Abstract
In fed-batch culture of Klebsiella pneumoniae, 1,3-propanediol production was growth associated, while the by-products, including lactic acid and ethanol, increased sharply as the cells grew slowly. When the fed-batch culture was supplied with a mixture of organic acids including citrate, fumarate and succinate, cell growth and 1,3-propanediol production increased significantly, whereas the by-products, especially lactic acid and ethanol, decreased sharply. High concentrations of PDO and acetate inhibited cell growth and PDO production. To improve the PDO production, repeated fed-batch culture with addition of the organic acid mixture was performed in a 5-l reactor. The fed-batch culture was repeated five times, and the 1,3-propanediol yield and concentration reached above 0.61 mol mol−1 and 66 g l−1, respectively, in 20 h for each cycle. Furthermore, the PDO productivity reached above 3.30 g l−1 h−1 in each cycle, which was much higher than that of the original fed-batch culture.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
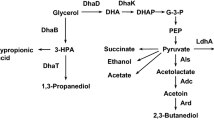
1,3-Propanediol (PDO) has become one of the most interesting feedstocks in recent years because of its wide industrial applications, such as synthesis of polytrimethylene terephthalate and other polyester fibers [1]. PDO can be produced by chemical synthesis or by biotransformation [2]. Current commercial production is based on the former route, which uses acrolein or ethylene as the starting material [1]. Biotransformation is mainly based on fermentation of glycerol by microorganisms including Clostridium, Citrobacter, Lactobacillus and Klebsiella [3–5]. Among the microorganisms that can convert glycerol to PDO, C. butyricum and K. pneumoniae are paid much attention because of their substrate tolerance, high yield and productivity [6–8]. Compared with the chemical synthesis method, biotransformation has the advantage of utilizing inexpensive, renewable resources such as glycerol or glucose, and becomes increasingly attractive [2].
To make the price more attractive, many efforts have been made to enhance the yield, productivity and concentration of PDO in the biotransformation process. In continuous culture, high 1,3-propanediol productivities between 4.9 and 8.8 g l−1 h−1 were obtained, but the PDO concentration was low (only 35.2–48.5 g l−1) [9]. In fed-batch culture, the 1,3-propanediol concentration of around 70 g l−1 can be achieved, while the productivity is below 1.5 g l−1 h−1 [10, 11]. The possible reason for the low productivity can be attributed to high concentrations of products in the later phase of fed-batch culture [12]. Accumulation of the by-products, especially lactic acid and ethanol, not only inhibits cell growth, but also consumes much reduction equivalent of NADH, to directly compete with the formation of PDO, and results in the low yield of PDO [13]. Genetic manipulation was performed to decrease the formation of by-products. A lactate dehydrogenase deficient mutant of K. oxytoca [14] and a strain of aldehyde dehydrogenase inactivated K. pneumoniae [15] were employed to successfully decrease lactic acid and ethanol production, respectively. In addition, the strategy of fumarate addition also benefited PDO production, but the PDO productivity still remained low [16].
In fed-batch cultures, the initial PDO productivity is high, while with the increased concentrations of PDO and other by-products, the PDO productivity drops quickly during the late period of cultivation. If part of the culture is withdrawn at this moment, and a fresh medium with the same volume is then filled, product inhibition can be released, and a high productivity can be maintained. Repeated fed-batch culture is an effective method to enhance the productivity of microbial cultures and has been applied to many fermentation processes, such as those for kojic acid and acetic acid production [17, 18]. In this work, we employed the strategy of repeated fed-batch culture to recover the PDO production rate. In addition, some organic acids were supplied in the cell growth phase. These strategies effectively enhanced the yield, productivity and final concentration of PDO and decreased the by-product formation.
Materials and methods
Strain and media
The strain K. pneumoniae LX3 was provided by Lixing Chemical Co., Anhui, China. This strain was obtained by repeated selection from K. pneumoniae ATCC10280 and was used exclusively in this study. The strain was preserved in 25% (w/v) glycerol at −20°C. The slant medium was composed of (in g l−1) glycerol, 20; peptone (Oxoid, UK), 3; MgSO4·7H2O, 0.2; NaCl, 5; agar (Huisheng Co., Shanghai, China), 20. The preculture medium was composed of (in g l−1) yeast extract (Oxoid, UK), 3; MgSO4·7H2O, 0.2; citric acid, 0.46; KH2PO4, 2; K2HPO4, 1.6; NH4Cl, 5.4; glycerol, 20. The medium for the main culture was composed of (in g l−1) yeast extract, 1; MgSO4·7H2O, 0.4; citric acid, 0.46; KH2PO4, 1.3; K2HPO4, 0.67; NH4Cl, 3; CaCl2 1; trace element solution, 1 ml. The trace element solution was composed of (in g l−1) FeCl3·6H2O, 5; MnCl2·4H2O, 2; ZnCl2, 0.684; CoCl2·6H2O, 0.476; CuCl2·2H2O, 0.17; H3BO3, 0.062; Na2MoO4·2H2O, 0.005; concentrated HCl (37%), 10 ml.
Cultivation
The strain was activated on the slant medium by incubation at 37°C for 15 h. Several inoculating loops of the cells on the slant were transferred to 100 ml of the preculture medium contained in a 250-ml shake flask that was aerobically grown at 37°C for 15 h to obtain the primary preculture. Then the primary preculture was inoculated into four 250-ml flasks containing 50 ml of the same preculture medium at an inoculum size of 10% (v/v) and incubated under the same conditions to obtain the secondary preculture. Batch cultivation was carried out in 50-ml anaerobic bottles containing 40 ml of medium for the main culture. The bottles were inoculated with 5 ml of the primary preculture and incubated at 37°C and 220 rpm for 15 h.
Fed-batch culture was carried out in a 5-l stirred bioreactor (BG-5, Baoxing Biotech Co., Shanghai, China) with a working volume of 3 l. The reactor was equipped with pH, temperature and agitation speed control and dissolved oxygen display. The temperature and agitation speed were maintained at 37°C and 400 rpm, respectively. pH was controlled at 7.0 by automatically adding a solution of 5 M sodium hydroxide. An anaerobic environment in the bioreactor was maintained by gassing nitrogen at 0.4 vvm. The initial concentration of glycerol was 40 g l−1, and then glycerol was maintained in the range between 15 and 25 g l−1 during the feeding phase. In fed-batch culture with the addition of an organic acid mixture (OAM), a solution containing citric acid, succinic acid and fumaric acid was added as the cell density (OD650) reached 3.0 to achieve the concentration of each acid of 3.80 mM.
Repeated fed-batch culture was carried out in the 5-l bioreactor and initiated in the fed-batch culture mode as mentioned above. At 20 h when the PDO production rate declined obviously, 2.7 l of the broth was withdrawn through the sampling pipeline. Then 2.7 l of fresh main culture medium was filled into the bioreactor aseptically to start the second run of fed-batch culture, and the same operations were repeated.
Analytical methods
Glycerol was assayed by a modified titration method of the previous report [19]. Ethanol and PDO were analyzed by a gas chromatograph (Shanfen GC112A, Shanghai, China) equipped with a 2-m column filled with Chromosorb 101 (Dikma, Shanghai, China) and a flame ionization detector. Temperature was controlled using a stepwise program (80°C for 2 min, then increased to 150°C at 30°C·min−1). Nitrogen was used as the carrier gas and 1-propanol as the internal standard. Cell density was determined by measuring the turbidity of appropriately diluted sample at 650 nm (OD650) using a UV-visible spectroscopy system (Unico, Dayton, NJ). Cell dry weight (CDW) was determined by centrifuging 20-ml samples, and the pellets were washed twice with deionized water before drying at 80°C to constant weight. One unit of OD650 was equivalent to 0.50 g l−1 of CDW. The organic acids, including lactic acid, acetic acid, succinic acid, citric acid and formic acid, were determined by ion chromatograph (ICS-1500, Dionex, USA).
Results and discussion
Fed-batch culture under the original control schemes
Fed-batch cultivation of K. pneumoniae was carried out in the 5-l reactor, and the results are shown in Fig. 1. The cells grew rapidly, and the cell density reached a maximum of 4.5 g l−1 at 14 h, but then declined slowly to 3.5 g l−1 at the end of culture (Fig. 1a). During the fast growth phase, both PDO and acetic acid increased rapidly with the cell growth, showing their growth-relating characteristics. The dependence of growth on acetate formation can be attributed to ATP formation in the acetate formation pathway, and growth favors reactivation of inactivated glycerol dehydratase in 3-hydroxypropyl aldehyde production. The ratio of PDO to acetic acid was about 2.0, consistent with the previous report [1]. From 14 to 20.3 h, the production rates of PDO and acetic acid slowed down following the decrease in the cell growth rate, while the by-products, including lactic acid, ethanol and succinic acid, increased constantly (Fig. 1b). Among these by-products, lactic acid and ethanol increased significantly from 1.74 and 3.23 g l−1 at 14.4 h to 5.09 and 7.63 g l−1 at 20.3 h, respectively, and their productivities were both higher than those in the other period. The succinic acid formation had a similar trend to lactic acid and ethanol. At the same time, the yields of lactic acid (Y LA/Gly) and ethanol (Y EtOH/Gly) based on consumed glycerol increased from 0.02 and 0.07 mol mol−1 to 0.26 and 0.16 mol mol−1, respectively (Table 1). The increased yields of these by-products resulted in a decrease of PDO yield that was only 0.29 mol mol−1 at the end of culture. Cheng et al. [12] showed that acetic acid was the major inhibitor to cell growth, followed by lactic acid and ethanol. The inhibitory effect of organic acids on microorganisms is mainly caused by their undissociated form [20].
Fed-batch culture of K. pneumoniae LX3 in a 5-l reactor. a Profiles of cell density, glycerol, acetic acid and PDO concentrations; b profiles of lactic acid, formic acid, succinic acid and ethanol. (Filled circle) CDW, (solid line) glycerol, (filled triangle) acetic acid, (filled square) PDO, (diamond) lactic acid, (open triangle) formic acid, (open circle) succinic acid, (multiplication symbol) ethanol
Fed-batch culture with OAM addition
It is reported that addition of organic acids, including succinic acid, citric acid and other intermediates in the TCA cycle, benefits PDO production in aerobic culture of K. oxytoca on glycerol [21]. PDO production was also enhanced in the anaerobic culture of K. pneumoniae with the addition of organic acids [22]. These organic acids can be converted to the precursors for biosynthesis of cellular components [23]. In addition, fumarate can be a terminal electron acceptor of the electron transfer chain under anaerobic conditions; thus, adding fumaric acid can benefit cell growth and PDO production because of more efficient ATP production and the decreased ratio of NAD+ to NADH caused by enhanced glycerol dehydrogenase [16]. In the present experiment, OAM was added as the biomass (OD650) reached about 3.0, and other culture conditions were the same as those in the previous experiment. The PDO production in the 5-l bioreactor with OAM addition was higher than that when each organic acid was added alone at the same concentration (not shown). The effects of different amounts of OAM addition on cell growth and product formation are shown in Fig. 2. With the addition of OAM, both cell growth and PDO production increased significantly as compared with those in the control. The CDW with OAM addition at different concentrations was higher than that of the control, and the maximal CDW of 4.88 g l−1 at 19.3 h was obtained when 3.80 mM OAM was added. In the control experiment, the PDO concentration increased rapidly before 11 h, but then slowed down in the late period. However, with the OAM addition, the PDO concentration increased constantly until 20 h. Among the three modes of OAM addition, the highest PDO concentration was achieved at the OAM concentration of 3.80 mM, and the maximal PDO reached above 70 g l−1 at 30 h, a 25% increase of the control. This indicated that OAM addition not only improved the cell growth, but also benefited PDO formation. The dynamic behavior of acetic acid production was also similar to CDW and PDO. Among these experiments, the highest concentration of acetic acid was 10.31 g l−1, which was obtained at 10.5 h at the OAM concentration of 3.80 mM. Then acetate increased slowly up to 13.4 g l−1 at the end of culture.
The by-product formation at different OAM concentrations is also shown in Fig. 2. Compared with the control, the final lactic acid concentration decreased sharply with the OAM addition, and the lowest final lactic acid of 6.0 g l−1 was achieved when 3.80 mM OAM was added, i.e., only half of that of the control. At the same time, the terminal ethanol concentration decreased obviously when OAM of 3.80 or 5.93 mM was added, and was 6.74 and 5.62 g l−1, respectively, lower than that of the control. In addition, succinic acid formation in all the experiments with OAM addition decreased, and the highest succinic acid concentration was 3.29 g l−1 as 1.93 mM OAM was added, still lower than that of the control. Therefore, addition of OAM significantly decreased the formation of lactic acid, ethanol and succinic acid, and was beneficial to PDO production due to less requirement for NADH.
Effects of the products on PDO production
The PDO production in the late phase of fed-batch culture slowed down, and the phenomenon can be attributed to the accumulation of PDO and by-products. The by-products, including acetic acid, lactic acid and ethanol, and especially their mixtures, strongly inhibit cell growth [12]. Among these by-products, acetic acid is the most potent inhibitor of cell growth, especially in the undissociated form [12, 20]. Although lactic acid and ethanol are less toxic than acetic acid to cell growth [12], their rapid formation in the late phase of cultivation leads to consumption of more NADH and competes with PDO formation, resulting in decreased yield of PDO [1]. To understand the effects of acetic acid and PDO on the strain used in this study, different concentrations of acetic acid and PDO and their mixture were added to the anaerobic bottles, and the results are shown in Table 2.
As the concentrations of acetic acid and PDO in the medium were low (at 1 and 6 g l−1), the cell growth, glycerol consumption and PDO production were all similar to the control. The biomass (OD650) and glycerol consumption decreased sharply as the concentration of acetic acid was 10 g l−1 or PDO was 60 g l−1, and the PDO production was 62.65 and 30.36 mM, i.e., decreased by 22% and 62%, respectively, compared with that of the control. Cell growth was inhibited strongly as a mixture containing acetic acid of 13.8 g l−1 and PDO of 60 g l−1 was added. The biomass was only 0.36 (OD650), and glycerol consumption and PDO production ceased. These indicate that cell growth, glycerol consumption and PDO production are affected significantly by the concentrated acetic acid and PDO, especially in the presence of both. In the late phase of fed-batch culture, the inhibitors included not only acetic acid and PDO, but also lactic acid, ethanol and other products, and thus the inhibition is more serious [6].
Repeated fed-batch culture with OAM addition
Repeated fed-batch culture is an effective method to enhance the productivity of microbial cultures to release the inhibition caused by products by repeatedly replacing a portion of culture with fresh medium. The above experiments indicate that the PDO production is growth associated, and PDO, acetic acid and other by-products inhibit cell growth and PDO production. Therefore, in the early period of cultivation, both the rates of cell growth and PDO production were high, but decreased rapidly later due to the accumulation of PDO and the by-products. If part of the culture with high product concentrations is replaced with fresh medium, the products can be diluted and the inhibition caused by them can be released.
To examine the feasibility of repeated batch culture for PDO production, experiments were firstly carried out in 50-ml anaerobic bottles with a working volume of 45 ml. The initial batch culture was performed at 37°C and 220 rpm for 15 h with an inoculum size of 10% (v/v). Then 40 ml of the culture was withdrawn, and the same volume of medium was filled. The mixture was incubated for another 15 h, and the same cultivation procedure was repeated eight times. At the end of each batch culture, the cell density, PDO and glycerol concentrations were measured, and the results are shown in Table 3. It can be seen that the cell density, glycerol consumption and PDO production at the end of each batch culture stayed relatively constant. These indicated that replacement of the culture with medium recovered cell growth and PDO production, the strain used in this study was stable, and the ability of PDO production was maintained during the repeated batch cultivation.
Repeated fed-batch culture was further carried out in the 5-l fermentor. Since the OAM addition improved PDO production, it was also added in the repeated fed-batch culture as the OD650 reached about 3.0 in each cycle of the repeated fed-batch culture. The experimental results are shown in Fig. 3. The cell growth in each cycle had similar dynamics, and the maximal biomass (CDW) was in the range between 4.5 and 4.7 g l−1. The PDO concentration in different cycles also had a similar trend and increased rapidly after the partial replacement of the culture with medium; it reached more than 66 g l−1 in each cycle. The trend of acetic acid was similar to cell growth, and the maximal acetic acid was above 12 g l−1, higher than that in the original fed-batch culture (Fig. 2). In addition, the concentrations of lactic acid and ethanol in each cycle were below 6 and 4 g l−1, respectively, much lower than those in the original fed-batch culture. In the last cycle of cultivation, further extending the culture time to more than 20 h (total culture time more than 100 h) resulted in rapid increases in lactic acid and ethanol, but slower increases in PDO and acetic acid.
Table 4 compares the consumption of glycerol and alkali, molar yields of PDO and by-products, and PDO productivity in the repeated fed-batch culture with those in other cultivation modes. The PDO productivity in each cycle of the repeated fed-batch culture with OAM addition was above 3.3 g l−1 h−1, much higher than that in the original fed-batch culture (about 1.5 g l−1 h−1). At the same time, the molar yield of PDO in the repeated fed-batch culture was also increased significantly because of decreased ethanol production, even though production of acetate and formate was slightly increased. After the replacement of culture broth with fresh medium, a high growth rate could be recovered because of the reduction of the concentrations of products, and a high cell growth rate is favorable to PDO production. Furthermore, lower ethanol formation saved the precious reducing power of NADH, which could be more effectively used for PDO production.
Conclusion
In fed-batch culture of K. pneumoniae, PDO production is growth-associated, whereas lactic acid and ethanol increased rapidly as the cell growth rate dropped. The accumulation of by-products not only inhibits cell growth, but also causes decreased PDO formation. Addition of OAM effectively enhances PDO production. The maximal PDO reached more than 70 g l−1 at 30 h by addition of 3.80 mM OAM. At the same time, by-products, especially lactic acid and ethanol, decreased sharply. Repeated fed-batch cultivation effectively released the inhibition caused by the products, and the PDO concentration and yield reached above 66 g l−1 and 0.61 mol mol−1, respectively; the PDO productivity was higher than 3.3 g l−1 h−1 in each cycle. The production of by-products, especially lactic acid and ethanol, declined sharply in the repeated fed-batch cultivation.
References
Biebl H, Menzel K, Zeng AP, Deckwer WD (1999) Microbial production of PDO. Appl Microbiol Biotechnol 52:289–297
Zeng AP, Biebl H (2002) Bulk chemicals from biotechnology: the case of PDO production and the new trends. Adv Biochem Eng Biotechnol 74:239–259
Reimann A, Biebl H, Deckwer WD (1998) Production of 1,3 propanediol by Clostridium butyricum in continuous culture with cell recycling. Appl Microbiol Biotechnol 49:359–363
El-Ziney MG, Arneborg N, Uyttendaele M, Debevere J, Jakobsen M (1998) Characterization of growth and metabolite production of Lactobacillus reuteri during glucose/glycerol fermentation in batch and continuous cultures. Biotechnol Lett 20:913–916
Biebl H (1991) Glycerol fermentation to PDO by Clostridium butyricum: measurement of product inhibition by use of a pH-auxostat. Appl Microbiol Biotechnol 35:701–705
Menzel K, Ahrens K, Zeng AP, Deckwer WD (1998) Kinetic, dynamic, and pathway studies of glycerol metabolism by Klebsiella pneumoniae in anaerobic continuous culture: IV. Enzymes and fluxes of pyruvate metabolism. Biotechnol Bioeng 60:617–626
Colin T, Bories A, Moulin G (2000) Inhibition of Clostridium butyricun by PDO and diols during glycerol fermentation. Appl Microbiol Biotechnol 54:201–205
Huang H, Gong CS, Tsao GT (2002) Production of 1,3-propanediol by Klebsiella pneumoniae. Appl Biochem Biotechnol 98:687–698
Menzel K, Zeng AP, Deckwer WD (1997) Hight concentration and productivity of PDO from continuous fermentation of glycerol by Klebsiella pneumoniae. Enzyme Microb Technol 20:82–86
Ji XJ, Huang H, Zhu JG, Hu N, Li S (2009) Efficient PDO production by fed-batch culture of Klebsiella pneumoniae: the role of pH fluctuation. Appl Biochem Biotechnol 159:605–613
Zheng ZM, Hu QL, Hao J, Xu F, Guo N, Sun Y, Liu DH (2008) Statistical optimization of culture conditions for PDO by Klebsiella pneumoniae AC15 via central composite design. Bioresour Technol 5:1052–1056
Cheng KK, Liu HJ, Liu DH (2005) Multiple growth inhibition of Klebsiella pneumoniae in PDO fermentation. Biotechnol Lett 27:19–22
Zeng AP, Menzel K, Deckwer WD (1996) Kinetic, dynamic, and pathway studies of glycerol metabolism by Klebsiella pneumoniae in anaerobic continuous culture: II analysis of metabolic rates and pathways under oscillation and steady-state conditions. Biotechnol Bioeng 52:561–571
Yang G, Tian J, Li J (2007) Fermentation of PDO by a lactate deficient mutant of Klebsiella oxytoca under microaerobic conditions. Appl Microbiol Biotechnol 73:1017–1024
Zhang YP, Liu M, Du CY, Shen JY, Cao ZA (2006) Effect of by-products on cell growth and biosynthesis of PDO by Klebsiella pneumoniae. Chin J Proc Eng 6:804–808 (in Chinese)
Lin RH, Liu HJ, Hao J, Cheng KK, Liu DH (2005) Enhancement of PDO production by Klebsiella pneumoniae with fumarate addition. Biotechnol Lett 27:1755–1759
Wan HM, Chen CC, Giridhar R, Chang TS, Wu WT (2005) Reapeated-bach production of kojic acid in a cell-retention fermenter using Aspergillus oryzae M3B9. J Ind Microbiol Biotechnol 32:227–233
Ito T, Sota H, Honda H, Shimizu K, Kobayashi T (1991) Efficient acetic acid production by repeated fed-batch fermentation using two fermentors. Appl Microbiol Biotechnol 36:295–299
Bradford P, Pohle WD, Gunther JK, Mehlenbacher VC (1942) Determination of glycerol by oxidation with periodic acid. Oil Soap 19:189–193
Fond O, Jansen NB, Tsao GT (1985) A model of acetate and 2,3-butanediol inhibition of the growth and metabolism of Klebsiella oxytoca. Biotechnol Lett 7:727–732
Guan GP, Wang HB, Tian JH (2008) Effect of succinate on aerobic fermentation of 1,3-propanediol from glycerol by Klebsiella oxytaca. Food Ferment Ind 34(5):14–17 (in Chinese)
Wang LM, Gan RY, Jin P, Dong MY (2006) Effect of some organic acids on cell growth and 1,3-propanediol production. Fine Chem 5:439–442 (in Chinese)
Nelson DL, Cox MM (2000) Lehninger principles of biochemistry, 3rd edn. Worth Publishers, New York
Acknowledgments
This work was supported by BG2006036, the Department of Science and Technology, Jiangsu Province, China, and partly supported by Shanghai Leading Academic Discipline Project, no. B505.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xue, X., Li, W., Li, Z. et al. Enhanced 1,3-propanediol production by supply of organic acids and repeated fed-batch culture. J Ind Microbiol Biotechnol 37, 681–687 (2010). https://doi.org/10.1007/s10295-010-0711-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-010-0711-z