Abstract
Here, we demonstrated the one-step production of cadaverine from starch using a Corynebacterium glutamicum strain coexpressing Streptococcus bovis 148 α-amylase (AmyA) and Escherichia coli K-12 lysine decarboxylase (CadA). We constructed the E. coli–C. glutamicum shuttle vector, which produces CadA under the control of the high constitutive expression (HCE) promoter, and transformed this vector into C. glutamicum CSS secreting AmyA. The engineered C. glutamicum expressed both CadA and AmyA, which retained their activity. We performed cadaverine fermentation using 50 g/l soluble starch as the sole carbon source without pyridoxal-5’-phosphate, which is the coenzyme for CadA. C. glutamicum coexpressing AmyA and CadA successfully produced cadaverine from soluble starch and the yield of cadaverine was 23.4 mM after 21 h. CadA expression levels under the control of the HCE promoter were assumed to be sufficient to convert l-lysine to cadaverine, as there was no accumulation of l-lysine in the culture medium during fermentation. Thus, we demonstrated that C. glutamicum has great potential to produce cadaverine from biomass resources.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In order to develop a biobased economy for sustainable economic growth, it is necessary to produce chemicals and fuels from renewable resources, such as biomass (Chotani et al. 2000; Kamm and Kamm 2004; Ohara 2003; Ragauskas et al. 2006). Although production of bio-ethanol from biomass has been widely studied, the production of chemicals such as amino acids and organic acids from biomass would also be of significant importance.
Corynebacterium glutamicum, a Gram-positive bacterium belonging to the order Actinomycetales, is one of the most useful microorganisms for producing chemicals and fuels, as C. glutamicum is generally regarded as safe and it is used to produce amino acid industrially (Hermann 2003; Leuchtenberger et al. 2005). Some researchers have reported production of the biodegradable polyester poly-3-hydroxybutyrate (Jo et al. 2006), organic acids such as lactic acid and succinic acid (Inui et al. 2004a; Okino et al. 2005), and fuels such as ethanol (Inui et al. 2004b) using C. glutamicum. One of the problems with C. glutamicum mediated production, however, is that a saccharification process is needed, as C. glutamicum cannot utilize substrates such as starch directly. In our previous study, we constructed C. glutamicum CSS, which secretes Streptococcus bovis 148 α-amylase (AmyA), in order to successfully produce l-lysine directly from starch (Tateno et al. 2007b).
In this study, we focused on cadaverine and demonstrated its direct production from starch using engineered C. glutamicum. Cadaverine is an attractive chemical, as it can be used as a replacement for the oil-derived hexamethylenediamine as a raw material for polyamide 66 (nylon 66). Cadaverine can be obtained from l-lysine using lysine decarboxylase (Sabo et al. 1974). Mimitsuka et al. (2007) reported the production of cadaverine from glucose as a carbon source using recombinant C. glutamicum expressing Escherichia coli lysine decarboxylase (CadA), which is known to maintain external pH in order to protect the cells from the acidic conditions (Meng and Bennett 1992; Neely and Olson 1996; Yamamoto et al. 1997; Lemonnier and Lane 1998; Samartzidou and Delcour 1999).
In order to produce cadaverine from starch directly, we introduced E. coli K-12 lysine decarboxylase (CadA) into C. glutamicum CSS secreting AmyA, thus enabling the production of cadaverine from biomass resources. We investigated the effect of a carbon source on cadaverine production. As a result, effective production of cadaverine directly from starch was achieved using C. glutamicum secreting AmyA and expressing CadA.
Materials and methods
Bacterial strains and media
E. coli SCS110 (Invitrogen, Carlsbad, CA, USA) was used for genetic manipulation. E. coli NovaBlue (Novagen, Madison, WI, USA) was used to isolate lysine decarboxylase. E. coli was grown in Luria–Bertani (LB) medium (10 g/l tryptone, 5 g/l yeast extract, 5 g/l sodium chloride). C. glutamicum CSS secreting AmyA (Tateno et al. 2007b) was grown in BY medium (10 g/l peptone, 10 g/l meat extract, 5 g/l yeast extract, 5 g/l sodium chloride) at 30°C (Katsumata et al. 1984). When necessary, LB medium and BY medium containing 50 and 25 mg/l kanamycin, respectively, or BY solid medium containing 10 g/l sucrose was used.
Construction of l-lysine-producing C. glutamicum
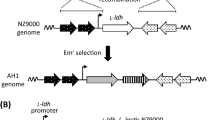
As a control, l-lysine-producing C. glutamicum, which does not produce α-amylase, was constructed. The integration plasmids for the chloramphenicol acetyltransferase gene (cat), pTM44-cm, were constructed using the basic plasmid pTM44 (Tateno et al. 2007a) containing the homoserine dehydrogenase gene (hom) and the Bacillus subtilis levansucrase gene (sacB). The cat gene was amplified by polymerase chain reaction (PCR) from the plasmid pHLA (Narita et al. 2006) using NgoMIV-cm_F primer (5’-GGAGCCGGCAAATTTAGGAGGCATATCAAATGAACT-3’) and cm-NgoMIV_R primer (5’-GGAGCCGGCCAGTCATTAGGCCTATCTGACAATTC-3’). The amplified fragment was digested with NgoMIV and was introduced into the NgoMIV site of hom on pTM44. The resulting plasmid was designated pTM44-cm (Fig. 1a).
Transformation of C. glutamicum ATCC 13032 was performed by electroporation; two recombination events were performed using kanamycin resistance and sacB selection (Tateno et al. 2007a). Integration of hom was confirmed using PCR and the auxotrophy of l-homoserine. The recombinant strain integrated using pTM44-cm was designated Cm strain.
Construction and transformation of plasmid for expression of CadA
The gene encoding lysine decarboxylase (cadA) was amplified by PCR using BamHI-cadA_F primer (5’-CGCGGATCCATGAACGTTATTGCAATATTGAATCACATGG-3’) and cadA-FLAG-HindIII_R primer (5’-CCCAAGCTTTTACTTGTCATCGTCATCCTTGTAGTCTTTTTTGCTTTCTTCTTTCAATACCTTAACGG-3’) with E. coli NovaBlue genomic DNA as the template. Amplified cadA-FLAG gene was introduced into the BamHI and HindIII sites of the plasmid pHSG298 (Takara Bio Inc., Otsu, Japan) for cloning. The resulting plasmid was designated pHSG298-cadAF. The cadA-FLAG fusion gene was amplified by PCR using SacI-cadA_F primer (5’-CCCGATATCGAGCTCATGAACGTTATTGCAATATTGAATCA-3’) and FLAG-NheI-XhoI_R primer (5’-CCCCTCGAGGCTAGCTCACTTGTCATCGTCATCCTTG-3’) with pHSG298-cadAF as the template. Amplified cadA-FLAG gene was introduced into the SacI and XhoI sites of the plasmid pCH (Tateno et al. 2007b). The resulting plasmid was designated pCH-cadAF (Fig. 1b).
Plasmid pCH-cadAF was introduced into C. glutamicum Cm and CSS by electroporation, as described previously (Tateno et al. 2007a). C. glutamicum Cm and CSS harboring pCH-cadAF were designated Cm-cadAF and CSS-cadAF, respectively. As a control, plasmid pCC, which contains only cspB promoter (Tateno et al. 2007a), was introduced into C. glutamicum Cm and CSS by electroporation. C. glutamicum Cm and CSS harboring pCC were designated Cm-pCC and CSS-pCC, respectively. The strains used are summarized in Table 1.
Expression of CadA and Western blotting
C. glutamicum cells were cultured at 30°C for 24 h in 5 ml of BY medium. After cultivation, cells were centrifuged at 1,000×g for 5 min at room temperature. Cells were washed and resuspended in 700 μl of phosphate-buffered saline (pH 7.2) containing 1% (vol/vol) Protease Inhibitor Cocktail (Nacalai Tesque, Kyoto, Japan). Cells were then disrupted using a Multi Beads Shocker (Yasuikikai, Osaka, Japan) according to a previously described protocol (Tateno et al. 2007b). The insoluble fraction and glass beads were removed by centrifugation at 21,000×g for 10 min at 4°C. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer was added to the supernatant, followed by boiling at 100°C for 5 min. Proteins were analyzed by SDS-PAGE using an SDS-polyacrylamide gel (8%: w/v), after which proteins were electroblotted onto a polyvinylidene difluoride membrane (Millipore Co., Boston, MA, USA) and were allowed to react with the primary mouse anti-FLAG and the secondary goat antimouse immunoglobulin G alkaline-phosphatase-conjugated antibodies (Promega Co., Madison, WI, USA). The membrane was then stained with nitroblue tetrazolium (Promega) and 5-bromo-4-chloro-3-indolylphosphate (Promega) according to the manufacturer’s protocol.
Fermentation experiments
Cadaverine fermentation by recombinant C. glutamicum was performed in a 2.0-l jar fermentor with a 1.0-l working volume. GMMYE-1 containing 50 g/l glucose as the sole carbon source and SMMYE-1 containing 50 g/l soluble starch (Wako Pure Chemical Industries, Osaka, Japan) as the sole carbon source (Tateno et al. 2007a) were used as fermentation media. GMMYE-1 and SMMYE-1 contained 25 mg/l kanamycin. C. glutamicum cells grown on BY solid medium containing 25 mg/l kanamycin at 30°C for 1 day were used to inoculate 5 ml of BY liquid medium containing 25 mg/l kanamycin in a test tube. After incubation at 30°C for 24 h, the culture was transferred into 40 ml of BY medium containing 25 mg/l kanamycin in a 100-ml flask. After incubation in a shaking incubator at 30°C for 24 h, the culture (3% v/v) was used to inoculate fermentor medium, and fermentation was performed at an agitation speed of 650 rpm and an aeration rate of 2.0 l/min at 30°C. To maintain the medium pH at 7.0, 5 N NH3 was automatically added to the fermentation culture.
Supernatant was used to determine α-amylase activity with an α-amylase measurement kit (Kikkoman, Tokyo, Japan; Tateno et al. 2007b). The activity of CadA was measured according to the protocols described in Mimitsuka et al. (2007). Protein concentration was measured with a BCA Protein Assay Kit (Takara Bio Inc.). Glucose concentration was determined using a Wako Glucose CII-Test kit (Wako Pure Chemical Industries, Osaka, Japan). A colorimetric method based on the phenol-sulfuric acid reaction (Dubois et al. 1956) was used to determine the amount of total sugars corresponding to starch and starch hydrolysis products. Cadaverine and l-lysine concentrations were determined by reverse-phase high-performance liquid chromatography (GL Science Co., Osaka, Japan; Tateno et al. 2007a).
Results
Expression of CadA in C. glutamicum
In order to test the expression of CadA in C. glutamicum, the intracellular fractions of C. glutamicum cells were analyzed by Western blot analysis after 24 h of cultivation. In the case of Cm-cadAF and CSS-cadAF, a clear band corresponding to CadA (82 kDa) was observed (Fig. 2, lanes 3 and 4). Expression of cadA in CSS-pCC was not observed (Fig. 2, lane 2). Furthermore, CadA activity was confirmed using l-lysine as a substrate with the intracellular fractions of Cm-cadAF and CSS-cadAF. The CadA activity of Cm-cadAF and CSS-cadAF was almost the same, but CadA activity was not detected in CSS-pCC (data not shown). These results indicate the successful intracellular expression of CadA in Cm-cadAF and CSS-cadAF.
Fermentation experiments with glucose as sole carbon source
We then evaluated cadaverine production using glucose as the sole carbon source with CSS-pCC, Cm-cadAF, and CSS-cadAF.
Figure 3a–c shows the results for cell growth, α-amylase activity, and sugar consumption, respectively. Cell growth of CSS-pCC and CSS-cadAF was slower when compared with Cm-cadAF. Cm-cadAF showed almost the same growth as cells harboring the control plasmid (data not shown). The Cm-cadAF strain consumed all of the glucose within 18 h. On the other hand, CSS-pCC and CSS-cadAF had consumed 40.0 and 23.4 g/l after 24 h, respectively. The CSS-pCC and CSS-cadAF strains consumed less glucose when compared with Cm-cadAF. These results showed that AmyA expression, as well as AmyA and CadA coexpression, in C. glutamicum led to a reduction in cell growth and glucose consumption. Figure 3d, e shows the results for l-lysine and cadaverine production, respectively. The CSS-pCC strain produced 33.3 mM l-lysine. The Cm-cadAF strain produced 49.4 mM cadaverine from glucose, and only 4.2 mM l-lysine remained after 24 h. Surprisingly, CSS-cadAF did not produce l-lysine and produced only 3.2 mM cadaverine after 24 h, despite expressing both AmyA and CadA.
Results of cadaverine fermentation experiments with glucose as the sole carbon source using CSS-pCC (triangles), Cm-cadAF (diamonds), and CSS-cadAF (squares). Changes in cell growth by measurement of OD600 (a), α-amylase activity (b), total sugar concentration (c), l-lysine concentration (d), and cadaverine concentration (e) are shown in the figure. Data points represent means and standard deviation of three independent experiments
Fermentation experiments with soluble starch as the sole carbon source
In order to evaluate cadaverine production with soluble starch as the sole carbon source, CSS-pCC and CSS-cadAF were used.
Figure 4a–c shows the results for cell growth, α-amylase activity, and sugar consumption, respectively. CSS-pCC and CSS-cadAF showed similar cell growth and reached almost the same maximum OD600 value. CSS-pCC and CSS-cadAF consumed 40.6 and 35.6 g/l soluble starch after 24 h, respectively. The sugar consumption of both strains was also similar. Notable differences between cell growth and sugar consumption were not observed between AmyA-expressing and AmyA–CadA-coexpressing C. glutamicum strains. Figure 4d, e shows the l-lysine and cadaverine concentrations, respectively. CSS-pCC produced 32.7 mM l-lysine. The l-lysine productivity of CSS-pCC from soluble starch was almost equal to that from glucose. CSS-cadAF strain produced 22.9 mM cadaverine directly from soluble starch after 24 h and did not accumulate l-lysine.
Results of cadaverine fermentation experiments with soluble starch as the sole carbon source using CSS-pCC (triangles) and CSS-cadAF (squares). Changes in cell growth by measurement of OD600 (a), α-amylase activity (b), total sugar concentration (c), l-lysine concentration (d), and cadaverine concentration (e) are shown in the figure. Data points represent means and standard deviation of three independent experiments
Discussion
The aim of this study is the direct production of cadaverine from starch using C. glutamicum coexpressing AmyA and CadA. We previously reported the direct production of l-lysine from starch using C. glutamicum CSS, which secretes AmyA. Therefore, we introduced the E. coli lysine decarboxylase cadA gene into C. glutamicum CSS to produce cadaverine from starch.
We constructed C. glutamicum Cm harboring pCH-cadAF (Cm-cadAF strain). Figure 3d, e shows that Cm-cadAF could efficiently convert l-lysine to cadaverine without accumulating intracellular l-lysine. Our results for cadaverine production (49.4 mM) from 50 g/l of glucose using Cm-cadAF were approximately two-fold higher than in previous studies (Mimitsuka et al. 2007) using pyridoxal-5’-phosphate, which suggests that CadA activity using pCH-cadAF under the control of HCE promoter is sufficient to convert l-lysine to cadaverine. CadA requires pyridoxal-5’-phosphate as a coenzyme (Sabo et al. 1974; Sabo and Fischer 1974), similar to other important enzymes such as alanine racemase (Oikawa et al. 2006), threonine dehydrogenase (Möckel et al. 1992), cystathionine γ-synthase, and o-acetylhomoserine sulfhydrylase (Lee 2005) and purified CadA activity without pyridoxal-5’-phosphate was approximately one tenth compared with pyridoxal-5’-phosphate in vitro (Sabo and Fischer 1974). Nevertheless, we achieved the efficient production of cadaverine using Cm-cadAF without the addition of such coenzymes to the culture medium. It is advantageous to perform fermentation without pyridoxal-5’-phosphate with regard to costs.
Glucose is the most preferable carbon source for almost all microorganisms. Interestingly, our results show that cell growth of CSS-pCC and CSS-cadAF using soluble starch as the sole carbon source was much better than when glucose was used as the sole carbon source. Figure 4e also shows that CSS-cadAF was able to produce cadaverine from soluble starch as the sole carbon source, although it did not produce cadaverine from glucose. When maltose was supplied as the sole carbon source, the maximum OD600 value for CSS-cadAF reached 49.3 and CSS-cadAF produced 34.3 mM cadaverine (data not shown). Therefore, C. glutamicum strain CSS-pCC and CSS-cadAF are apparently adversely affected due to AmyA expression with glucose, as the cell growth of CSS-pCC and CSS-cadAF was lower than that of Cm-CadAF (Fig. 3a). These effects might be explained by the reduction of stress resistance caused by AmyA expression. C. glutamicum can produce trehalose, which has a role in protecting the cells from hyperosmotic stress (Tzvetkov et al. 2003; Wolf et al. 2003), using three pathways (OtsAB, TreYZ, TreS). The OtsAB pathway produces trehalose from UDP-glucose and glucose-6-phosphate. The TreYZ pathway produces trehalose from oligo–polymaltodextrins–glycogen by maltooligosyltrehalose synthase (TreY) and maltooligosyltrehalose trehalohydrolase (TreZ). The TreS pathway produces trehalose from maltose by trehalose synthase (TreS). As soluble starch degraded by AmyA contains TreY or TreS substrates such as maltose, CSS-pCC and CSS-cadAF may obtain resistance to the stresses caused by AmyA expression. Wolf et al. (2003) also reported that an intracellular trehalose content in C. glutamicum using maltose was higher than using glucose. Therefore, the results using maltose also indicate that CSS-cadAF is able to tolerate the stresses caused by AmyA expression via the TreS pathway. In fact, fluorescent-probe-based real-time PCR analysis using CSS-cadAF culture after 15 h revealed that the treY gene expression levels using soluble starch was 1.4-fold higher than using glucose and maltose, and the treZ gene expression levels using maltose and soluble starch were 3.3-fold and 4.0-fold higher than using glucose, respectively, and the treS gene expression level using maltose was 1.2-fold higher than using glucose (data not shown).
In conclusion, we demonstrated a direct fermentation system from starch using C. glutamicum coexpressing AmyA and CadA. Our results indicate that starch is a more suitable substrate for C. glutamicum expressing AmyA than glucose in some cases. Furthermore, the application of AmyA and CadA coexpression to the industrial l-lysine-producing C. glutamicum strain may enable the production of cadaverine directly from starchy materials on an industrial scale.
References
Chotani G, Dodge T, Hsu A, Kumar M, LaDuca R, Trimbur D, Weyler W, Sanford K (2000) The commercial production of chemicals using pathway engineering. Biochem Biophys Acta 1543:434–455
Dubois M, Gilles KA, Hamilton JK, Reberse PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Hermann T (2003) Industrial production of amino acids by coryneform bacteria. J Biotechonol 104:155–172
Inui M, Murakami S, Okino S, Kawaguchi H, Vertès AA, Yukawa H (2004a) Metabolic analysis of Corynebacterium glutamicum during lactate and succinate productions under oxygen deprivation conditions. J Mol Microbiol Biotechnol 7:182–196
Inui M, Kawaguchi H, Murakami S, Vertès AA, Yukawa H (2004b) Metabolic engineering of Corynebacterium glutamicum for fuel ethanol production under oxygen-deprivation conditions. J Mol Microbiol Biotechnol 8:243–254
Jo SJ, Maeda M, Ooi T, Taguchi S (2006) Production system for biodegradable polyhydroxybutyrate by Corynebacterium glutamicum. J Biosci Bioeng 102:233–236
Kamm B, Kamm M (2004) Principles of biorefineries. Appl Microbiol Biotechnol 64:137–145
Katsumata R, Ozaki A, Oka T, Furuya A (1984) Protoplast transformation of glutamate-producing bacteria with plasmid DNA. J Bacteriol 159:306–311
Lee HS (2005) Sulfur metabolism and its regulation. In: Eggeling L, Bott M (eds) Handbook of Corynebacterium glutamicum. CRC, Boca Raton, pp 351–371
Lemonnier M, Lane D (1998) Expression of the second lysine decarboxylase gene of Escherichia coli. Microbiology 144:751–760
Leuchtenberger W, Huthmacher K, Drauz K (2005) Biotechnological production of amino acids and derivatives: current status and prospects. Appl Microbiol Biotechnol 69:1–8
Meng SY, Bennett GN (1992) Nucleotide sequence of the Escherichia coli cad operon: a system for neutralization of low extracellular pH. J Bacteriol 174:2659–2669
Mimitsuka T, Sawai H, Hatsu M, Yamada K (2007) Metabolic engineering of Corynebacterium glutamicum for cadaverine fermentation. Biosci Biotechnol Biochem 71:2130–2135
Möckel B, Eggeling L, Sahm H (1992) Functional and structural analyses of threonine dehydrogenase from Corynebacterium glutamicum. J Bacteriol 174:8065–8072
Narita J, Okano K, Tateno T, Tanino T, Sewaki T, Sung MH, Fukuda H, Kondo A (2006) Display of active enzymes on the cell surface of Escherichia coli using PgsA anchor protein and their application to bioconversion. Appl Microbiol Biotechnol 70:564–572
Neely MN, Olson ER (1996) Kinetics of expression of the Escherichia coli cad operon as a function of pH and lysine. J Bacteriol 178:5522–5528
Ohara H (2003) Biorefinery. Appl Microbiol Biotechnol. 62:474–477
Oikawa T, Tauch A, Schaffer S, Fujioka T (2006) Expression of alr gene from Corynebacterium glutamicum ATCC 13032 in Escherichia coli and molecular characterization of the recombinant alanine racemase. J Biotechnol 125:503–512
Okino S, Inui M, Yukawa H (2005) Production of organic acids by Corynebacterium glutamicum under oxygen deprivation. Appl Microbiol Biotechnol 68:475–480
Ragauskas AJ, Williams CK, Davison BH, Britovsek G, Cairney J, Eckert CA, Frederick WJ Jr, Hallett JP, Leak DJ, Liotta CL, Mielenz JR, Murphy R, Templer R, Tschaplinski T (2006) The path forward for biofuels and biomaterials. Science 311:484–489
Sabo DL, Fischer EH (1974) Chemical properties of Escherichia coli lysine decarboxylase including a segment of its pyridoxal 5’-phosphate binding site. Biochemistry 13:670–676
Sabo DL, Boeker EA, Byers B, Waron H, Fischer EH (1974) Purification and physical properties of inducible Escherichia coli lysine decarboxylase. Biochemistry 13:662–670
Samartzidou H, Delcour AH (1999) Excretion of endogenous cadaverine leads to a decrease in porin-mediated outer membrane permeability. J Bacteriol 181:791–798
Tateno T, Fukuda H, Kondo A (2007a) Production of l-lysine from starch by Corynebacterium glutamicum displaying α-amylase on its cell surface. Appl Microbiol Biotechnol 74:1213–1220
Tateno T, Fukuda H, Kondo A (2007b) Direct production of l-lysine from raw corn starch by Corynebacterium glutamicum secreting Streptococcus bovis α-amylase using cspB promoter and signal sequence. Appl Microbiol Biotechnol 77:533–541
Tzvetkov M, Klopprogge C, Zelder O, Liebl W (2003) Genetic dissection of trehalose biosynthesis in Corynebacterium glutamicum: inactivation of trehalose production leads to impaired growth and an altered cell wall lipid composition. Microbiology 149:1659–1673
Wolf A, Krämer R, Morbach S (2003) Three pathways for trehalose metabolism in Corynebacterium glutamicum ATCC 13032 and their significance in response to osmotic stress. Mol Microbiol 49:1119–1134
Yamamoto Y, Miwa Y, Miyoshi K, Furuyama J, Ohmori H (1997) The Escherichia coli ldcC gene encodes another lysine decarboxylase, probably a constitutive enzyme. Genes Genet Syst 72:167–172
Acknowledgment
This work was supported by a Grant-in-Aid for JSPS Fellows (20859 to T. Tateno).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tateno, T., Okada, Y., Tsuchidate, T. et al. Direct production of cadaverine from soluble starch using Corynebacterium glutamicum coexpressing α-amylase and lysine decarboxylase. Appl Microbiol Biotechnol 82, 115–121 (2009). https://doi.org/10.1007/s00253-008-1751-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-008-1751-4