 μl of the reaction mixtures was taken out in triplicate and determined at OD620. The data were presented as the mean of six readings.
Composition analysis of SCO and SL
Fatty acid profile of the obtained SCO was determined by directly methylating fatty acids to fatty acid methyl esters according to the method of Zhang et al. (2011). One gram of the lipid was treated in a flask with 20 ml of 0.6 M KOH–methanol solution and 20 ml hexane, mixed on a vortex for 2 min and placed at 60 °C for 30 min. Then, 50 ml distilled water was added to the mixed liquor and allowed to stratify. The fatty acid methyl esters in organic layer were subjected to fatty acid composition analysis. They were determined by GCMS-QP2010 (Shimadzu, Japan) with a RTX-5 column (30 m × 0.25 mm, 0.25-μm coating, Agilent Technologies Inc., USA). The column temperature was programmed as being increased from 80 to 280 °C at a rate of 10 °C/min and kept for 3 min. Nitrogen was used as the carrier gas. Split ratio was 1∶100 (v/v). The injector and the detector temperatures were set at 250 and 280 °C, respectively. After lipid extraction, the cell suspension was then filtered and the cell debris cake on the filter paper was dried at 105 °C to obtain SCP.
The composition of crude total SLs was analyzed by analytical HPLC (Shimadzu, Japan) with a Venusil MP-C18 column (250 × 4.6 mm, Bonna-Agela Technologies Inc., USA) and a UV detector at 207 nm. Acetonitrile/water was used as the mobile phase at an acetonitrile gradient from 40 to 60 % for 15 min followed by an acetonitrile gradient from 60 to 70 % for 35 min at a flow rate of 1.0 ml/min (Ma et al. 2011). The data of gas chromatography-mass spectrometry (GC-MS) and HPLC analytical determination were presented as the mean of three independent experimental results with the same injection volume.
Results
In our study, new cheap substrates were utilized to reduce production cost of SL, SCO, and SCP. Firstly, commercial cellulase was used in the preparation of DCCRHs. While sugar in DCCRH was almost consumed, C. curvatus cells were harvested. After cell disruption, SCO was obtained by extraction with organic solvents or cell homogenate containing both SCO and cell debris (SCP) that was obtained without lipid extraction. The resulting SCO or cell homogenate was mixed with DCCRH and used for growth and SL production of the yeast W. domercqiae. The detoxification pretreatment by adsorption using activated carbon was used to improve the fermentation quality of DCCRH by removing the inhibitors. Our idea was to use the DCCRHs, hydrolysate-originated SCO, and cell debris to replace the required hydrophilic and hydrophobic carbon sources and nitrogen source in the process of cell growth and SL production of W. domercqiae.
Composition of DCCRHs
There are different kinds of sugar and main inhibitory compound (i.e., acetic acid) in the DCCRH. DCCRH contained 60.59 g/l digestible monosaccharides including glucose (54.96 g/l), xylose (2.78 g/l), arabinose (2.85 g/l), 5.11 g/l cellobiose, and 2.23 g/l inhibitor (acetic acid). Glucose was the most abundant monosaccharide in the DCCRH and the concentration of hexose was about ten times higher than pentose after removal of the majority lignin by alkali pretreatment. Inhibitor of acetic acid was completely removed after treatment of activated carbon adsorption due to its high specific surface and numerous micropores. These results further suggested that DCCR might be considered as a good raw material for microbial oil and SL production.
SCO production from DCCRH
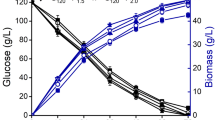
Growth of C. curvatus and production of SCO after 72-h cultivation by this microorganism were shown in Table 1. High lipid content of 56.67 % and low biomass of 8.71 g/l were obtained in synthetic medium when glucose and yeast extract were used. C. curvatus could not grow in untreated DCCRH cultivation medium because of the presence of high concentration of acetic acid. It can also be seen that pretty low biomass of 7.18 g/l was obtained from detoxified DCCRH medium without supplement of yeast extract (DCCRH-YE) due to nitrogen limitation. However, after 72-h cultivation, 17.36 g/l dry weight of biomass containing 7.92 g/l SCO was produced from DCCRH treated by activated carbon adsorption and supplement of yeast extract (DCCRH-C-YE). The lipid content of the yeast cells cultivated in detoxified DCCRH was 44.35 % of the cell dry weight and lipid yield was determined to be 0.16 g lipid/g glucose.
The fatty acid composition of the lipids was assayed by GC/MS. Oleic acid, and palmitic and stearic acids were the richest among the extracted intracellular lipids. Fatty acids with long aliphatic chain were detected. The fatty acid distribution was also compared to that of some vegetable oils including rapeseed oil and soybean oil (Table 2). It was interesting to note that the fatty acid composition of the SCO from glucose showed great differences to that obtained from DCCRH. Unsaturation and high unusual fatty acid occupation rate also appeared to occur in cultivation from DCCRH. This suggests that yeast cells grown in synthetic medium and DCCRH medium have different fatty acid compositions.
It was reported that SL production was significantly higher when two carbon sources, a glucidic and a lipidic substrate, were simultaneously provided (Davila et al. 1997). It can be concluded from the present results that the lipids produced by C. curvatus could serve as a lipidic substrate and the disrupted cells could represent the source of trace elements and vitamins for SL production. These two nutritional sources, together with the sugar in DCCRH, could comprise an optimal medium for SL production. However, it was reported that β-glycosidase and lipase activities in cell extracts of C. curvatus may cause the degradation of SLs. Therefore, autoclaving was necessary not only for sterilization but also for inactivating these enzymes (Daniel et al. 1999).
Effects of acetic acid on cell growth and SL production
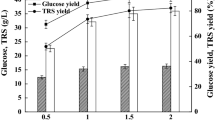
Influence of acetic acid on glucose consumption, yeast growth, and SL production was studied by adding acetic acid of different concentrations to the cultivation medium before cultivation. The results listed in Table 3 indicated that the optimum acetic acid concentration for lactonic and total SL production was 1.5 and 2.0 g/l, respectively. Lactonic SL production and proportion of lactonic SL to total SL increased with increasing concentration of acetic acid under 1.5 g/l, which demonstrated that acetic acid of appropriate concentration is more suitable for lactonic SL production. When acetic acid addition increased to 2.0 g/l, the highest total SL of 66.79 g/l was obtained. The reason was that low pH of the broth caused by acetic acid addition is more beneficial for acidic SL production. An increase of biomass and decrease of both lactonic and total SL production were obtained with an addition of 2.4 g/l acetic acid in the medium. Furthermore, glucose utilization, cell growth, and SL production were all inhibited when acetic acid concentration exceeded 2.4 g/l. W. domercqiae cells could not survive in the presence of 3.4 g/l acetic acid.
SL production from DCCRH and oleic acid
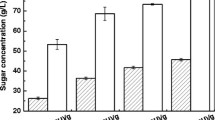
Sugars in DCCRH and oleic acid were used as complex carbon sources to produce SL by W. domercqiae (Table 4). In each case, residual glucose and SL production were determined at the end of cultivation. The results in Table 4 showed that sugars in DCCRH can substitute for glucose in the microbial production of SLs at a yield of 16.10 g/l of lactonic SL and 32.79 g/l of total SL. Besides, less acidic SL was observed when compared to the cultivation occurring in synthetic medium. DCCRH treated by activated carbon adsorption was found to increase SL production and the percentage of lactonic SL to total SL (DCCRH-C). The same results were obtained for cultivation when some glucose was added to DCCRH to lift the total glucose concentration to 60 g/l (DCCRH-Glc and DCCRH-C-Glc). It was also shown that the highest production of SLs was obtained when DCCRH-C with glucose addition and oleic acid were used as substrates. The results in Table 4 indicated that glucose addition enhanced both the production of lactonic SL and total SL. This fact can be explained by the elevation of C:N ratio of media.
SL produced from synthetic medium was crystalline and deposited at the bottom of the flask (Fig. S1a). Differently, SLs produced from DCCRH were brown particles with a diameter of around 0.5 mm, which made them easier to separate from yeast cells (Fig. S1b). Filtration with a strainer was enough for SL recovery from culture broth and cells. This formation of particle-shaped SLs may be attributed to the minute particles existing in DCCRH.
Composition analysis of the purified SLs obtained from the cultivation with DCCRH and oleic acid showed that 62 % of the SL molecules existed in lactonic form. The main SL molecules were the diacetylated lactones with fatty acid of C18:2 (6.6 %) and C18:1 (9.7 %). For cultivation on glucose and oleic acid, 7.1 and 8.7 % of the diacetylated lactones with fatty acid of C18:2 and C18:1 was produced, respectively. These results were quite different with the SLs obtained from C. bombicola, where fully 87 or 97 % of the SL molecules existed in the form of lactone (Solaiman et al. 2007, 2004). Regardless, our results indicated that W. domercqiae could utilize the sugar in DCCRHs for SL production. Furthermore, the produced SL could be collected by a fast and cheap method of filtration in downstream processing and organic solvents may be omitted.
SL production from DCCRH and SCO
SL production by W. domercqiae from sugar in DCCRH and SCO in shaking flasks was shown in Table 5. Glucose in DCCRH was almost consumed after 7-day cultivation. W. domercqiae could utilize sugar in DCCRH and SCO for cell growth and SL production. Compared to oleic acid, SCO was more suitable for acidic SL production and showed almost no difference for total SL production from that in synthetic media. The results showed that DCCRH treated by activated carbon adsorption was beneficial to both lactonic and total SL production and increased the percentage of lactonic SL to total SL, which were in agreement with the results observed in Table 4.
The composition of SLs produced from SCO was analyzed by gradient elution HPLC. The results in the present study demonstrated that lipidic substrates (different fatty acids contained in SCO or oleic acid) had hardly any influence on the composition of SL mixture but altered the proportion of each SL molecules in the mixture. For cultivation on glucose and SCO, 5.6 and 11.9 % of the diacetylated lactones with fatty acid of C18:2 and C18:1 was obtained, respectively. Nevertheless, the two main components accounted for 15.5 and 7.6 % of the crude product from 80 g/l of glucose and oleic acid. Regardless of which kinds of carbohydrate or fatty acid were used, the produced SL mixtures by W. domercqiae have the same SL molecule composition. Furthermore, W. domercqiae can, de novo, synthesize either the lipids or the sophorose moieties when glucose or oleic acid was used as sole carbon source and compositions of the SL mixtures produced from glucose or oleic acid were the same as the SL mixture from complex carbon source.
SL production from DCCRH and cell homogenate
In the present study, we investigated the complete omission of glucose and yeast extract in the cultivation broth to further reduce the material cost of SL production. We hypothesized that the nitrogenous substances such as the proteinaceous materials (5.2 % w/v) in the cell homogenate could serve as the nitrogen source for the fermentative production of SLs by W. domercqiae. Our results showed that when cell homogenate alone was used as combined nitrogen and lipidic carbon sources in conjunction with glucose as hydrophilic cosubstrate, acidic SL was predominantly obtained and only 2.79 and 21.75 g/l of lactonic and total SL were produced because of lipidic carbon source limitation (SM-CH in Table 6). However, when commonly used nitrogen and complex carbon sources in SL production were replaced by cell homogenate and DCCRH combined with oleic acid, lactonic and total SL production by W. domercqiae reached 13.23 and 37.19 g/l, respectively. Detoxification of DCCRH was found to improve both lactonic and total SL production (Table 6). In comparison with the SL yield obtained from cultivation with glucose, yeast extract, and oleic acid, the lactonic and total SL production by using DCCRH-C reached 137 and 116 %. These results demonstrated the feasibility of lowering SL production cost by using DCCRH and C. curvatus cell homogenate as a combined carbon and nitrogen source.
Discussion
Biosurfactant of SLs is scarcely able to compete with established chemical surfactants for their relatively high price. To expand the range of applications of SL, cheap fermentative feedstock development and SL molecules with different structures specifically applied in different fields have attracted much attention (Van Bogaert et al. 2011 ). There are various factors influencing SL yield and composition, e.g., C:N ratio, nature of precursors, organic nitrogen source addition, pH value, oxygen supply, and temperature (Stüwer et al. 1987). Influences of hydrophilic carbon source, hydrophobic carbon source, and nitrogen source on SL production are mostly reported (Ma et al. 2011). Relatively poor information is available about full use of low-cost lignocellulosic material on SL production and composition. In the present study, the whole process includes three stages: DCCRH preparation by cellulase, SCO and SCP production by oleaginous yeast of C. curvatus, and SL production by SL-producing yeast of W. domercqiae.
In the previous studies, different substrates and cultivation conditions have been used to maximize lipid production of C. curvatus. Evans and Ratledge (1983) studied growth of C. curvatus on glucose, sucrose, lactose, ethanol, and xylose in continuous fermentation and lipid contents of 29, 28, 31, 37, and 35 % were obtained, respectively. Higher lipid contents of 49.7 and 68.9 % were reported by Iassonova et al. (2008) in media containing fish oil and clarinol. The results described in this study demonstrated that lipid production from sugar in DCCRH was considerable for large-scale production. Further improvement of the biomass and cellular lipid content could be accomplished by optimizing the cultivation process and medium composition.
Daniel et al. (1999) managed to fully use the deproteinized whey as a substrate for SL production by a two-stage cultivation process with C. bombicola and C. curvatus. SL production of 12 g/l was gained when 10 g/l SCO and 80 g/l glucose were used. Although 422 g/l of SLs was obtained from 20 g/l SCO and 400 g/l rapeseed oil by an optimized two-stage fed-batch process, long cultivation time of 145 h for C. curvatus and 410 h for C. bombicola was needed. Also, low-cost soy molasses can act as glucose substitute with C. bombicola, but again, lower yields were observed (Solaiman et al. 2004). Different from the previous reports, SL production from W. domercqiae was demonstrated using DCCR as low-cost raw material. Furthermore, detoxification pretreatment of activated carbon adsorption greatly enhanced the fermentability of sugar in DCCRH. Both cell growth and oil accumulation in C. curvatus biomass and SL production reached high levels.
Both results of Davila et al. (1994) and Otto et al. (1999) showed that fatty acid composition of the lipidic substrate and hydrophilic carbon source had a distinct influence on the composition of the crude SL mixture. However, the results in the present study demonstrated that lipidic substrates (different fatty acids contained in SCO or oleic acid) and hydrophilic carbon source (DCCRH or glucose) had hardly any influence on the composition of SL mixture but altered the proportion of each SL molecules in the mixture.
No information on the utilization of the sugar in DCCRH for SL production was available in the previous literatures. The present study provides some results concerning the feasibility of using the low-value DCCRH for the production of SLs by W. domercqiae, which provide more useful information for the selection of the most common industrial raw materials for SL production. For economic reasons, future work should concentrate on the optimization of SCO production and obtainment of higher yield of SLs. In the process of C. curvatus cultivation, cells should not only grow fast and accumulate large quantities of oil, but the cultivation should be able to be performed at low cost and within a short time. For either the process of W. domercqiae growth or SL production from DCCRH, SCO, or cell homogenate, the cost and amount of oil were the limiting factors. This problem could probably be overcome by the addition of cheap oils to DCCRH media, thus further lowering cost of SL production.
References
Daniel HJ, Reuss M, Syldatk C (1998) Production of sophorolipids in high concentration from deproteinized whey and rapeseed oil in a two stage fed batch process using Candida bombicola ATCC 22214 and Cryptococcus curvatus ATCC 20509. Biotechnol Lett 20:1153–1156
Daniel HJ, Otto R, Binder M, Reuss M, Syldatk C (1999) Production of sophorolipids from whey: development of a two-stage process with Cryptococcus curvatus ATCC 20509 and Candida bombicola ATCC 22214 using deproteinized whey concentrates as substrates. Appl Microbiol Biotechnol 51:40–45
Davila AM, Marchal R, Vandecasteele JP (1994) Sophorose lipid production from lipidic precursors: predictive evaluation of industrial substrates. J Ind Microbiol Biotechnol 13:249–257
Davila AM, Marchal R, Vandecasteele JP (1997) Sophorose lipid fermentation with differentiated substrate supply for growth and production phases. Appl Microbiol Biotechnol 47:496–501
Evans CT, Ratledge C (1983) A comparison of the oleaginous yeast, Candida curvata, grown on different carbon sources in continuous and batch culture. Lipids 18:623–629
Fakas S, Papanikolaou S, Batsos A, Galiotou-Panayotou M, Mallouchos A, Aggelis G (2009) Evaluating renewable carbon sources as substrates for single cell oil production by Cunninghamella echinulata and Mortierella isabellina. Biomass Bioenergy 33:573–580
Ghose T (1987) Measurement of cellulase activities. Pure Appl Chem 59:257–268
Iassonova DR, Hammond EG, Beattie SE (2008) Oxidative stability of polyunsaturated triacylglycerols encapsulated in oleaginous yeast. J Am Oil Chem Soc 85:711–716
Kim YB, Yun HS, Kim EK (2009) Enhanced sophorolipid production by feeding-rate-controlled fed-batch culture. Bioresour Technol 100:6028–6032
Lee YJ, Choi JK, Kim EK, Youn SH, Yang EJ (2008) Field experiments on mitigation of harmful algal blooms using a sophorolipid–yellow clay mixture and effects on marine plankton. Harmful Algae 7:154–162
Li ZF, Shen XJ, Lai BS, Sun SQ (2001) A comparative study on four method of fungi lipid extraction. Microbiology 28:72–75
Liu K, Lin X, Yue J, Li X, Fang X, Zhu M, Lin J, Qu Y, Xiao L (2010) High concentration ethanol production from corncob residues by fed-batch strategy. Bioresour Technol 101:4952–4958
Ma XJ, Li H, Shao LJ, Shen J, Song X (2011) Effects of nitrogen sources on production and composition of sophorolipids by Wickerhamiella domercqiae var. sophorolipid CGMCC 1576. Appl Microbiol Biotechnol 91:1623–1632
Mann RM, Boddy MR (2000) Biodegradation of a nonylphenol ethoxylate by the autochthonous microflora in lake water with observations on the influence of light. Chemosphere 41:1361–1369
Otto RT, Daniel HJ, Pekin G, Muller DK, Furstenberger G, Reuss M, Syldatk C (1999) Production of sophorolipids from whey. II. Product composition, surface active properties, cytotoxicity and stability against hydrolases by enzymatic treatment. Appl Microbiol Biotechnol 52:495–501
Rau U, Hammen S, Heckmann R, Wray V, Lang S (2001) Sophorolipids: a source for novel compounds. Ind Crop Prod 13:85–92
Solaiman DKY, Ashby RD, Nunez A, Foglia TA (2004) Production of sophorolipids by Candida bombicola grown on soy molasses as substrate. Biotechnol Lett 26:1241–1245
Solaiman DK, Ashby RD, Zerkowski JA, Foglia TA (2007) Simplified soy molasses-based medium for reduced-cost production of sophorolipids by Candida bombicola. Biotechnol Lett 29:1341–1347
Stüwer O, Hommel R, Haferburg D, Kleber HP (1987) Production of crystalline surface-active glycolipids by a strain of Torulopsis apicola. J Biotechnol 6:259–269
Van Bogaert IN, Roelants S, Develter D, Soetaert W (2010) Sophorolipid production by Candida bombicola on oils with a special fatty acid composition and their consequences on cell viability. Biotechnol Lett 32:1509–1514
Van Bogaert INA, Zhang J, Soetaert W (2011) Microbial synthesis of sophorolipids. Process Biochem 46:821–833
Vrati S (1984) Single cell protein production by photosynthetic bacteria grown on the clarified effluents of biogas plant. Appl Microbiol Biotechnol 19:199–202
Wadekar SD, Kale SB, Lali AM, Bhowmick DN, Pratap AP (2012) Utilization of sweetwater as a cost-effective carbon source for sophorolipids production by Starmerella bombicola (ATCC 22214). Prep Biochem Biotechnol 42:125–142
Wodarczak S, Buschmann N (1995) Analytical methods for alkylpolyglucosides. GIT Lab Fachz 5:410–411
Zhang J, Fang X, Zhu XL, Li Y, Xu HP, Zhao BF, Chen L, Zhang XD (2011) Microbial lipid production by the oleaginous yeast Cryptococcus curvatus O3 grown in fed-batch culture. Biomass Bioenergy 35:1906–1911
Acknowledgments
This study was funded by the Key Scientific and Technological project of Shandong Province (2007GG10002002), the National Natural Science Foundation of China (no. 30870048 and no. 30970052), and the National Key Technology R&D Program (no. 2008BAI63B08) and the National Key Technology R&D Program (2011BAC02B04).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 4116 kb)
Rights and permissions
About this article
Cite this article
Ma, Xj., Li, H., Wang, Dx. et al. Sophorolipid production from delignined corncob residue by Wickerhamiella domercqiae var. sophorolipid CGMCC 1576 and Cryptococcus curvatus ATCC 96219. Appl Microbiol Biotechnol 98, 475–483 (2014). https://doi.org/10.1007/s00253-013-4856-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-013-4856-3