Abstract
Systemic inflammatory response is closely related to the pathogenesis and prognosis in critical patients. Recently, systemic immune-inflammation index (SII), an indicator of systemic inflammatory response, was proved to predict the outcome in cancerous and non-cancerous diseases. The aim of this study is to investigate the association between SII on admission and 6-month outcome in patients with aneurysmal subarachnoid hemorrhage (aSAH). The clinical data and prognosis of 76 patients with aSAH were analyzed. The 6-month outcome was assessed by the modified Rankin scale(mRS). The unfavorable outcome was defined as mRS score ≥ 3. In addition, multivariate analysis was conducted to investigate factors independently associated with the favorable outcome. Receiver operating characteristic (ROC) curve analysis was undertaken to identify the best cut-off value of SII for the discriminate between favorable and unfavorable outcome in these patients. Thirty-six patients (47.4%) in our study had an unfavorable outcome (mRS ≥ 3) at 6 months, and twenty-four (66.7%) of them were in the high-SII group. A significantly higher SII on admission was observed in patients with unfavorable functional outcome at 6 months. Binary logistic regression analysis showed that there was an independent association between SII on admission and 6-month clinical outcome (adjusted OR = 4.499, 95%CI: 1.242–16.295, P < 0.05). The AUC of the SII for predicting unfavorable outcome was 0.692 (95% CI: 0.571–0.814, P < 0.05). Systemic immune-inflammation index (SII) could be a novel independent prognostic factor for aSAH patients at the early stage of the disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aneurysmal subarachnoid hemorrhage (aSAH), which has a high fatality rate (8.3 ~ 66.7%) and high disability rate (10–20%), accounts for 5% of all strokes [4, 10, 12, 25, 31, 32]. Because of complex pathophysiology in early and delayed brain damage, inflammation after bleeding is an important factor affecting the prognosis of aSAH [3, 25, 28]. Peripheral biomarkers may result from the inflammatory mechanisms involved in the aSAH-induced injury on the disease course [29].
Previous studies showed that leukocytosis, thrombocytosis, and platelet activation were correlated with delayed cerebral ischemia (DCI) and neurological outcome following aSAH [9, 31]. At present, indicators such as neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), prognostic nutrition index (PNI), MMP-9, TNF-a, and IL-6 have shown associations with clinical complications and outcomes in aSAH [6,7,8, 13]. And Tao et al. reported that the combined use of NLR and PLR (combined NLR-PLR) might have a better predictive value than each alone [33].
Meanwhile, systemic immune-inflammation index (SII), which includes peripheral lymphocytes, neutrophils, and platelets, was first used by Hu et al. to predict poor outcome in patients with hepatocellular carcinoma (HCC) and showed powerful predictive value [16]. As an integrated indicator, SII might be better able to reflect the balance of host inflammatory and immune status, and it was shown to be a more useful prognostic indicator than NLR and PLR in patients with esophageal squamous cell carcinoma, gastroesophageal adenocarcinoma, and so on [11, 16, 18]. Currently, SII is increasingly recognized as a readily available biomarker for systemic inflammation and plays an important role in predicting the prognosis and survival rate of patients with brain malignant tumor, spontaneous cerebral hemorrhage, cerebral infarction, and dementia [15, 22, 23, 35, 36, 39]. Furthermore, aSAH is a complex disease with a state of systematic inflammation, immunosuppression, and hypercoagulation [5, 33]. So, we hypothesized that SII may serve as a reliable biomarker for predicting outcomes in patients with aSAH. This retrospective study aimed to investigate the prognostic value of SII on admission for the 6-month outcome after aSAH.
Material and methods
Patients
We retrospectively analyzed the clinical data of patients with aSAH who were hospitalized in the department of neurosurgery or intensive care unit of the Second Affiliated Hospital of Chongqing Medical University from March 2018 to July 2020. The inclusion criteria were as follows:
-
1)
Patients with a definite diagnosis of intracranial aneurysm (IA) by craniocerebral computerized tomography angiography (CTA) and digital subtraction angiography (DSA), and this IA is the cause of SAH.
-
2)
Initial blood sampling for laboratory test was limited within 72 h after ictus of hemorrhage.
-
3)
Age: from 18 to 80.
-
4)
Admitted within 72 h after aSAH.
In addition, the exclusion criteria were as follows:
-
1)
Other intracranial vascular diseases.
-
2)
Acute and chronic infectious inflammation before admission (pneumonia supported by imaging examination, urinary tract infection by urine examination, chronic active viral hepatitis, etc.).
-
3)
History of autoimmune disease.
-
4)
Systemic diseases including malignant tumor, uremia, liver cirrhosis, chronic heart, disease, and chronic lung disease.
-
5)
Seizures and acute obstructive hydrocephalus occurred prior to admission.
-
6)
Previous history of severe trauma, stroke, etc.
-
7)
Diseases of the blood system other than anemia.
-
8)
Patients who suffered aneurysm rebleeding.
This study has been approved by the Ethics Committee of the Second Affiliated Hospital of Chongqing Medical University.
Methods
Demographic data and the medical history of patients, including age and gender, hypertension, diabetes, and history of anticoagulation therapy, were collected from the hospital database. In addition, lifestyle risk factors such as smoking habits and drinking status were also recorded, as well as treatment during hospitalization. The number of white blood cells [WBC], platelet [PLT], absolute neutrophil [ANC], and absolute lymphocyte counts [ALC] was obtained by automated test systems Hitachi 7600 and BM2000 on admission. The severity of aSAH was assessed by World Federation of Neurosurgical Societies (WFNS) grade, Hunt and Hess grade, and Fisher grade. The SII (109/L) was calculated by the following formula: PLT (109/L) × ANC (109/L)/ALC (109/L) [16]. Receiver operating characteristic (ROC) analysis was undertaken to evaluate the predictive ability of SII and determine the optimal cut-off point. The patients were divided into two groups according to cut-off value, high SII group (SII > 2344.65) and low SII group (SII ≤ 2344.65) (Table 1), and univariate analysis was carried out to detect between-group differences in baseline data. Follow-up was undertaken by telephone after 6 months of aSAH, and the 6-month neurological function was evaluated with modified Rankin scale (mRS). Patients were divided into a favorable outcome group with a low mRS (< 3) and an unfavorable outcome group with a high mRS (≥ 3).
Statistical analysis
The analysis was performed with SPSS V22 and MedCalc Statistical Software version 19.0.7. Continuous data were expressed as mean ± standard deviation (SD) or median (quartile range), and categorical data were presented as frequency and percentage (%). Comparisons of categorical variables were performed using the chi-square test or Fisher’s exact test, while the Mann–Whitney U test or unpaired t-test was applied for continuous variables. Receiver operating characteristic (ROC) analysis was performed to evaluate associations between the SII on admission and the 6-month clinical outcomes to determine the best cut-off point for the outcome prediction. To assess the importance of SII in the clinical context, already established clinical grading scales, such as the WFNS and Hunt and Hess grade, were included in the abovementioned ROC analysis. The AUCs were compared by the DeLong test. The independent predictors of aSAH were determined by univariate analysis and binary logistic regression; odds ratios (OR) and 95% confidence interval (CI) were calculated. All P values were on the 2 sides, and significance was set at P < 0.05.
Results
Patient characteristics
The patient characteristics were summarized in Table 2. Seventy-six patients were enrolled in this study, with a mean age of 57.3 years (range from 18 to 80 years). The median WFNS grade was 2.29 with the range from 1 to 5, and the median Fisher grade was 2.7 with the range from 1 to 5. The average length of hospital stay was 18.4 days. Forty-five patients (59.2%) in this study were female. Sixty-eight patients (89.5%) were admitted to the intensive care unit (ICU). Forty-eight patients (63.2%) had one aneurysm and 19 patients (25%) had two. And 36 patients (47%) had an unfavorable outcome (mRS ≥ 3) at 6 months.
Association of the SII with functional outcome
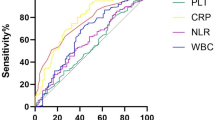
As shown in Table 3, thirty-six patients (47.4%) in our study had an unfavorable outcome (mRS ≥ 3) at 6 months. To gain a closer view of the relationship between the 6-month outcome and the clinical characteristics, the univariate analysis was conducted, and significantly older age; higher WFNS, Hunt and Hess, and Fisher grades; and higher infection rate were detected in patients with unfavorable outcome than those with the favorable outcome (P < 0.05). Further, a significantly higher SII on admission was observed in patients with unfavorable functional outcome at 6 months. Multivariate analysis showed that the SII might be an independent predictor for the unfavorable outcome (Table 4), and the adjusted odds ratio was 4.499 (95%CI: 1.242–16.295, P < 0.05).Furthermore, the ROC curve for unfavorable outcome also manifested the predictive value of the SII, and a SII of 2344.65 was recognized as the optimal cut-off threshold to discriminate between favorable and unfavorable outcomes at 6 months (Fig. 1 and Table 5). The AUC of the SII for predicting unfavorable outcome was 0.692 (95% CI: 0.571–0.814, P < 0.05). The sensitivity and specificity of the expected performance of SII were 66.7% and 75.0%. Then, the DeLong method was used to compare SII with other established clinical grading scales such as WFNS grade, Hunt and Hess grade, and Fisher grade. No statistical difference was found, which suggested that SII might be as useful as established clinical grading scales (Fig. 1 and Table 5).
Differences between high SII and low SII groups
In Table 1, there was no significant difference between high and low SII groups in baseline clinical characteristics data such as age, gender, and length of hospital stays. But higher WFNS grade, higher Hunt and Hess grade, and higher Fisher grade were detected in patients with high SII. Also, Spearman correlation analysis showed that the SII was correlated with WFNS grade, Hunt and Hess grade, and Fisher grade (r = 0.534, P < 0.05; r = 0.413, P < 0.05; r = 0.301, P < 0.05, respectively).
Comparison of SII with NLR and PLR
As shown in Fig. 2, the AUC of NLR for predicting unfavorable outcome was 0.620 (95% CI: 0.502–0.729, P = 0.069), while that of PLR was 0.697 (95% CI: 0.581–0.797, P < 0.001). The sensitivity and specificity of the expected performance of NLR were 66.7% and 75.0%, while that of PLR were 69.4% and 70.0%. The Youden index of SII, NLR, and PLR was 0.417, 0.286, and 0.394, respectively (Table 5). No statistical difference (SII vs NLR, P = 0.367; SII vs PLR, P = 0.958, respectively) was identified when comparing the AUC of SII with NLR and PLR by the DeLong method.
Discussion
In this retrospective study, our findings were as follows: (1) SII was correlated with the score of WFNS grade, Hunt and Hess grade, and Fisher grade on admission. (2) higher SII on admission was associated with poor 6-month outcome after aSAH. Taken together, the findings of the present study confirmed the hypothesis that SII on admission might be an important independent predictor for functional outcomes in patients with aSAH.
Aneurysmal subarachnoid hemorrhage has a very complex pathophysiological process and has been shown to be a state of systematic inflammation and immunosuppression [5, 29]. Clinical data demonstrated that increased blood leukocyte count was associated with higher disease severity and worse outcomes in strokes [2, 7, 26]. Besides, the elevation of neutrophils within 0–14 days after SAH was relevant to higher risk for subsequent vasospasm and poor long-term outcome [7]. Recent study recognized great decrease in circulating T lymphocytes during acute phase SAH [30]. Decreased T lymphocyte count may be associated with higher risk for pneumonia and 3-month mortality [21, 37]. Moreover, previous studies showed that elevated platelets during aSAH were associated with DCI and a worse prognosis, while antiplatelet therapy could reduce the incidence of DCI [5, 19]. In summary, neutrophil, platelet, and lymphocyte counts were associated with the occurrence of complications and prognosis of aSAH. But, individual blood parameters can be altered by some variables such as dehydration, excessive hydration, blood specimen handling, and even race. So researchers used ratios such as NLR and PLR to minimize the influence of above confounders and gain much more reliable predictive effects for the prognosis of aSAH patients [20, 27, 33]. And the role of NLR in reflecting inflammation and immune status has been confirmed in some diseases such as glioma [14]. Our study consistently demonstrated that patients with poor prognoses had higher neutrophil counts and lower lymphocyte counts than those with favorable prognoses. Patients with poor prognoses also had higher mean platelet counts, although no statistical significance was observed in the study. Similarly, SII has been shown to be a good predictor of aSAH prognosis at 6 months in the study.
At present, WFNS grade, Hunt and Hess grade, and Fisher grade are often used to estimate the severity and prognosis of aSAH patients. But, all the scales above were based on clinical manifestations rather than laboratory data; hence, it is a research direction to find appropriate serological indicators to predict the prognosis of aSAH. Our results suggested that SII is a novel integrated inflammatory marker in the area of aSAH research. In our study, ROC analysis indicated comparable predictive capability of SII to WFNS grade, Hunt and Hess grade, and Fisher grade in adverse prognosis, according to the non-significant statistical differences between the above parameters analyzed by the Delong test. Similarly, the Delong test did not show statistical significance in comparison of SII with NLR or PLR. However, the Youden index of SII was larger than that of NLR and PLR, respectively, which might be a hint of a more accurate predictive effect of SII on the prognosis of aSAH. Such superiority in accuracy might be due to the subtle differences in the effects of SII compared with NLR and PLR. NLR mainly reflected inflammatory injury, PLR showed the effects of hemostasis and thrombosis, while SII provided holistic information on inflammation, immunity, hemostasis, and thrombosis [5, 29, 34]. Since the adverse effects of inflammation, immunosuppression, and thrombosis had been validated in experimental and clinical data after aSAH, it was rational that SII would show a stronger predictive effect [24, 34]. Thus, the SII which combined ANC, ALC, and PLT together may be a comprehensive biomarker for assessing aSAH severity and predicting 6-month outcome. However, it is not clear whether there is a difference in SII between the acute and chronic stages of aSAH, because it has been shown that the pathophysiological processes are actually different in acute and chronic diseases. Previous studies have suggested that early inflammatory response is a protective mechanism in intracerebral hemorrhage disease, while persistent inflammatory response is a harmful mechanism [24, 29]. Furthermore, with the development of SAH, patients might develop vasospasm, DCI, hydrocephalus, epilepsy, and other serious complications, leading to a worse prognosis for patients. Previous studies have shown that NLR can effectively predict the occurrence of DCI [1, 17, 38]. As a more comprehensive indicator than NLR, SII should be also effective in predicting the occurrence of late complications of SAH.
Based on our findings, the current standard treatment of SAH could be further optimized. Individuals considering SAH should be evaluated with SII on admission. Those with high SII need to be screened for additional risks including inflammatory injury, immunosuppression, and thrombosis. More detailed assessment could be performed according to the patient’s condition. Following individualized treatment plans should be formulated according to available information, such as reducing inflammatory damage, strengthening nutritional support to improve immunity, reducing the risk of thrombosis, and preventing platelet overactivation. These treatments might improve the prognosis of patients with high SII. However, the specific treatment measures still depend on the experience of clinicians.
It’s easy to obtain specific serological indicators for calculating SII in clinic. What’s more, our research proved a great predictive value of SII. It is reasonable to believe that the SII could be used to guide the stratification of the risk of aSAH with poor prognosis and provide personalized treatment in the early stage of aSAH, which might improve the patient’s prognosis. Furthermore, our data also suggested that it was necessary to consider established clinical grades, such as Hunt and Hess grade and WFNS grade. However, further studies are needed to determine whether the cut-off values of SII are different in different regions and populations and whether SII can be used as a monitoring indicator of changes in condition of aSAH patients during hospitalization.
Limitation
There are potential limitations to our study. Firstly, our sample size is small, and it is necessary to increase the sample size for further verification. Secondly, the study lacks prospective and multicenter design, and the threshold value obtained from the total sample size may be biased. What’s more, the cut-off values may change with regions and populations. Thirdly, the telephone follow-up after 6 months was only subjective evaluation, lacking objective standards. Fourthly, the level of peripheral blood inflammatory indicators varies greatly during the acute attack and progression of aSAH, and inflammation is often observed to be biphasic in nature, with elements that are both protective and deleterious. We only collected blood samples from patients at the time of admission, and there was a lack of blood samples during hospitalization.
Conclusion
In conclusion, high SII was associated with a poor 6-month outcome in patients with aSAH. SII could be a novel independent prognostic factor for aSAH patients at the early stage of the disease. Further prospective studies about SII need to be evaluated in the future.
Data availability
The data used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Code availability
Not applicable.
Abbreviations
- aSAH:
-
Aneurysmal subarachnoid hemorrhage
- AUC:
-
Area under the curve
- ANC:
-
Absolute neutrophil counts
- ALC:
-
Absolute lymphocyte counts
- CT:
-
Computerized tomography
- CTA:
-
Computerized tomography angiography
- CNS:
-
Central nervous system
- CI:
-
Confidence interval
- DND:
-
Delayed neurological dysfunction
- DSA:
-
Digital subtraction angiography
- H–H grade:
-
Hunt and Hess grade
- IA:
-
Intracranial aneurysm
- mRS:
-
Modified Rankin scale
- NLR:
-
Neutrophil-to-lymphocyte ratio
- OR:
-
Odds ratios
- PLR:
-
Platelet-to-lymphocyte ratio
- PNI:
-
Prognostic nutrition index
- PLT:
-
Platelet
- PLC:
-
Platelet counts
- ROC:
-
Receiver operating characteristics
- SII:
-
Systemic immune-inflammation index
- SD:
-
Standard deviation
- WBC:
-
White blood cells
- WFNS grade:
-
World Federation of Neurosurgical Societies grade
References
Al-Mufti F, Amuluru K, Damodara N et al (2019) Admission neutrophil-lymphocyte ratio predicts delayed cerebral ischemia following aneurysmal subarachnoid hemorrhage. J Neurointerv Surg 11(11):1135–1140. https://doi.org/10.1136/neurintsurg-2019-014759
Al-Mufti F, Misiolek K, Roh D et al (2019) White blood cell count improves prediction of delayed cerebral ischemia following aneurysmal subarachnoid hemorrhage. Neurosurgery 84(2):397–403. https://doi.org/10.1093/neuros/nyy045
Aronowski J, Zhao X (2011) Molecular pathophysiology of cerebral hemorrhage: secondary brain injury. Stroke 42(6):1781–1786. https://doi.org/10.1161/strokeaha.110.596718
Broderick J, Brott T, Duldner J, Tomsick T, Leach AJS (1994) Initial and recurrent bleeding are the major causes of death following subarachnoid hemorrhage. Stroke 25(7):1342–1347. https://doi.org/10.1161/01.str.25.7.1342
Budohoski K, Guilfoyle M, Helmy A et al (2014) The pathophysiology and treatment of delayed cerebral ischaemia following subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry 85(12):1343–1353. https://doi.org/10.1136/jnnp-2014-307711
Chou S, Feske S, Atherton J et al (2012) Early elevation of serum tumor necrosis factor-α is associated with poor outcome in subarachnoid hemorrhage. J Investig Med 60(7):1054–1058. https://doi.org/10.2310/JIM.0b013e3182686932
Chou S, Feske S, Simmons S et al (2011) Elevated peripheral neutrophils and matrix metalloproteinase 9 as biomarkers of functional outcome following subarachnoid hemorrhage. Transl Stroke Res 2(4):600–607. https://doi.org/10.1007/s12975-011-0117-x
Fassbender K, Hodapp B, Rossol S et al (2001) Inflammatory cytokines in subarachnoid haemorrhage: association with abnormal blood flow velocities in basal cerebral arteries. J Neurol Neurosurg Psychiatry 70(4):534–537. https://doi.org/10.1136/jnnp.70.4.534
Frontera JA, Provencio JJ, Sehba FA et al (2017) The role of platelet activation and inflammation in early brain injury following subarachnoid hemorrhage. Neurocrit Care 26(1):48–57. https://doi.org/10.1007/s12028-016-0292-4
Fujii M, Yan J, Rolland W et al (2013) Early brain injury, an evolving frontier in subarachnoid hemorrhage research. Transl Stroke Res 4(4):432–446. https://doi.org/10.1007/s12975-013-0257-2
Geng Y, Shao Y, Zhu D, et al (2016) Systemic immune-inflammation index predicts prognosis of patients with esophageal squamous cell carcinoma: a propensity score-matched analysis. Scientific reports 6(39482). https://doi.org/10.1038/srep39482
Geraghty J, Testai FJCar (2017) Delayed cerebral ischemia after subarachnoid hemorrhage: beyond vasospasm and towards a multifactorial pathophysiology. Curr Atheroscler Rep 19(12):50 https://doi.org/10.1007/s11883-017-0690-x
Ghali M, Srinivasan V, Johnson J et al (2018) Therapeutically targeting platelet-derived growth factor-mediated signaling underlying the pathogenesis of subarachnoid hemorrhage-related vasospasm. J Stroke Cerebrovas Dis Official J Nat Stroke Assoc 27(9):2289–2295. https://doi.org/10.1016/j.jstrokecerebrovasdis.2018.02.017
Gomes Dos Santos A, de Carvalho R, de Morais A, et al (2021) Role of neutrophil-lymphocyte ratio as a predictive factor of glioma tumor grade: a systematic review. Crit Rev Oncol Hematol 163(103372) https://doi.org/10.1016/j.critrevonc.2021.103372
Hou D, Wang C, Luo Y, et al. (2020) Systemic immune-inflammation index (sii) but not platelet-albumin-bilirubin (palbi) grade is associated with severity of acute ischemic stroke (ais). Int J Neurosci:1–6 https://doi.org/10.1080/00207454.2020.1784166
Hu B, Yang X, Xu Y et al (2014) Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res Official J Am Assoc Cancer Res 20(23):6212–6222. https://doi.org/10.1158/1078-0432.Ccr-14-0442
Jabbarli R, Pierscianek D, Darkwah Oppong M et al (2020) Laboratory biomarkers of delayed cerebral ischemia after subarachnoid hemorrhage: a systematic review. Neurosurg Rev 43(3):825–833. https://doi.org/10.1007/s10143-018-1037-y
Jomrich G, Paireder M, Kristo I et al (2021) High systemic immune-inflammation index is an adverse prognostic factor for patients with gastroesophageal adenocarcinoma. Ann Surg Oncol 273(3):532–541. https://doi.org/10.1097/sla.0000000000003370
Kasius K, Frijns C, Algra A, Rinkel GJCd (2010) Association of platelet and leukocyte counts with delayed cerebral ischemia in aneurysmal subarachnoid hemorrhage. Cerebrovasc Dis 29(6):576–583. https://doi.org/10.1159/000306645
Lattanzi S, Brigo F, Trinka E et al (2019) Neutrophil-to-lymphocyte ratio in acute cerebral hemorrhage: a system review. Transl Stroke Res 10(2):137–145. https://doi.org/10.1007/s12975-018-0649-4
Li P, Mao L, Liu X et al (2014) Essential role of program death 1-ligand 1 in regulatory t-cell-afforded protection against blood-brain barrier damage after stroke. Stroke 45(3):857–864. https://doi.org/10.1161/strokeaha.113.004100
Liang R, Chen N, Li M et al (2018) Significance of systemic immune-inflammation index in the differential diagnosis of high- and low-grade gliomas. Clin Neurol Neurosurg 164:50–52. https://doi.org/10.1016/j.clineuro.2017.11.011
Liang R, Li J, Tang X, Liu Y (2019) The prognostic role of preoperative systemic immune-inflammation index and albumin/globulin ratio in patients with newly diagnosed high-grade glioma. Clin Neurol Neurosurg 184(105397) https://doi.org/10.1016/j.clineuro.2019.105397
Lucke-Wold B, Logsdon A, Manoranjan B et al (2016) Aneurysmal subarachnoid hemorrhage and neuroinflammation: a comprehensive review. Int J Mol Sci 17(4):497. https://doi.org/10.3390/ijms17040497
Macdonald RL, Schweizer TA (2017) Spontaneous subarachnoid haemorrhage. Lancet (London, England) 389(10069):655–666. https://doi.org/10.1016/s0140-6736(16)30668-7
McMahon C, Hopkins S, Vail A et al (2013) Inflammation as a predictor for delayed cerebral ischemia after aneurysmal subarachnoid haemorrhage. J Neurointerv Surg 5(6):512–517. https://doi.org/10.1136/neurintsurg-2012-010386
Nóbrega Lima Rodrigues de Morais A, Ribeiro Baylão VM, Martins Silva T, et al (2021) Is neutrophil-lymphocyte ratio a useful tool for predicting outcome in subarachnoid hemorrhage? A systematic review. Neurosurg Rev https://doi.org/10.1007/s10143-021-01484-7
Olsen MH, Lilja-Cyron A, Bache S, Eskesen V, Møller K (2019) [Aneurysmal subarachnoid haemorrhage]. Ugeskrift for laeger 181(31)
Saand A, Yu F, Chen J et al (2019) Systemic inflammation in hemorrhagic strokes - a novel neurological sign and therapeutic target? J Cereb Blood Flow Metab 39(6):959–988. https://doi.org/10.1177/0271678x19841443
Sarrafzadeh A, Schlenk F, Meisel A et al (2011) Immunodepression after aneurysmal subarachnoid hemorrhage. Stroke 42(1):53–58. https://doi.org/10.1161/strokeaha.110.594705
Srinivasan A, Aggarwal A, Gaudihalli S et al (2016) Impact of early leukocytosis and elevated high-sensitivity c-reactive protein on delayed cerebral ischemia and neurologic outcome after subarachnoid hemorrhage. World Neurosurg 90:91–95. https://doi.org/10.1016/j.wneu.2016.02.049
Swirski F, Nahrendorf MJS (2013) Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science (New York, NY) 339(6116):161–166. https://doi.org/10.1126/science.1230719
Tao C, Wang J, Hu X et al (2017) Clinical value of neutrophil to lymphocyte and platelet to lymphocyte ratio after aneurysmal subarachnoid hemorrhage. Neurocrit Care 26(3):393–401. https://doi.org/10.1007/s12028-016-0332-0
Terpolilli N, Brem C, Bühler D, Plesnila NJS (2015) Are we barking up the wrong vessels? Cereb Microcircul After Subarach Hemorrh Stroke 46(10):3014–3019. https://doi.org/10.1161/strokeaha.115.006353
Trifan G, Testai FD (2020) Systemic immune-inflammation (sii) index predicts poor outcome after spontaneous supratentorial intracerebral hemorrhage. J Stroke Cerebrovasc Dis Official J Nat Stroke Assoc 29(9):105057. https://doi.org/10.1016/j.jstrokecerebrovasdis.2020.105057
van der Willik KD, Fani L, Rizopoulos D et al (2019) Balance between innate versus adaptive immune system and the risk of dementia: a population-based cohort study. J Neuroinflammation 16(1):68. https://doi.org/10.1186/s12974-019-1454-z
Vogelgesang A, Grunwald U, Langner S et al (2008) Analysis of lymphocyte subsets in patients with stroke and their influence on infection after stroke. Stroke 39(1):237–241. https://doi.org/10.1161/strokeaha.107.493635
Wu Y, He Q, Wei Y et al (2019) The association of neutrophil-to-lymphocyte ratio and delayed cerebral ischemia in patients with aneurysmal subarachnoid hemorrhage: possible involvement of cerebral blood perfusion. Neuropsychiat Dis Treatment 15:1001–1007. https://doi.org/10.2147/ndt.S190477
Xu W, Wang D, Zheng X, Ou Q, Huang L (2018) Sex-dependent association of preoperative hematologic markers with glioma grade and progression. J Neurooncol 137(2):279–287. https://doi.org/10.1007/s11060-017-2714-3
Funding
This work was supported by the Kuanren Talents Program of the Second Affiliated Hospital of Chongqing Medical University (No. 201959), Chongqing Science and Health Joint Project (No. 2020GDRC006), and Venture and Innovation Support Program for Chongqing Overseas Returnees (No. CX2019156).
Author information
Authors and Affiliations
Contributions
Author contributions to the study and manuscript preparation include the following: Conception and design: Fushu Luo, Yuanyou Li, Zongyi Xie. Acquisition of data: Yuanyou Li, Mingjiang Sun. Analysis and interpretation of data: Fushu Luo, Yuanyou Li, Yuantong Zhao. Drafting the article: Fushu Luo, Yuanyou Li. Critically revising the article: all authors. Reviewed submitted version of manuscript: all authors. Approved the final version of the manuscript for publication on behalf of all authors: Zongyi Xie. Study supervision: Zongyi Xie.
Corresponding author
Ethics declarations
Ethics approval
This study has been approved by the Ethics Committee of the Second Affiliated Hospital of Chongqing Medical University (No.34).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflicts of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Luo, F., Li, Y., Zhao, Y. et al. Systemic immune-inflammation index predicts the outcome after aneurysmal subarachnoid hemorrhage. Neurosurg Rev 45, 1607–1615 (2022). https://doi.org/10.1007/s10143-021-01681-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-021-01681-4