Abstract
Aneurysmal subarachnoid hemorrhage (aSAH) is a devastating and life-threatening stroke subtype, that has a high disability and fatality rate. By the use of the systemic immune-inflammation index (SII), it is possible to understand the pathophysiology that underlies immune and inflammatory responses and anticipate consequences including delayed cerebral ischemia (DCI), delayed cerebral vasospasm, and functional outcome. A systematic search of the English-language literature in PubMed and Embase was performed to locate articles addressing the usage of SII in aSAH patients. The cutoff value, sensitivity, specificity, and area-under-the curve (AUC) of the receiver operating characteristic (ROC) curve were collected. Four publications were reviewed after applying the exclusion criteria from the 53 included articles. All the studies indicated that higher SII on admission was significantly associated with poor prognosis. The research examined in this paper provides the earliest indications that higher SII predicts DCI, delayed cerebral vasospasm, and functional outcome, even though other medical subspecialties have used this ratio for a long time to make such predictions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aneurysmal subarachnoid hemorrhage (aSAH) is a devastating and life-threatening stroke subtype, that accounts for five percent of all strokes, and has a high disability and fatality rate. Approximately one-quarter of patients with aSAH do not reach the hospital or die in the emergency room [1]. Acute subarachnoid hemorrhage mortality has gradually dropped to 35% in recent years [2, 3], which can be attributed to improvements in contemporary surgical and medical treatment strategies. Despite these advancements, it remains a significant threat that can cause irreversible neurological injury and results in a variety of neurologic diseases, substantial disability, and other unfavorable outcomes, such as decreased quality of life, additional costly and care burden on affected families, and substantial expenses to the health care industry [2,3,4].
The mechanisms of cerebral injury following aSAH remain unclear. However, there is growing proof that systemic inflammatory responses usually take place during the early stage of aSAH, play a crucial role in early brain injury and pathophysiological progression [5], cause harm to the hemorrhagic brain, and impact patients’ prognosis [6]. It is pressing to create a useful prognostic indicator for aSAH patient prognosis analysis. Despite the fact that many biomarkers have been identified as indicators of prognosis, the majority of them is not useful in clinical settings or needs specialized laboratory tools or sophisticated sample collection [7]. Simple, convenient, cheap, quick, and accurate biomarkers are far more useful for aSAH therapy than the majority of biomarkers, which call for laborious cerebrospinal fluid sample collection and laboratory analyses. A novel inflammatory marker, the systemic immune-inflammation index (SII), calculated using the following formula: SII = platelet count × neutrophil count/lymphocyte count, has recently been introduced for aSAH. As a ratio calculated based on platelets, neutrophils, and lymphocytes, compared with other common inflammatory parameters, such as the neutrophil–lymphocyte ratio (NLR), lymphocyte to monocyte ratio (LMR), and platelet-lymphocyte ratio (PLR), this parameter concurrently includes three different kinds of peripheral blood inflammatory cells, which can offer a more accurate and thorough reflection of the balance between the patient’s inflammation and immune reaction [8]. SII can be simply detected by the routine blood test for complete blood count, the most normally used test in clinical applications.
In the last decade, SII has been demonstrated to be useful in some pathologies, such as cancer, acute coronary syndromes, and neurosurgery [9,10,11]. Recently, some studies have proposed that SII could be related to prognosis in aSAH patients [6, 12]. However, because of the various experimental regions and subjects, there have been no conclusive findings among the results of previous studies. Hence, we conducted a systematic review to comprehensively examine the value of SII as a reasonable and accessible indicator for predicting the risk of aSAH.
Materials and methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were used to report this study. Additionally, we prospectively registered the study protocol in PROSPERO (registration number: CRD 42022364661).
Literature search
We conducted a thorough search of the published literature on PubMed and Ovid EMBASE, from inception to October 04, 2022. The following search terms were used for each database: (“systemic immune inflammatory index” OR “systemic immune-inflammatory index” OR “systemic-immune-inflammation index” OR “systemic immune-inflammation index” OR “neutrophil × platelets/lymphocyte” OR “SII”) AND (“SAH” OR “subarachnoid hemorrhage” OR “spontaneous hemorrhage” OR “ruptured intracranial aneurysm” OR “ruptured cerebral aneurysm” OR “ruptured brain aneurysm” OR “aneurysmal subarachnoid hemorrhage”).
Inclusion and exclusion criteria
The study topics were defined by participant or patient, intervention, comparison, outcome, study design (PICOS) criteria, and the publications had to meet the following requirements to be included: (a) comprised of adult patients (≥ 18 years old) with an aSAH diagnosis and (b) addressed the use of the SII as a predictive factor for delayed cerebral ischemia (DCI), delayed cerebral vasospasm, or functional outcome after aSAH. Studies were excluded if they met any of the following criteria: (a) reviews, editorials, commentaries, or conference abstracts; (b) SAH caused by factors other than aneurysm, such as trauma and other cerebrovascular diseases; (c) included pediatric patients; (d) studies presenting solely unadjusted univariate analysis data; and (e) not written in English.
Study selection and data extraction
The identified studies were independently reviewed by two authors in accordance with the requirements. Titles and abstracts of the retrieved records were first skimmed, and then, full texts of any potentially relevant research were examined. Justifications for study exclusion were noted and verified. The following information was collected by using a preestablished checklist: first author, country, study design, sample size, sex ratio, median age of the patients, outcome, optimal cutoff value of SII, follow-up period, outcome measures, and area under the curve (AUC). Any inconsistencies were clarified by discussion among the authors or by consulting a third reviewer.
Data analysis
To evaluate the quality of the eligible studies, which included the terms selection, comparability, and outcome/exposure, we utilized the Newcastle–Ottawa scale (NOS) [13]. Higher scores indicated higher quality, with the total score ranging from 0 to 9. A study with an NOS score of ≥ 6 was regarded as high quality; otherwise, it was considered to be at high risk of bias.
The predictive power of SII in the studies was then classified by the AUC-ROC as poor (0.5–0.6), acceptable (0.6–0.8), and good (0.8–1.0).
Results
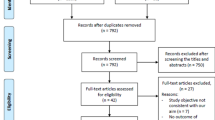
In the initial literature search, we used a string query to systematically search 53 studies. There were 40 studies left after duplicates were eliminated. By examining the abstracts and titles, and applying the inclusion and exclusion criteria to the full text, 4 studies [6, 12, 14, 15] were included in the current study, totaling 1335 patients. The procedure flow for the research screening is depicted in Fig. 1. Two of them were performed in China; one each were performed in Korea and the USA. The oldest publication year was 2021, and the most recent was 2022. The endpoints were all concerned with the likelihood of adverse outcomes being predicted, including DCI, delayed cerebral vasospasm, and functional outcome. The quality assessment using NOS is shown in Supplemental Table. The NOS score for each of the included studies exceeded 6, which represented high quality. The information acquired through the systematic review is summarized in Table 1 and presented in the paragraphs that follow.
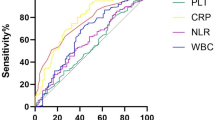
Chen [12] retrospectively gathered and assessed data from 333 patients, of whom 101 suffered DCI. Blood specimens were collected before treatment at admission. SII was significantly higher in the DCI group than in the non-DCI group: 2260 (1747–3218) vs. 1061 (737–1671), p < 0.001. With an AUC of 0.860 (95% CI: 0.818–0.896, p < 0.001), ROC curves demonstrated that SII might predict DCI. Although the AUC for SII was plainly higher than that for PLR, there was no discernible difference between NLR and SII. An SII ≥ 1424 might predict DCI with a sensitivity of 93.1% and a specificity of 68.1%. This was determined to be the best cutoff value for SII to predict DCI.
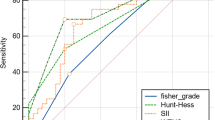
In determining whether delayed cerebral vasospasm follows aneurismal rupture, Geraghty [14] discovered, using the information from 246 individuals, of which 166 (67.5%) developed sonographic or angiographic evidence of cerebral vasospasm, that the SII index remained an independent predictor of vasospasm after adjustment for age, aneurysm location, diabetes mellitus, hyperlipidemia, and modified Fisher scale, with an OR of 1.386 (1.118–1.719, p = 0.003). The prediction model demonstrated that the optimal cutoff for the SII level was 1924, whereby individuals with a SII index higher than this threshold is more prone to developing vasospasm. The model showed a sensitivity of 58.9% and specificity of 80.6% (AUC 0.767). The laboratory data were further collected within 24 h of admission.
Luo [15] discussed the influence of SII, which was obtained on admission, on the clinical functional outcome at the 6-month period after aSAH. In 36 of the 76 patients who were included, a modified Rankin scale (mRS) score equal to or higher than 3 was considered to indicate a poor functional outcome. SII at admission and 6-month clinical outcome were independently correlated, according to binary logistic regression (OR = 4.271, 95% CI: 1.047–17.422, p < 0.05). The prediction model revealed that the best cutoff value of 2344.65 resulted in a sensitivity and specificity of 66.7% and 75.0%, respectively, with an AUC-ROC of 0.692 (95% CI:0.571–0.814, p < 0.05).
Additionally, evaluating unfavorable clinical outcomes, Yun [6] assessed functional outcomes at the 3-month mark, and an unfavorable outcome was defined as an mRS score of 3–6. A total of 680 patients with aSAH were enrolled. The 190 patients in the group with a poor clinical outcome had an average age of 55.2 ± 12.4 years. The SII index was an independent indicator of poor prognosis after aSAH (OR: 1.68, 95% CI: 1.24–2.74, p = 0.040). It was discovered that 960 was the best cutoff value for the SII index to predict prognosis (AUC:0.702, 95% CI: 0.638–0.765, p < 0.001). Laboratory findings were obtained using blood collected upon admission to the emergency department.
Discussion
In this study, we primarily reviewed the existing literature and summarized that the prognostic value of SII as a novel biomarker for poor outcome prediction in aSAH patients, including the occurrence of DCI and delayed cerebral vasospasm, and poor functional outcome. Four studies were included in our review, totaling 1335 patients. We discovered that two of the studies looked at the connection between SII and poor functional outcomes, while one assessed the association of SII and the occurrence of DCI, and one assessed the association of SII and the occurrence of delayed cerebral vasospasm. All the studies indicated that higher SII on admission was significantly associated with poor prognosis. To the best of our knowledge, this is the first systematic review providing comprehensive insights into this topic.
Over the past few years, neuroinflammation has been the subject of intense scholarly research in neurological disorders, particularly in cerebrovascular diseases, and several studies have been conducted on the association between the inflammatory response and aSAH. Systemic inflammation is thought to be a significant pathophysiological mechanism in aSAH and probably another crucial factor in the development of early brain injury, which takes place within the first 72 h but has persistent aftereffects that endure a lifetime and affect the development of DCI. Typically, changes in the concentrations of white blood cells, lymphocytes, neutrophils, and monocytes are used to describe the inflammatory response [5]. In recent years, a rising number of studies have explored some novel composite inflammation and immunity biomarkers based on these peripheral blood parameters, for example, PLR, NLR, LMR, systemic inflammatory response index (SIRI), and SII, with findings indicating that these combined markers are superior to single-cell counts as indicators of the prognosis for aSAH [5, 17,18,19]. These inflammatory biomarkers may contribute to endothelial damage, apoptosis, blood–brain barrier disruption, and cerebral edema, which may exacerbate brain damage (brain edema and secondary brain injury, including vasospasm and DCI) [16].
At present, there is accumulating evidence that the SII index can provide valuable prognostic information in a number of disorders, such as gastrointestinal cancer [10, 20], urinary system cancers [21], and cardiovascular diseases [11]. In the neurosciences, a higher SII index is known as a predictive factor for poor prognosis in brain pathologies such as intracerebral hemorrhage [22], sinus thrombosis [23], cerebral infarction [24], and glioma [9]. For intracerebral hemorrhage patients [22], it was demonstrated that SII, particularly day-1 SII, was strongly correlated with 90-day functional outcome.
It is crucial to detect potentially severe patients as soon as feasible in facilitate transfer to intensive care and effective time management for patients with aSAH. The most widely utilized clinical prediction models for recognizing aSAH patients with poor prognosis at the moment are the WFNS and Hunt Hess scale [25]. These models, however, do not take into account the predictive power of biological components and are only dependent on clinical data. SII index, which incorporates alterations in peripheral neutrophils, lymphocytes, and platelets, is a novel, easily available, and noninvasive biomarker with enhanced specificity to assist identify individuals at high risk of developing vasospasm. Previous studies suggested that patients with higher SII values upon admission may be at an increased risk of having a poor prognosis. In addition, SII may also help decision-makers decide which patients need prophylaxis against poor outcomes. For instance, numerous trials have demonstrated the safety and effectiveness of antiplatelet medication in SAH [26, 27]. They can lessen their risk of DCI and delayed cerebral vasospasm by using antiplatelet medications that have both antiplatelet and anti-inflammatory effects. Patients with greater SII may benefit from the initiation of antiplatelet medication since they are more likely to be experiencing a significant inflammatory response and to be in a pro-thrombotic condition. All the included studies measured SII upon admission, but this measure simply reflects admission status, and more studies that incorporate known risk factors and prognostic indicators are required to establish a schema for guidance.
There were some drawbacks to our study as well. First, only four studies received a full evaluation and were eligible for inclusion in this systematic review. Second, all included studies had the weaknesses associated with a single center, a small sample size and a retrospective design. Third, of those studies, a meta-analysis was impossible because there was high heterogeneity among the three outcomes that were taken into account. Consequently, the utility of SII remains limited, as its predictive factor is based solely on a single study per outcome. These viewpoints illuminate the way for additional primary investigations that have to concentrate on a prospective multicenter approach, large samples, reducing bias, and medications that might downregulate SII values. This study ultimately provides a foundation for upcoming meta-analyses.
Conclusions
The major conclusions of the papers included in this systematic review are as follows: (1) SII upon admission is markedly correlated with poor functional outcomes and the occurrence of delayed cerebral vasospasm and DCI in aSAH patients, and (2) SII may serve as a novel crucial indicator for prognosis prediction and risk stratification for patients with aSAH. Given the limitations of observational studies, the quality of the evidence was rated as low to very poor, indicating that more thorough investigation is required before drawing firm conclusions. Future research should also further establish the ideal cutoff value, define the group that will benefit, and explore whether SII can be utilized in conjunction with other recognized risk variables to create a reliable risk assessment system.
Data availability
Not applicable.
References
Claassen J, Park S (2022) Spontaneous subarachnoid haemorrhage. Lancet (London, England) 400(10355):846–862. https://doi.org/10.1016/s0140-6736(22)00938-2
Macdonald RL, Schweizer TA (2017) Spontaneous subarachnoid haemorrhage. Lancet (London, England) 389(10069):655–666. https://doi.org/10.1016/s0140-6736(16)30668-7
Petridis AK, Kamp MA, Cornelius JF, Beez T, Beseoglu K, Turowski B, Steiger HJ (2017) Aneurysmal subarachnoid hemorrhage. Deutsch Arztebl Int 114(13):226–236. https://doi.org/10.3238/arztebl.2017.0226
Kundra S, Mahendru V, Gupta V, Choudhary AK (2014) Principles of neuroanesthesia in aneurysmal subarachnoid hemorrhage. J Anaesthesiol Clin Pharmacol 30(3):328–337. https://doi.org/10.4103/0970-9185.137261
Zhang P, Li Y, Zhang H, Wang X, Dong L, Yan Z, She L, Wang X, Wei M, Tang C (2020) Prognostic value of the systemic inflammation response index in patients with aneurismal subarachnoid hemorrhage and a Nomogram model construction. Br J Neurosurg 1–7. https://doi.org/10.1080/02688697.2020.1831438
Yun S, Yi HJ, Lee DH, Sung JH (2021) Systemic inflammation response index and systemic immune-inflammation index for predicting the prognosis of patients with aneurysmal subarachnoid hemorrhage. J Stroke Cerebrovasc Dis: the official journal of National Stroke Association 30(8):105861. https://doi.org/10.1016/j.jstrokecerebrovasdis.2021.105861
Khey KMW, Huard A, Mahmoud SH (2020) Inflammatory pathways following subarachnoid hemorrhage. Cell Mol Neurobiol 40(5):675–693. https://doi.org/10.1007/s10571-019-00767-4
Hu B, Yang XR, Xu Y, Sun YF, Sun C, Guo W, Zhang X, Wang WM, Qiu SJ, Zhou J et al (2014) Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res: an official journal of the American Association for Cancer Research 20(23):6212–6222. https://doi.org/10.1158/1078-0432.ccr-14-0442
Liang R, Li J, Tang X, Liu Y (2019) The prognostic role of preoperative systemic immune-inflammation index and albumin/globulin ratio in patients with newly diagnosed high-grade glioma. Clin Neurol Neurosurg 184:105397. https://doi.org/10.1016/j.clineuro.2019.105397
Qiu Y, Zhang Z, Chen Y (2021) Prognostic value of pretreatment systemic immune-inflammation index in gastric cancer: a meta-analysis. Front Oncol 11:537140. https://doi.org/10.3389/fonc.2021.537140
Ye Z, Hu T, Wang J, Xiao R, Liao X, Liu M, Sun Z (2022) Systemic immune-inflammation index as a potential biomarker of cardiovascular diseases: a systematic review and meta-analysis. Front Cardiovasc Med 9:933913. https://doi.org/10.3389/fcvm.2022.933913
Chen L, Pandey S, Shen R, Xu Y, Zhang Q (2021) Increased systemic immune-inflammation index is associated with delayed cerebral ischemia in aneurysmal subarachnoid hemorrhage patients. Front Neurol 12:745175. https://doi.org/10.3389/fneur.2021.745175
Stang A (2010) Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25(9):603–605. https://doi.org/10.1007/s10654-010-9491-z
Geraghty JR, Lung TJ, Hirsch Y, Katz EA, Cheng T, Saini NS, Pandey DK, Testai FD (2021) Systemic immune-inflammation index predicts delayed cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Neurosurgery 89(6):1071–1079. https://doi.org/10.1093/neuros/nyab354
Luo F, Li Y, Zhao Y, Sun M, He Q, Wen R, Xie Z (2022) Systemic immune-inflammation index predicts the outcome after aneurysmal subarachnoid hemorrhage. Neurosurg Rev 45(2):1607–1615. https://doi.org/10.1007/s10143-021-01681-4
Osgood ML (2021) Aneurysmal subarachnoid hemorrhage: review of the pathophysiology and management strategies. Curr Neurol Neurosci Rep 21(9):50. https://doi.org/10.1007/s11910-021-01136-9
Chen L, Zhang Q (2020) Increased mean platelet volume is associated with poor outcome in patients with aneurysmal subarachnoid hemorrhage. World Neurosurg 137:e118–e125. https://doi.org/10.1016/j.wneu.2020.01.068
Jamali SA, Turnbull MT, Kanekiyo T, Vishnu P, Zubair AC, Raper CC, Tawk RG, Freeman WD (2020) Elevated neutrophil-lymphocyte ratio is predictive of poor outcomes following aneurysmal subarachnoid hemorrhage. J Stroke Cerebrovasc Dis: the official journal of National Stroke Association 29(4):104631. https://doi.org/10.1016/j.jstrokecerebrovasdis.2019.104631
Feghali J, Kim J, Gami A, Rapaport S, Caplan JM, McDougall CG, Huang J, Tamargo RJ, Jackson CM (2021) Monocyte-based inflammatory indices predict outcomes following aneurysmal subarachnoid hemorrhage. Neurosurg Rev 44(6):3499–3507. https://doi.org/10.1007/s10143-021-01525-1
Li X, Lin H, Ouyang R, Yang Y, Peng J (2021) Prognostic significance of the systemic immune-inflammation index in pancreatic carcinoma patients: a meta-analysis. Biosci Rep 41(8). https://doi.org/10.1042/bsr20204401
Li X, Gu L, Chen Y, Chong Y, Wang X, Guo P, He D (2021) Systemic immune-inflammation index is a promising non-invasive biomarker for predicting the survival of urinary system cancers: a systematic review and meta-analysis. Ann Med 53(1):1827–1838. https://doi.org/10.1080/07853890.2021.1991591
Li Y, Wen D, Cui W, Chen Y, Zhang F, Yuan M, Xiao H, Li H, Ma L, Hu X et al (2021) The prognostic value of the acute phase systemic immune-inflammation index in patients with intracerebral hemorrhage. Front Neurol 12:628557. https://doi.org/10.3389/fneur.2021.628557
Li S, Liu K, Gao Y, Zhao L, Zhang R, Fang H, Tao Y, Liu H, Zhao J, Xia Z et al (2020) Prognostic value of systemic immune-inflammation index in acute/subacute patients with cerebral venous sinus thrombosis. Stroke Vasc Neurol 5(4):368–373. https://doi.org/10.1136/svn-2020-000362
Hou D, Wang C, Luo Y, Ye X, Han X, Feng Y, Zhong P, Wu D (2021) Systemic immune-inflammation index (SII) but not platelet-albumin-bilirubin (PALBI) grade is associated with severity of acute ischemic stroke (AIS). Int J Neurosci 131(12):1203–1208. https://doi.org/10.1080/00207454.2020.1784166
Aggarwal A, Dhandapani S, Praneeth K, Sodhi HBS, Pal SS, Gaudihalli S, Khandelwal N, Mukherjee KK, Tewari MK, Gupta SK et al (2018) Comparative evaluation of H&H and WFNS grading scales with modified H&H (sans systemic disease): A study on 1000 patients with subarachnoid hemorrhage. Neurosurg Rev 41(1):241–247. https://doi.org/10.1007/s10143-017-0843-y
Darkwah Oppong M, Gembruch O, Pierscianek D, Köhrmann M, Kleinschnitz C, Deuschl C, Mönninghoff C, Kaier K, Forsting M, Sure U et al (2019) Post-treatment antiplatelet therapy reduces risk for delayed cerebral ischemia due to aneurysmal subarachnoid hemorrhage. Neurosurgery 85(6):827–833. https://doi.org/10.1093/neuros/nyy550
Nagahama Y, Allan L, Nakagawa D, Zanaty M, Starke RM, Chalouhi N, Jabbour P, Brown RD, Derdeyn CP, Leira EC et al (2018) Dual antiplatelet therapy in aneurysmal subarachnoid hemorrhage: association with reduced risk of clinical vasospasm and delayed cerebral ischemia. J Neurosurg 129(3):702–710. https://doi.org/10.3171/2017.5.jns17831
Author information
Authors and Affiliations
Contributions
All authors contributed to the article and approved the submitted version. B. L.: conception, design of the manuscript, and wrote the main manuscript text. B. L., Q. X., P. L.: database search, literature review, and data extraction. Y. Z.: supervision and made critical revision of the manuscript for important intellectual content.
Corresponding author
Ethics declarations
Ethical approval
This study did not require ethical approval.
Competing interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liao, B., Xu, Q., Lu, P. et al. The prognostic value of systemic immune-inflammation index in patients with aneurysmal subarachnoid hemorrhage: a systematic review. Neurosurg Rev 46, 219 (2023). https://doi.org/10.1007/s10143-023-02133-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10143-023-02133-x