Abstract
Cadaverine (1,5-pentanediamine, diaminopentane), the desired raw material of bio-polyamides, is an important industrial chemical with a wide range of applications. Biosynthesis of cadaverine in Corynebacterium glutamicum has been a competitive way in place of petroleum-based chemical synthesis method. To date, the cadaverine exporter has not been found in C. glutamicum. In order to improve cadaverine secretion, the cadaverine–lysine antiporter CadB from Escherichia coli was studied in C. glutamicum. Fusion expression of cadB and green fluorescent protein (GFP) gene confirmed that CadB could express in the cell membrane of C. glutamicum. Co-expression of cadB and ldc from Hafnia alvei in C. glutamicum showed that the cadaverine secretion rate increased by 22 % and the yield of total cadaverine and extracellular cadaverine increased by 30 and 73 %, respectively. Moreover, the recombinant strain cultured at acid and neutral pH separately hardly had any difference in cadaverine concentrations. These results suggested that CadB could be expressed in the cell membrane of C. glutamicum and that recombinant CadB could improve cadaverine secretion and the yield of cadaverine. Moreover, the pH value did not affect the function of recombinant CadB. These results may be a promising metabolic engineering strategy for improving the yield of the desired product by enhancing its export out of the cell.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cadaverine, also known as 1,5-pentanediamine, is a foul-smelling compound produced by protein hydrolysis during putrefaction of animal tissue [16]. Cadaverine is a kind of polyamine existing in a wide range of living beings with different kinds of bioactivity [7]. Cadaverine also has a wide range of applications in aspects of agriculture, medicine, and industry. Firstly, in agriculture, cadaverine can promote the growth of fruits and improve their production yields [4]. Secondly, in medicine, cadaverine could be clinically used as an efficient anti-diarrheal drug [3]. Last, and most importantly, it is also an important platform chemical with many industrial applications, especially serving as an important raw material for synthesis of novel nylon, polyamides54 (PA54), and polyamides56 (PA56). Polyamide is one of the most desirable engineered plastics, the production of which was in the first of the five engineered plastics, accounting for one-third of the global market. It has been widely used in aeronautical and space technologies, automobile components, machinery parts, electronic apparatuses, packing materials, cementing compounds, and cosmetics, for its high mechanical strength, high melting point, and high endurance for different organic solvents [15].
In vivo, cadaverine is formed directly from lysine by catalysis of lysine decarboxylase and the regulation mechanism on biosynthesis of cadaverine in Escherichia coli has been clarified [12, 23, 24]. In E. coli, there are two kinds of lysine decarboxylase, LdcC and CadA. There is another lysine decarboxylase (LDC) gene from Hafnia alvei that is cloned in the Escherichia coli strain HB101 [5]. LdcC is constitutively expressed and its expression from its natural promoter is very weak for cells growing in different media, but it is active over a broad pH range with an optimum pH 7.6 [18]. However, CadA is an inducible enzyme and prefers acidic conditions with an optimum pH of 5.5 [11]. The cadA gene encoding lysine decarboxylase and cadB gene encoding a cadaverine–lysine antiporter protein form the structural genes of cad operon, while cadC acts as a regulating gene. In effect, these proteins contribute to acid stress adaptation in E. coli. The expression of cadA and cadB is induced when acidic stress occurs in a lysine-rich environment, and CadA converts lysine into cadaverine, CadB imports the substrate lysine and exports the product cadaverine. At neutral pH, cadA and cadB almost do not express, although CadB can uptake cadaverine dependent on proton motive force [14], and Tyr73, Tyr89, Tyr90, Glu204, Tyr235, Asp303, and Tyr423 are strongly involved in both uptake and excretion [21]. The structure and function of a cadaverine–lysine antiporter CadB and a putrescine–ornithine antiporter PotE in E. coli were evaluated using model structures based on the crystal structure of AdiC, an agmatine–arginine antiporter, and the activities of various CadB and PotE mutants. The central cavity of CadB, containing the substrate binding site, was wider than that of PotE, mirroring the different sizes of cadaverine and putrescine [22]. Binding of cadaverine within the pair of central cavities in CadC deactivates the pH sensor CadC, which overlaps with the pH-responsive patch of amino acids located at the dimer interface of the periplasmic domain [6].
Biotechnological production of cadaverine from renewable feedstock is a promising alternative to the chemical synthesis that originates from non-renewable petroleum. Many engineered C. glutamicum strains producing cadaverine have been constructed by cloning lysine decarboxylase gene such as cadA [13] or ldcC [10] into them because C. glutamicum has been used in the production of lysine and can produce more than 1,000,000 metric tons of lysine per year [8]. In order to improve the yields of cadaverine, systems-wide metabolic engineering of the biosynthetic pathway was performed by supporting reactions [8] and eliminating the undesired secretion of lysine as well as the competing pathway towards N-acetyl-diaminopentane [9]. Cadaverine was also biosynthesized in engineered C. glutamicums using xylose [1, 2] or soluble starch as carbon source [17]. However, the yield of cadaverine was still rather low in the engineered C. glutamicum strains because only part of cadaverine was secreted and part of cadaverine remained in the cells, thereby inhibiting the activity of the lysine decarboxylase [10]. This research demonstrated that cadaverine export was a potential bottleneck affecting the yield of cadaverine. Therefore, it is very necessary to increase the export of cadaverine. Previous research showed that the proposed lysine exporter LysE was not involved in cadaverine export, although cadaverine and lysine have a similar structure [8]. The deletion of the cg2893 gene, encoding a permease in C. glutamicum reduced cadaverine secretion, but the exact function of the permease remained unclear [8]. The toxic effects seem to be relevant for few polyamines in general, as recent publications concerning cadaverine production by C. glutamicum is 3.4 g l−1 at the level of shake flask [20]. Cadaverine–lysine antiporter CadB, a membrane protein, whose physiological functions are already studied in E. coli, is responsible for the secretion of cadaverine in E. coli [19]. Therefore, the function of CadB from E. coli was studied in C. glutamicum.
In this work, in order to identify the functions of CadB, the engineered C. glutamicum strains were constructed by cloning cadB into C. glutamicum. The results showed that cadaverine secretion rate and the yield of extracellular cadaverine greatly increased in the engineered C. glutamicum strain containing a cadB (GenBank and accession number 388476123) gene from E. coli and the ldc (GenBank and accession number 43438) gene from Hafnia alvei [12], compared with that only containing ldc, confirming that recombinant CadB in C. glutamicum can improve cadaverine secretion and the yield of cadaverine. Moreover, the pH value does not affect the function of recombinant CadB.
Materials and methods
Strains and growth conditions
The bacterial strains used in this study are listed in Table 1.
E. coli DH5α and JM110 were used for general gene cloning studies. During plasmid construction, E. coli strains were grown at 37 °C in Luria–Bertani (LB) medium (10 g l−1 tryptone, 5 g l−1 yeast extract, 5 g l−1 sodium chloride), which was supplemented with 100 μg ml−1 ampicillin (Amp) or with 20 μg ml−1 chloramphenicol (Cm) when required for selection. E. coli K12 and Hafnia alvei AS 1.1009 were used for cloning cadB and ldc, respectively, and were cultivated in LB at 37 °C and 30 °C, respectively. C. glutamicum ATCC 13,032 was used as a host strain and was cultivated in LBG medium (LB supplemented with glucose 5 g l−1) at 30 °C. When required, chloramphenicol was added additionally (10 μg ml−1).
Construction of plasmids and engineered strains
The plasmids and engineered strains used in this study are listed in Table 1. The PCR primers which are listed in Table 2 for plasmid construction were designed by primer premier 5.0. Pyrobest DNA polymerase, restriction enzymes were purchased from TaKaRa and T4 DNA ligase was purchased from Promega. The gene sequences cloned were confirmed by sequencing at Sangon Biotech.
The lysine decarboxylase gene (ldc, 2,220 bp) from Hafnia alvei genomic DNA was amplified by PCR using primers ldcF and ldcR. The PCR fragments were cloned into pGEM-T Vector, resulting in plasmid pT-ldc, and then sequenced. The ldc gene was digested with Hind III and BamH I from pT-ldc, cloned into the expression vector pXMJ19 to construct plasmid pXMJ19-ldc (8,836 bp).
The cadaverine–lysine antiporter gene (cadB, 1,335 bp) from E. coli K12 genomic DNA was amplified by PCR using primers cadBF and cadBR. The PCR fragments were cloned into pGEM-T Vector, resulting in plasmid pT-cadB, and then sequenced. The cadB gene was digested with Hind III and BamH I from pT-cadB, cloned into the expression vector pXMJ19 to construct plasmid pXMJ19-cadB (7,936 bp).
The green fluorescent protein gene (gfp, 717 bp) from plasmid pMUTIN-GFP (preserved in our laboratory) was amplified by PCR using primers gfpF1/gfpF2 and gfpR. The PCR fragments were cloned into pGEM-T Vector, resulting in plasmid pT-gfp1/pT-gfp2, and then sequenced. The fragment digested with Hind III and EcoR I from pT-gfp1 was inserted between Hind III and EcoR I sites to construct plasmid pXMJ19-gfp. The gfp gene digested with Kpn I and EcoR I from pT-gfp was cloned into the expression vector pXMJ19-cadB digested with the same enzymes to construct plasmid pXBG (8,553 bp) by fusing it to C-terminus of cadB.
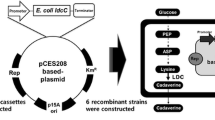
The co-expression vector pXLB was constructed according to the scheme shown in Fig. 1. Firstly, a terminator sequence (466 bp) was amplified from plasmid pXMJ19 by using primers terminatorsF and terminatorsR and inserted between Xba I and BamH I of pXMJ19 to generate plasmid pXMJ19-terminators (7,067 bp). Secondly, a tac-cadB gene (1,416 bp) was amplified from plasmid pXMJ19-cadB by using primers tac-cadBF and tac-cadBR. The PCR product was digested with BamH I and Sma I, and ligated into pXMJ19-terminators digested by the same enzymes to generate the recombinant plasmid pXTTB (8,482 bp). Finally, the DNA fragment (1,882 bp) containing terminators-Ptac-cadB gene digested with XbaI-SmaI from pXTTB was inserted into the XbaI-SmaI site of pXMJ19-ldc, and the obtained plasmid was designated pXLB (10,718 bp).
The recombinant plasmids pXMJ19-ldc, pXMJ19-gfp, pXBG, and pXLB isolated from E. coli JM110 were transformed to C. glutamicum ATCC13032 by electroporation [18]. All transformants were identified by digestion and/or PCR of the plasmid extracted from transformants with appropriate restriction enzymes and primers. The identified engineered strains containing plasmid pXMJ19-ldc, pXMJ19-gfp, pXBG, and pXLB were named, respectively, CDV-1, CgGFP, BG, and CDV-2.
Culturing of the engineered C. glutamicum strains
The engineered C. glutamicum strain was inoculated into 5 ml of LBG medium containing chloramphenicol (10 μg ml−1) and grown overnight at 30 °C in a rotary shaker (200 r min−1). One milliliter of the overnight culture was transferred subsequently into 100 ml of LBG medium containing chloramphenicol (10 μg ml−1) at 30 °C in a 500-ml flask (200 r min−1). When cell density reached OD600 of 0.6–0.8, IPTG (0.1 mmol l−1) was added to induce the expression of protein and cultivation was continued at 30 °C in rotary flasks (200 r min−1) for 36 h.
Lysine decarboxylase activity assay
The engineered C. glutamicum strain CDV-1 and CDV-2 induced with IPTG for 36 h were harvested by centrifugation at 10,000×g for 5 min at 4 °C, washed twice with phosphate-buffered saline (PBS, pH 7.2), and sonicated using an ultrasonic cell disruptor in an ice water bath for 30 min (working time, 3 s, cooling interval time, 9 s), controls for levels of Ldc activities were also detected by the corresponding strains not being induced. Subsequently, the supernatant was used to determine the activity of lysine decarboxylase as Kind described previously [6]. One unit of l-lysine decarboxylase activity was defined as the amount of enzyme that formed 1 μmol of cadaverine per min at 30 °C. The value is the mean of triplicates from three independent experiments.
Polyacrylamide gel analysis and fluorescence microscopy analysis
The engineered C. glutamicum strain BG and the control C. glutamicum strain CgGFP induced with IPTG for 36 h were collected by centrifugation at 4,000×g at 4 °C for 10 min and washed with phosphate-buffered saline (PBS, pH 7.2) three times. The cells were then resuspended and used as samples to be loaded in a polyacrylamide gel. In addition, the samples were observed by fluorescence microscope (OLYMPUS, Japan).
Determination of cadaverine and l-lysine
Cadaverine and l-lysine concentrations were determined by reverse-phase high-performance liquid chromatography (HPLC) after derivatization with 2,4-dinitrofluorobenzene. The specific steps were based on the method described by Tateno [17]. Chromatographic conditions conclude as follows: (a) Chromatographic column: C18 or performance is quite; (b) Column temperature: room temperature; (c) The mobile phase: A (acetonitrile), B (0.02 mol l−1 ammonium acetate). The detection wavelength is 254 nm. The injection volume is 20 μl. Each value is the mean ± SD of triplicates from three independent experiments.
Results
Construction of expression vectors and engineered strains
According to construction of plasmids and engineered strains in the part of materials and methods, expression vectors pXMJ19-ldc, pXMJ19-gfp, pXBG, and pXLB were successfully constructed by cloning the corresponding gene to the E. coli-C. glutamicum shuttle vector pXMJ19, which contains inducible promoter Ptac. The ldc gene in pXMJ19-ldc was from H. alvei and was 2,220 bp in length, which encodes a lysine decarboxylase containing 739 amino acids. pXBG contained the cadB gene from E. coli which was fused to N-terminus of gfp gene and encodes a cadaverine-lysine antiporter containing 444 amino acids. The sequences of ldc, cadB, and gfp genes cloned were confirmed by sequencing and shared 100 % identity with the reported gene sequences in NCBI databases. pXLB was a co-expression vector containing ldc and cadB genes (Fig. 1).
C. glutamicum ATCC13032, the wild-type strain, was used as the original strain to construct engineered C. glutamicum producing cadaverine and CadB-GFP fusion protein, respectively. The engineered strains CDV-1, CgGFP, BG, and CDV-2 were successfully obtained by transforming pXMJ19-ldc, pXMJ19-gfp, pXBG, and pXLB to C. glutamicum ATCC13032, respectively, and screening. By induction of IPTG, CDV-1 can express LDC, CgGFP can express GFP, BG can express CadB-GFP fusion protein, whereas CDV-2 can simultaneously express LDC and CadB.
Biosynthesis of cadaverine in CDV-1
To produce cadaverine, the engineered CDV-1 harboring pXMJ19-ldc was cultured in the LBG medium supplemented with 0.1 mmol l−1 IPTG, and the engineered strain harboring pXMJ19 acted as a control strain. The CDV-1 strain produced 2.12 g l−1 of cadaverine and excreted 1.11 g l−1 of cadaverine into the culture medium with the depletion of glucose, whereas the control strain did not produce cadaverine These results showed that LDC from H. alvei expressed in C. glutamicum and could convert lysine to cadaverine. However, the yield of cadaverine was still low. In order to find the reason for this, activity of LDC and the concentration of lysine were detected. In CDV-1, activity of LDC was about 36,000 U l−1 broth, and the concentration of residual lysine was 1.23 g l−1 broth, whereas in the control strain, there was no activity of LDC. The yield of lysine was 3.72 g l−1 of broth, which showed that a large number of lysines were not converted to cadaverine by LDC. Theoretically, as for those enzyme activities, it should also be possible to convert l-lysine to cadaverine completely. So, a possible reason was that the cadaverine present in CDV-1 inhibited activity of LDC. In CDV-1, the secretion efficiency of cadaverine was about 52.4 %, and so 47.6 % of the cadaverine still remained in vivo. When the induced CDV-1 (which was washed by phosphate buffered saline) was broken, about 85.6 % of residual lysine was converted to cadaverine, confirming that high concentrations of cadaverine in vivo might cause a decrease in l-lysine decarboxylase activity with competitive inhibition. Therefore, in addition to improving the yield of lysine, increasing the secretion efficiency of cadaverine is also an effective way of removing the inhibition of cadaverine on the LDC activity and improving the yield of cadaverine.
Expression of CadB in BG
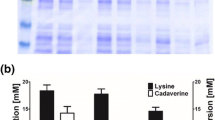
To improve the secretion of cadaverine in C. glutamicum, research on cadaverine transporter proteins is necessary. To date, the specific transporter protein has not been found in C. glutamicum. CadB, a membrane protein, is responsible for the secretion of cadaverine in E. coli. So, the expression of the cadB gene in C. glutamicum ATCC13032 was first researched. To confirm the exhibition of CadB on the cell membrane, the engineered BG harboring pXBG was constructed and the expression of fusion protein CadB-GFP was identified under a fluorescence microscope. As a control, the engineered CgGFP harboring pXMJ19-gfp was also constructed. As is shown in Fig. 2, polyacrylamide gel analysis showed that BG could express CadB-GFP of about 76 kD and the controls could express GFP of about 27 kD in CgGFP. In addition, the engineered BG with the green fluorescence in the cell surface was observed under a fluorescence microscope, whereas the engineered GFP, a control strain, was not observed (Fig. 3), which indicated that CadB could locate on the cell membrane of C. glutamicum. The result laid the foundation of researching the function of CadB in C. glutamicum.
Biosynthesis of cadaverine in CDV-2
In order to improve the secretion of cadaverine and relieve the competitive inhibition of cadaverine on LDC activity, the engineered CDV-2 co-expressing LDC and CadB was constructed. In CDV-2, activity of LDC was about 42,000 U l−1 broth, compared to CDV-1 in which the activity was about 36,000 U l−1. As shown in Fig. 4, when LDC and CadB co-expressed in CDV-2, the yield of total cadaverine reached 2.75 g l−1 and increased by 29.7 %, compared with the yield of total cadaverine in CDV-1. More importantly, the extracellular cadaverine reached 1.99 g l−1 and accounted for 72.4 % of total cadaverine, so the secretion efficiency of cadaverine in CDV-2 was improved by about 20 %, compared with that in CDV-1, whereas the concentration of residual lysine was 0.75 g l−1 in CDV-2, which dropped about 37.5 % in CDV-1. These results confirmed that the recombinant cadaverine-lysine antiporter CadB in C. glutamicum not only can transport intracellular cadaverine to culture medium but can also improve the yields of total cadaverine and extracelluar cadaverine by decreasing the accumulation of cadaverine in vivo, which relieves the inhibition of cadaverine on the LDC activity.
Influence of pH on function of CadB
In E. coli, the function of CadB was impacted by pH. At acidic condition, CadB functions as a cadaverine–lysine antiporter, whereas at neutral condition CadB acts as a cadaverine and proton symporter. In order to analyze function of recombinant CadB in C. glutamicum, the engineered CDV-2 was induced at pH 5.5 and pH 7.0, respectively, and the yields of total and extracelluar cadaverine were detected. As is shown in Fig. 5, at pH 5.5 and pH 7.0 conditions, the yields of total and extracelluar cadaverine as well as the secretion of cadaverine were almost the same. That is to say, they had no statistically significant difference at different pH conditions. Therefore, pH does not affect the function of recombinant CadB, which transport cadaverine in C. glutamicum.
Discussion
Polyamides have an annual global market of 3,500,000 tons and are completely derived from petroleum chemistry, meaning that the biotechnological platforms producing these polymers possess enormous ecological and economical potential. Cadaverine is an important building block for synthesizing bio-based polyamides, especially innovative polyamide 54 (PA54) and polyamide 56 (PA56), which are polymerized from succinic acid/adipic acid and cadaverine. Because succinic acid produced by microbial fermentation has been well evolved, it is crucial to produce cadaverine to polymerize to nylon. As a production host, it appeared highly desirable that C. glutamicum overproducing lysine is the precursor of the synthesis of cadaverine. Therefore, the production of cadaverine by C. glutamicum from renewable biomass is one of the most promising biotechnological developments as an alternative of fossil resources.
In order to produce cadaverine by C. glutamicum, we cloned ldc from Hafnia alvei into this organism. Data showed that in the CDV-1 stain, the total cadaverine and extracellular cadaverine concentrations were 2.12 and 1.11 g l−1, separately, and the cadaverine secretion rate was 52.4 %. Moreover, activity of LDC reached 36,000 U l−1. Theoretically, LDC expressed in the CDV-1 stain should convert l-lysine to cadaverine completely, however, 1.23 g l−1 of the lysine remained. Therefore, it was possible that high concentrations of cadaverine (47.6 %) in vivo inhibited the activity of LDC. These results are consistent with Mimitsuka’s report [13]. In order to relieve the competitive inhibition of high concentrations of cadaverine in vivo, the induced CDV-2 was broken and both cadaverine and LDC were released from the CDV-2. About 85.6 % of residual lysine was converted to cadaverine, confirming that the improvement of secretion of cadaverine can relieve the competitive inhibition and improve the yield of cadaverine. Kind [8, 9] performed systems-wide metabolic engineering of the synthetic pathway of cadaverine, in addition to improving secretion of cadaverine; the yield was also low. Therefore, the excretion of cadaverine is a key bottleneck for improving the production of cadaverine, and the conclusions are consistent with other reports [15, 17].
In C. glutamicum, cadaverine export is unclear. The previously proposed lysine exporter lysE was shown not to be involved in cadaverine export. The deletion of the cg2893 gene, encoding a permease in C. glutamicum, reduced cadaverine secretion, but the exact function of the permase remained unclear [10]. The transmembrane protein CadB, which has both cadaverine uptake activity and excretion activity, is responsible for the secretion of cadaverine in E. coli [17]. In order to research the function of CadB in C. glutamicum, CadB was first fused to the N-terminus of GFP and the expression of CadB-GFP fusion protein was detected. Green fluorescence was observed in the cell surface showed that CadB could express and might locate on the cell membrane of C. glutamicum.
With the purpose of transporting intracellular cadaverine to extracellular, cadB was cloned into C. glutamicum and co-expressed with ldc from Hafnia alvei. When compared with CDV-1, secretion efficiency of cadaverine improved 20 %, moreover, the yield of total cadaverine and extracellular cadaverine also improved 29.7 and 79.3 %, respectively. In contrast, the residual lysine decreased by 37.5 %. The results showed that the recombinant CadB in C. glutamicum could export cadaverine and enhance product export out of the cell. Therefore, it is an effective way of improving the secretion of cadaverine by enhancing the expression of CadB to improve the yield of cadaverine and conversion of substrate lysine to cadaverine in the engineered C. glutamicum producing cadaverine. Moreover, it is easier to purify extracellular cadaverine than intracellular cadaverine when the cadaverine in the engineering strain secrets into the culture medium.
In the engineered CDV-2 strain, the influence of pH on the function of cadaverine was detected. Results showed that there was no statistically significant difference in the yield of cadaverine and secretion efficiency of the cadaverine at pH 5.5 and pH 7.0 conditions, confirming that pH does not affect the function that CadB export cadaverine in C. glutamicum. This is where the difference from the function of CadB in E. coli is. In E. coli, CadB had both cadaverine uptake activity (dependent on proton motive force), and cadaverine excretion activity (acting as a cadaverine-lysine antiporter). In neutral conditions, CadB functions as a cadaverine and proton symporter, and cell growth was stimulated by cadaverine. However, expression of the cadBA operon was low at neutral pH. Consequently, the physiological effect of CadB at neutral pH is thought to be small. In acidic conditions, expression of the cadBA operon is induced, then CadA and CadB are synthesized, CadB functions as an electrogenic cadaverine-lysine antiporter, which benefits bacterial cell growth, and pH in the medium is increased by secretion of cadaverine. The fact that there was no CadC co-expressing could be a reason why the CadB showed a pH-independent behavior. However, the reason why pH does not affect the function of CadB in C. glutamicum needs to be further studied.
In conclusion, recombinant CadB can increase the secretion of cadaverine and improve the yield of cadaverine in C. glutamicum, and the function that recombinant CadB export cadaverine is not affected by pH. This provides an effective way of improving the yield of cadaverine by enhancing the synthesis of CadB in engineered C. glutamicum producing cadaverine.
In the future, the possible transporter of cadaverine, including CadB and Cg2893, should be further identified and compared so that the better transporter could exhibit its excretory ability and improve the yield of cadaverine in C. glutamicum. Then what can be considered is to combine with the systems-wide metabolic pathway engineering in C. glutamicum for bio-based production of cadaverine.
References
Buschke N, Becker J, Wittmann C et al (2013) Systems metabolic engineering of xylose-utilizing Corynebacterium glutamicum for production of 1, 5-diaminopentane. Biotechnol J 8:557–570
Buschke N, Schroder H, Wittmann C (2011) Metabolic engineering of Corynebacterium glutamicum for production of 1,5-diaminopentane from hemicellulose. Biotechnol J 6:306–317
Cassan F, Maiale S, Masciarelli O et al (2009) Cadaverine production by Azospirillum brasilense and its possible role in plant growth promotion and osmotic stress mitigation. Eur J Soil Biol 45:12–19
Casalino M, Latella MC, Prosseda G et al (2005) Molecular evolution of the lysine decarboxylase defective phenotype in Shigella sonnei. Int J Med Microbiol 294:503–512
Fecker LF, Beier H, Berlin J (1986) Cloning and characterization of a lysine decarboxylase gene from Hafnia alvei. Mol Gen Genet MGG 203(1):177–184
Haneburger I, Fritz G, Jurkschat N et al (2012) Deactivation of the E. coli pH stress sensor CadC by cadaverine. J Mol Biol 424(1–2):15–27
Igarashi K, Kashiwagi K (2010) Modulation of cellular function by polyamines. Int J Biochem Cell B 42:39–51
Kind S, Jeong WK, Schroder H et al (2010) Systems-wide metabolic pathway engineering in Corynebacterium glutamicum for bio-based production of diaminopentane. Metab Eng 12:341–351
Kind S, Jeong WK, Schröder H et al (2010) Identification and elimination of the competing N-acetyl-diaminopentane pathway for improved production of diaminopentane by Corynebacterium glutamicum. Appl Environ Microbiol 76:5175–5180
Kind S, Kreye S, Wittmann C (2011) Metabolic engineering of cellular transport for overproduction of the platform chemical 1,5-diaminopentane in Corynebacterium glutamicum. Metab Eng 13:617–627
Lemonnier M, Lane D (1998) Expression of the second lysine decarboxylase gene of Escherichia coli. Microbiology 144:751–760
Meng SY, Bennett GN (1992) Nucleotide sequence of the Escherichia coli cad operon: a system for neutralization of low extracellular pH. J Bacteriol 174:2659–2669
Mimitsuka T, Sawai H, Hatsu M, Yamada K (2007) Metabolic engineering of Corynebacterium glutamicum for cadaverine fermentation. Biosci Biotechnol Biochem 71:2130–2135
Neely MN, Olson ER (1996) Kinetics of expression of the Escherichia coli cad operon as a function of pH and lysine. J Bacteriol 178:5522–5528
Qian ZG, Xia XX, Lee SY (2011) Metabolic engineering of Escherichia coli for the production of cadaverine: a five-carbon diamine. Biotechnol Bioeng 108:93–103
Tabor CW, Tabor H (1985) Polyamines in microorganisms. Microbiol Rev 49:81–99
Tateno T, Okada Y, Tsuchidate T et al (2009) Direct production of cadaverine from soluble starch using Corynebacterium glutamicum coexpressing α–amylase and lysine decarboxylase. Appl Microbiol Biotechnol 82:115–121
Van der Rest ME, Lange C, Molenaar D (1999) A heat shock following electroporation induces highly efficient transformation of Corynebacterium glutamicum with xenogenetic plasmid DNA. Appl Microbiol Biotechnol 52:541–545
Soksawatmaekhin W, Kuraishi A, Sakata K et al (2004) Excretion and uptake of cadaverine by CadB and its physiological functions in Escherichia coli. Mol Microbiol 51:1401–1412
Schneider J, Wendisch VF (2011) Biotechnological production of polyamines by bacteria: recent achievements and future perspectives. Appl Microbiol Biotechnol 91(1):17–30
Soksawatmaekhin W, Uemura T, Fukiwake N et al (2006) Identification of the cadaverine recognition site on the cadaverine-lysine antiporter CadB. J Biol Chem 281(39):29213–29220
Tomitori H, Kashiwagi K, Igarashi K (2012) Structure and function of polyamine-amino acid antiporters CadB and PotE in Escherichia coli. Amino Acids 42(2–3):733–740
Watson N, Dunyak DS, Rosey EL, Slonczewski JL, Olson ER (1992) Identification of elements involved in transcriptional regulation of the Escherichia coli cad operon by external pH. J Bacteriol 174:530–540
Yamamoto Y, Miwa Y, Miyoshi K et al (1997) The Escherichia coli ldcC gene encodes another lysine decarboxylase probably a constitutive enzyme. Genes Genet Syst 72:167–172
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 21176190), Key Technology Research and Development Program of Tianjin, China (No. 11ZCKFSY00900) and Tianjin Research Program of Application Foundation and Advanced Technology (No. 11JCYBJC09600).
Conflict of Interest
We declare that we have no financial or personal relationships with other people or organizations that could inappropriately influence our work. There is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in, or the review of, the manuscript entitled ‘Improving the secretion of cadaverine in Corynebacterium glutamicum by co-expressing lysine decarboxylase and cadaverine–lysine antiporter’.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, M., Li, D., Huang, Y. et al. Improving the secretion of cadaverine in Corynebacterium glutamicum by cadaverine–lysine antiporter. J Ind Microbiol Biotechnol 41, 701–709 (2014). https://doi.org/10.1007/s10295-014-1409-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-014-1409-4