Abstract
Delayed cerebral ischemia (DCI) is a severe complication of subarachnoid hemorrhage (SAH). Clinical and radiographic features of SAH may be helpful in identification of individuals prone to DCI. The aim of this systematic review was to analyze the present evidence on predictive value of blood and cerebrospinal fluid (CSF) biomarkers of DCI after SAH. We systematically searched in PubMed, Scopus, Web of Science, and Cochrane Library databases for publications before July 15, 2018, reporting correlations between blood/CSF biomarkers and occurrence of DCI and/or vasospasm in SAH patients. Included studies underwent quality assessment according to QUIPS and STARD guidelines. Level of evidence (I–IV) for each of tested biomarkers was assessed according to GRADE guidelines. Of 2181 unique records identified in four databases, 270 original articles and 5 meta-analyses were included to this review. Of 257 blood and CSF parameters analyzed in 16.914 SAH patients, there was no biomarker with positive association with DCI/vasospasm showing level I evidence. Twenty-one biomarkers achieved level II evidence and could be confirmed as predictive biomarkers. In this review, six single nucleotide polymorphisms (for EET metabolic pathways, COMT, HMGB1, ACE, PAI-1 promoter, and Hp genes) and 15 non-genetic biomarkers (pNF-H, ADAMTS13, NPY, Copeptin, HMGB1, GFAP, periostin, Tau, BNP, NT pro-BNP, hs-TnT, PA-TEGMA, MPV:PLT, NLR, and PLR) were selected as predictive DCI biomarkers. We propose that a panel analysis of the selected genetic and protein biomarker candidates would be needed for further validation in a large SAH cohort.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Subarachnoid hemorrhage (SAH) due to aneurysm rupture is a complex neurovascular disease with multiple factors influencing the final functional outcome. Despite recent advances in aneurysm treatment and neurocritical care, almost half of SAH patients still develop unfavorable long-term outcome [13, 33].
Alongside with initial severity of SAH and aneurysm rebleeding prior to treatment [5, 19], certain secondary complications also contribute to morbidity and mortality of SAH [18]. Among them, delayed cerebral ischemia (DCI) is a major risk factor for SAH patients with successful aneurysm treatment and uncomplicated initial course [1]. DCI is supposed to be of multifactorial nature, but is integrally related to occurrence of symptomatic cerebral vasospasm [31]. Therefore, proper diagnosis and management of cerebral vasospasm and its ischemic complications are highly essential for outcome improvement.
DCI is traditionally assessed through regular clinical examination [15]. However, poor initial clinical condition of the majority of DCI candidates, as well as clinical manifestation usually occurring at the time of irreversible cerebral damages, necessitated development of novel diagnostic approaches [31]. In particular, there are a large number of studies focusing on predictors of vasospasm and DCI [6, 8, 12, 18, 21]. But the amount of intracranial bleeding is the only consistently demonstrated risk factor for vasospasm [16]. Transcranial Doppler (TCD) ultrasonography is the most common non-invasive bedside tool for identification of vasospasm, but is characterized with a moderate specificity and predictive value [22].
In light of this, suitable laboratory biomarkers would represent an attractive solution allowing a proper and timely DCI diagnosis in SAH patients. Extensive biomarker research and clinical integration is an important pillar of modern precision medicine [2]. Evaluation of clinical value of various laboratory parameters in SAH patients has nearly a semi-centennial history [28], including recent publications targeted on novel molecular genetic and protein biomarkers, via applied genomic and proteomic approaches [32, 36]. However, compared to other diseases, laboratory biomarkers have still no routine application as DCI predictors in SAH.
In this systematic review, we addressed publications analyzing the associations between molecular genetic and protein laboratory biomarkers (investigated in blood and cerebrospinal fluid [CSF]) and occurrence of cerebral vasospasm and/or DCI in SAH cohorts. In this context, we aimed at systemizing all currently tested DCI/vasospasm biomarkers into a unique biomarker database. In addition, we report on the present level of evidence with consecutive selection of the most promising candidate biomarkers for future validation.
Material and methods
This systematic review has been designed according to the PRISMA guidelines [27]. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Search strategy and selection criteria
In PubMed, Scopus, Cochrane Database, and Web of Science, we searched for articles published in English before the 15 July 2018 reporting data on DCI biomarkers in SAH patients. A full list of search terms is available in the Supplementary Materials (Table E1). The search results were recorded into a custom electronic database (Microsoft Access 2013; Microsoft Corporation, Redmond, WA, USA). After automatic exclusion of duplicate entries, RJ and DP independently screened the titles and abstracts of all collected publications. In case of disagreement, the senior author (US) reviewed the article and the disagreement was resolved by discussion between the three reviewers. Reference lists of relevant publications were screened for additional articles by RJ and DP in the same manner.
Data were taken from case-control, cross-sectional, and longitudinal studies containing patients with acute aneurysmal SAH. The studies were considered eligible, if they reported associations between laboratory biomarkers and occurrence of at least one of the following DCI-related study endpoints according to current recommendations [38, 39]: (a) cerebral infarction on follow-up computed tomography (CT) scans and/or magnetic resonance imaging (MRI), (b) delayed neurological deterioration commonly defined by authors as “symptomatic vasospasm” or “delayed ischemic neurological deficit (DIND)”, (c) angiographic vasospasm evidenced on digital subtraction angiography (DSA), and (d) increased flow velocities on TCD ultrasonography suspicious for cerebral vasospasm (according to study-specific cutoffs). The biomarkers must be collected in a routine manner either from blood (serum and plasma, via peripheral or central venous catheter) or from CSF (using external ventricular and/or lumbar drainage or single lumbar puncture). The exclusion criteria were (1) interventional studies and (2) use of non-conventional methods for biomarker sampling, such as cerebral microdialysis and jugular bulb catheterization. The publications based on meta-analysis were also considered; however, the results were interpreted according to the conformity to the criteria of evidence level applied in the current review (as described below).
Data analysis
Data collection
The full-text analysis of all manuscripts included to the systematic review was performed by RJ and quality controlled by DP. Extracted data contained the following information:
-
Study and population characteristics: study design, geographic origin, study years, number of participants, baseline demographic/clinical parameters of cohorts, analyzed study endpoints
-
Biomarker-related data: the list of tested biomarkers, sample origin, and frequency of sampling
-
Study results: association between laboratory biomarkers and each DCI endpoint, quality of statistical evaluation (adjusted or unadjusted for potential confounders, such as SAH severity)
Quality assessment
On the basis of QUIPS (Quality Assessment in Prognostic studies) tool [17] and STARD (Standards for Reporting Diagnostic Accuracy) criteria [4], an adapted quality assessment form was developed to cover the topic of our review (see Table E2 in the Supplementary Materials). To evaluate the methodological and content quality, RJ and DP independently calculated appropriate scores for each included study except meta-analyses (Quality Assessment Score [QAS], 0–40 points). The studies scoring 30 points and more were regarded as good quality studies. Accordingly, QAS values were incorporated into assessment of the evidence level for laboratory biomarkers.
Database integration and classification of laboratory biomarkers
Biomarkers collected from eligible studies were recorded into a unique database (see Table E2 in the Supplementary Materials) containing the information on source studies, total number of SAH patients with biomarker evaluation, tissue origin, tested DCI surrogate, and the results of associations for each endpoint. In addition, all biomarkers were classified into the different categories according to their origin and functional pathways.
The level of evidence and recommendations for laboratory biomarkers
According to the GRADE (Grading of Recommendations Assessment, Development and Evaluation) guidelines [14], the level of evidence for all biomarkers was assigned to the following four classes: high, moderate, low, and very low evidence (classes I, II, III, and IV respectively). Criteria for classification were adapted according to the scope of the review (Table 1). Similar to one previous report [7], the recommendations regarding the diagnostic value of biomarkers were classified intro three categories: predictive biomarkers, non-predictive biomarkers (both for biomarkers with the levels of evidence classes I or II), and non-conclusive biomarkers (levels of evidence classes III or IV). Allocation of all identified biomarkers to certain evidence and recommendation levels was performed independently by RJ and DP.
Results
Characteristics of publications
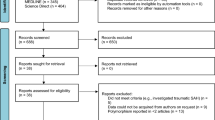
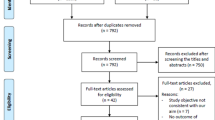
After the search in four academic databases and reference list check of relevant publications, 2181 non-duplicating records were screened for eligibility. Accordingly, 270 original papers and 5 meta-analyses published between 1974 and 2018 were included to this systematic review (see Fig. 1 for the flow chart, as well as Table E3 in the Supplementary Materials for detailed characteristics of the included publications).
Considering overlapping cohorts, 257 laboratory predictors of DCI (including 25 genetic biomarkers) were evaluated in 217 different SAH populations with a total of 16.914 patients. The mean number of patients in each study was 77.9 (range 3–849, median 42 patients). The overall mean age of all SAH patients was 54.2 years (ranging between 36.4 and 67.2 years in a given cohort); 62.3% were females (range 33–87.5%).
The vast majority of studies (n = 225) were published in the recent 20 years. Sixty-nine publications were based on US cohorts, followed by Japan (n = 43), Germany (n = 28), China (n = 19), and Italy (n = 14).
Regarding the quality of the studies, the mean QAS value was 30.6 points (range 18–40 points). One hundred fifty-six original papers scored ≥ 30 points, fulfilling the criteria for good quality.
Laboratory biomarkers for DCI after SAH: a pooled evidence
All identified DCI biomarkers (n = 257) were integrated into a unique electronic database and stratified into different functional categories (Fig. 2). The mean total number of analyzed patients for every single biomarker was 169.5 (range 3–4181, median 53.5). In the majority of cases (n = 155), the total patients’ load per biomarker was < 100 individuals. Mostly (n = 152), there was one study per biomarker. Eleven substances (1 genetic [ApoE (apolipoprotein E) SNP (single nucleotide polymorphism)] and 6 protein biomarkers [TNF-α (tumor necrosis factor-α), vWF (von Willebrand factor), S100B, ET-1 (endothelin-1), ICAM-1 (intercellular adhesion molecule-1), CRP (C-reactive protein), IL-6 (interleukin-6), MMP-9 (matrix metalloproteinase-9), WBC (white blood cells), and platelets count]) were tested in 10 and more studies. Detailed information on biomarkers is shown in the Supplementary Materials (Table E4).
Different functional categories of DCI biomarkers. This 3D circle diagram presents basic functional categories of DCI/vasospasm biomarkers. For each category, the number of identified biomarkers is given. Several biomarkers (n = 13) may be referred to more than one functional category as it is shown in the Table E4 in the Supplementary Materials. The following rare functional categories were included to the group “diverse biomarkers”: tumor biomarkers (n = 2), hormonal biomarkers (n = 6). See also Tables E4 and E5 in the Supplementary Materials for the detailed list of functional categories and tested biomarkers
Overall, there was mostly insufficient level of evidence for laboratory biomarkers (Fig. 3). In particular, 157 biomarkers (61.1%) presented with very low evidence (class IV). Moreover, 55 laboratory predictors showed low evidence (class III). Therefore, no definite recommendation could be derived for 212 biomarkers according to the current evidence in the literature.
Full list of laboratory DCI biomarkers allocated into different recommendation and evidence levels. Of the 45 laboratory parameters with the evidence level allowing a recommendation regarding DCI prediction (classes I–II), 21 biomarkers were allocated as “predictive” and 24 as “non-predictive” biomarkers. Only Hct showed the level I evidence (as non-predictive biomarker), whereas the remaining biomarkers with given recommendations reached evidence level II. The majority of the biomarkers (212, 82.5%) showed an insufficient evidence for a definite recommendation (classes III–IV). For a full list of biomarkers and used abbreviations, see Table E4 in the Supplementary Materials
Of the remaining 45 biomarkers, only one parameter reached class I level of evidence (Hct (hematocrit) as “non-predictive biomarker”). Accordingly, 44 laboratory parameters showed class II level of evidence. Twenty-one parameters could be classified as “predictive biomarkers” of DCI including six genetic SNP for EETs (epoxyeicosatrienoic acid) metabolic pathways, COMT (catechol-O-methyltransferase), HMGB1 (high-mobility group box 1), ACE (angiotensin-converting enzyme), PAI-1 (plasminogen activator inhibitor-1) promoter, and Hp (haptoglobin) genes. Moreover, pNF-H (phosphorylated major neurofilament H), ADAMTS13 (A disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13), NPY (neuropeptide Y), copeptin, HMGB1 protein, GFAP (glial fibrillary acidic protein), BNP (brain natriuretic peptide), NT pro-BNP (N-terminal pro B-type natriuretic peptide), hs-TnT (high-sensitive troponin T), Tau, periostin, NLR (neutrophil to lymphocyte ratio), PLR (platelet to lymphocyte ratio), MPV:PLT (mean platelet volume to platelet count ratio), and PA-TEGMA (platelet activation [thromboelastography maxial amplitude]) were also included to the list of “predictive” DCI biomarkers (Table 2).
Alongside with Hct, the following laboratory parameters were also classified as “non-predictive”: seven SNPs (for RYR1 (ryanodine receptor type 1), CBS (cystathionine β-synthase), factor V Leiden, prothrombin, MTHFR (methylenetetetrahydrofolate reductase), factor XIII, and TNF-α genes), as well as ED1-fn (extra domain 1-fibronectin), EET, DHET (dihydroxyeicosatetraenoic acid), RBC (red blood cell) count, aPTT (activated partial thromboplastin time), AT-III (antithrombin III), PAP (plasmin-α2-antiplasmin complex), TNF-α, IL-1ra, creatinine, α2-AP (α2-antiplasmin), plasminogen, protein C, procalcitonin, MCV (mean corpuscular volume), and LDH (lactate dehydrogenase).
Of the biomarkers with insufficient level of evidence (“non-conclusive biomarkers”), eNOS (endothelial nitric oxide synthase) gene SNP and four protein-biomarkers showed predominantly positive associations with DCI risk: S100B, ET-1, vWF, and TAT (thrombin-antithrombin complex) (all with class III evidence).
Discussion
In this systematic review, we present 257 different laboratory biomarkers that were associated with the occurrence of DCI and/or vasospasm in SAH patients. These substances were integrated into a new biomarker database with subsequent stratification into different functional categories. Based on the current level of evidence, we selected a panel consisting of 6 genomics and 15 protein biomarkers with the currently best evidence for DCI prediction.
Biomarkers in modern precision medicine
Precision medicine is “an emerging approach for disease treatment and prevention that takes into account individual variability in genes, environment, and lifestyle for each person” [11]. As opposed to standardized medicine with “one-size-fits-all” philosophy, precision medicine carries a change for a more effective and personalized approach to patient care [2]. Recent biotechnological and laboratory advances allowed a better understanding of disease processes, as well as their early identification and prediction, such as genomic sequencing [2]. This “omics” technology, comprising of various genomics, proteomics, metabolomics, metallomics, glycomics, and microbiomics, presents a key element of precision medicine [2, 24, 29]. For example, genomics analysis has completely changed the rules of the game in neuro-oncology and became the blueprint for the new classification of brain tumors [9].
Value of DCI biomarkers in SAH patients
In SAH patients, a considerable number of laboratory biomarkers have already been investigated as potential predictors of functional outcome and certain complications [2]. However, there is still no established laboratory surrogate for preclinical diagnosis of DCI or monitoring of its progression [23]. Identification of reliable biomarker for cerebral ischemia is a challenge not only for SAH, but also for all acute stroke conditions [26].
The pathophysiological basis of DCI after SAH is complex with a cascade of biochemical events [2]. Recent publications consistently point to multifactorial etiology of DCI including cerebral vasoconstriction, cortical spreading depolarization, microthrombosis, and impaired autoregulation [31, 34]. In turn, different biochemical and cellular pathways may underlie each of DCI contributors. In particular, clear associations between severity of inflammatory processes (both within and without central nervous system) and development of cerebral vasospasm could be demonstrated in clinical, histopathologic, and experimental studies [10]. In addition, genotypic variability may also define the susceptibility of individual patients to secondary damage caused by DCI [31]. Laboratory parameters selected in this review as “most promising biomarkers” represent the following functional groups: “brain injury,” “inflammatory,” “coagulation cascade,” “metabolic,” “vasoactive,” and “oxidative” markers. All these processes are considered to be involved in the pathogenesis of DCI in SAH [31, 34]. In summary, these pathways present promising targets for the identification of reliable DCI biomarkers.
DCI risk stratification with genomics
First clinical reports on the role of genetic factors in the course of SAH go back to the pre-next generation sequencing era in the early 2000s [20, 35, 37]. In this systematic review, we report 50 studies analyzing genomic data in clinical series of SAH patients. In addition, results of 3 meta-analysis papers were also reviewed [3, 25, 30]. In total, data on association of 25 different genetic biomarkers with DCI risk were analyzed.
Due to the small size of SAH cohorts, a variety of polymorphisms tested in the candidate genes, and partially discrepant results, the current level of evidence for “DCI genomics” is sparse. To date, the following six gene SNPs can be regarded as most promising genetic predictors of DCI after SAH: EET metabolic pathways, COMT, HMGB1, ACE, PAI-1 promoter, and Hp. However, we strongly recommend further prospective evaluation of the abovementioned candidate genes, in order to improve the level of evidence and confirm the clinical utility of genomics for DCI prediction.
Vasospasm monitoring with proteomics
Over 200 laboratory parameters have been tested as preclinical biomarkers of impending DCI. Similar to genomics research, data on diagnostic value of protein research as proteomics for DCI prediction are insufficient to derive certain recommendations for most of the identified biomarkers. An additional limitation for eventual data generalization consists in non-uniform methodological approaches utilized in these studies: time points and frequency of measurements, reported values (cutoffs), and analyzed vasospasm-related endpoints.
Nevertheless, in this large and heterogeneous data set, we selected a panel consisting of 15 laboratory parameters that fulfill the criteria for predictive biomarkers, and may become a valuable diagnostic tool for early DCI recognition after further prospective evaluation: pNF-H, ADAMTS13, NPY, copeptin, HMGB1 protein, GFAP, BNP, NT pro-BNP, Hs-TnT, Tau, periostin, NLR, PLR, MPV:PLT, and PA-TEGMA.
General limitations
As already mentioned above, there is a wide range of limitations regarding the presented data. Methodological difficulties begin already at the stage of literature search. In particular, studies on laboratory (bio)-markers in SAH patients existed even before the terms “biomarker” and “marker” were established in the literature in the present context. Moreover, it might be believed that studies with negative reports on associations with laboratory DCI biomarkers could not be properly identified during the initial screening, since such data are likely to be mentioned only in full texts or, even, only as online supplements.
As to the included studies, the majority of them present data based on relatively small SAH cohorts, especially regarding the novel biomarkers. In addition, non-unique methodological approach to biomarker sampling, addressed polymorphism types, used DCI surrogates, and data presentation diminish the chances for data pooling and cumulative conclusions.
Nevertheless, in face of increasing relevance of “omics” signatures in the modern precision medicine, it is of paramount importance to (1) perform an overview of currently analyzed laboratory biomarkers of DCI after SAH and to (2) identify the most promising candidates bearing a potential to become a reliable biomarker of DCI in future.
Conclusions
Of 257 laboratory biomarkers identified in this systematic review, we selected a panel with six genetic (EET metabolic pathways, COMT, HMGB1, ACE, PAI-1 promoter, and Hp) and 15 non-genetic (pNF-H, ADAMTS13, NPY, copeptin, HMGB1 protein, GFAP, BNP, NT pro-BNP, Hs-TnT, Tau, periostin, NLR, PLR, MPV:PLT, and PA-TEGMA) biomarkers that showed positive predictive value for DCI occurrence. Due to still limited evidence level, we recommend further prospective evaluation of these biomarkers in large SAH cohorts.
References
Bacigaluppi S, Zona G, Secci F, Spena G, Mavilio N, Brusa G, Agid R, Krings T, Ottonello G, Fontanella M (2015) Diagnosis of cerebral vasospasm and risk of delayed cerebral ischemia related to aneurysmal subarachnoid haemorrhage: an overview of available tools. Neurosurg Rev 38:603–618. https://doi.org/10.1007/s10143-015-0617-3
Burrell C, Avalon NE, Siegel J, Pizzi M, Dutta T, Charlesworth MC, Freeman WD (2016) Precision medicine of aneurysmal subarachnoid hemorrhage, vasospasm and delayed cerebral ischemia. Expert Rev Neurother 16:1251–1262. https://doi.org/10.1080/14737175.2016.1203257
Chen C, Hu Z (2016) ApoE polymorphisms and the risk of different subtypes of stroke in the Chinese population: a comprehensive meta-analysis. Cerebrovasc Dis 41:119–138. https://doi.org/10.1159/000442678
Cohen JF, Korevaar DA, Altman DG, Bruns DE, Gatsonis CA, Hooft L, Irwig L, Levine D, Reitsma JB, de Vet HC, Bossuyt PM (2016) STARD 2015 guidelines for reporting diagnostic accuracy studies: explanation and elaboration. BMJ Open 6:e012799. https://doi.org/10.1136/bmjopen-2016-012799
D'Souza S (2015) Aneurysmal subarachnoid hemorrhage. J Neurosurg Anesthesiol 27:222–240. https://doi.org/10.1097/ANA.0000000000000130
de Oliveira Manoel AL, Jaja BN, Germans MR, Yan H, Qian W, Kouzmina E, Marotta TR, Turkel-Parrella D, Schweizer TA, Macdonald RL, collaborators S (2015) The VASOGRADE: a simple grading scale for prediction of delayed cerebral ischemia after subarachnoid hemorrhage. Stroke 46:1826–1831. https://doi.org/10.1161/STROKEAHA.115.008728
de Rooij NK, Rinkel GJ, Dankbaar JW, Frijns CJ (2013) Delayed cerebral ischemia after subarachnoid hemorrhage: a systematic review of clinical, laboratory, and radiological predictors. Stroke 44:43–54. https://doi.org/10.1161/STROKEAHA.112.674291
Degos V, Apfel CC, Sanchez P, Colonne C, Renuit I, Clarencon F, Nouet A, Boch AL, Pourmohamad T, Kim H, Gourraud PA, Young WL, Puybasset L (2012) An admission bioclinical score to predict 1-year outcomes in patients undergoing aneurysm coiling. Stroke 43:1253–1259. https://doi.org/10.1161/STROKEAHA.111.638197
DeWitt JC, Mock A, Louis DN (2017) The 2016 WHO classification of central nervous system tumors: what neurologists need to know. Curr Opin Neurol 30:643–649. https://doi.org/10.1097/WCO.0000000000000490
Gallia GL, Tamargo RJ (2006) Leukocyte-endothelial cell interactions in chronic vasospasm after subarachnoid hemorrhage. Neurol Res 28:750–758. https://doi.org/10.1179/016164106X152025
Garrido P, Aldaz A, Vera R, Calleja MA, de Alava E, Martin M, Matias-Guiu X, Palacios J (2017) Proposal for the creation of a national strategy for precision medicine in cancer: a position statement of SEOM, SEAP, and SEFH. Clin Transl Oncol 20:443–447. https://doi.org/10.1007/s12094-017-1740-0
Gerber CJ, Lang DA, Neil-Dwyer G, Smith PW (1993) A simple scoring system for accurate prediction of outcome within four days of a subarachnoid haemorrhage. Acta Neurochir 122:11–22
Grasso G, Alafaci C, Macdonald RL (2017) Management of aneurysmal subarachnoid hemorrhage: state of the art and future perspectives. Surg Neurol Int 8:11. https://doi.org/10.4103/2152-7806.198738
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schunemann HJ, Group GW (2008) GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. Bmj 336:924–926. https://doi.org/10.1136/bmj.39489.470347.AD
Hanggi D, Participants in the International Multi-Disciplinary Consensus Conference on the Critical Care Management of Subarachnoid H (2011) Monitoring and detection of vasospasm II: EEG and invasive monitoring. Neurocrit Care 15:318–323. https://doi.org/10.1007/s12028-011-9583-y
Harrod CG, Bendok BR, Batjer HH (2005) Prediction of cerebral vasospasm in patients presenting with aneurysmal subarachnoid hemorrhage: a review. Neurosurgery 56:633–654 discussion 633-654
Hayden JA, van der Windt DA, Cartwright JL, Cote P, Bombardier C (2013) Assessing bias in studies of prognostic factors. Ann Intern Med 158:280–286. https://doi.org/10.7326/0003-4819-158-4-201302190-00009
Jabbarli R, Reinhard M, Roelz R, Shah M, Niesen WD, Kaier K, Taschner C, Weyerbrock A, Van Velthoven V (2015) Early identification of individuals at high risk for cerebral infarction after aneurysmal subarachnoid hemorrhage: the BEHAVIOR score. J Cereb Blood Flow Metab 35:1587–1592. https://doi.org/10.1038/jcbfm.2015.81
Jaja BN, Lingsma H, Schweizer TA, Thorpe KE, Steyerberg EW, Macdonald RL, collaboration S (2015) Prognostic value of premorbid hypertension and neurological status in aneurysmal subarachnoid hemorrhage: pooled analyses of individual patient data in the SAHIT repository. J Neurosurg 122:644–652. https://doi.org/10.3171/2014.10.JNS132694
Khurana VG, Fox DJ, Meissner I, Meyer FB, Spetzler RF (2006) Update on evidence for a genetic predisposition to cerebral vasospasm. Neurosurg Focus 21:E3
Kubben PL, Germans MR, Jabbarli R (2017) Delayed cerebral ischemia after subarachnoid hemorrhage: comparing and integrating classification systems. Surg Neurol Int 8:121. https://doi.org/10.4103/2152-7806.208805
Kumar G, Shahripour RB, Harrigan MR (2016) Vasospasm on transcranial Doppler is predictive of delayed cerebral ischemia in aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis. J Neurosurg 124:1257–1264. https://doi.org/10.3171/2015.4.JNS15428
Lad SP, Hegen H, Gupta G, Deisenhammer F, Steinberg GK (2012) Proteomic biomarker discovery in cerebrospinal fluid for cerebral vasospasm following subarachnoid hemorrhage. J Stroke Cerebrovasc Dis 21:30–41. https://doi.org/10.1016/j.jstrokecerebrovasdis.2010.04.004
Ladner TR, Zuckerman SL, Mocco J (2013) Genetics of cerebral vasospasm. Neurol Res Int 2013:291895–291811. https://doi.org/10.1155/2013/291895
Lanterna LA, Ruigrok Y, Alexander S, Tang J, Biroli F, Dunn LT, Poon WS (2007) Meta-analysis of APOE genotype and subarachnoid hemorrhage: clinical outcome and delayed ischemia. Neurology 69:766–775. https://doi.org/10.1212/01.wnl.0000267640.03300.6b
Laskowitz DT, Grocott H, Hsia A, Copeland KR (1998) Serum markers of cerebral ischemia. J Stroke Cerebrovasc Dis 7:234–241
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6:e1000097. https://doi.org/10.1371/journal.pmed.1000097
Neil-Dwyer G, Cruickshank J (1974) The blood leucocyte count and its prognostic significance in subarachnoid haemorrhage. Brain 97:79–86
Roos A, Thompson R, Horvath R, Lochmuller H, Sickmann A (2017) Intersection of proteomics and genomics to “solve the unsolved” in rare disorders such as neurodegenerative and neuromuscular diseases. Proteomics Clin Appl. https://doi.org/10.1002/prca.201700073
Rosalind Lai PM, Du R (2015) Role of genetic polymorphisms in predicting delayed cerebral ischemia and radiographic vasospasm after aneurysmal subarachnoid hemorrhage: a meta-analysis. World Neurosurg 84:e932. https://doi.org/10.1016/j.wneu.2015.05.070
Rowland MJ, Hadjipavlou G, Kelly M, Westbrook J, Pattinson KT (2012) Delayed cerebral ischaemia after subarachnoid haemorrhage: looking beyond vasospasm. Br J Anaesth 109:315–329. https://doi.org/10.1093/bja/aes264
Sahu S, Nag DS, Swain A, Samaddar DP (2017) Biochemical changes in the injured brain. World J Biol Chem 8:21–31. https://doi.org/10.4331/wjbc.v8.i1.21
Spetzler RF, McDougall CG, Zabramski JM, Albuquerque FC, Hills NK, Russin JJ, Partovi S, Nakaji P, Wallace RC (2015) The Barrow ruptured aneurysm trial: 6-year results. J Neurosurg 123:1–9. https://doi.org/10.3171/2014.9.JNS141749
Sugimoto K, Shirao S, Koizumi H, Inoue T, Oka F, Maruta Y, Suehiro E, Sadahiro H, Oku T, Yoneda H, Ishihara H, Nomura S, Suzuki M (2016) Continuous monitoring of spreading depolarization and cerebrovascular autoregulation after aneurysmal subarachnoid hemorrhage. J Stroke Cerebrovasc Dis 25:e171–e177. https://doi.org/10.1016/j.jstrokecerebrovasdis.2016.07.007
Takenaka KV, Sakai N, Murase S, Kuroda T, Okumura A, Sawada M (2000) Elevated transferrin concentration in cerebral spinal fluid after subarachnoid hemorrhage. Neurol Res 22:797–801
Tasneem N, Samaniego EA, Pieper C, Leira EC, Adams HP, Hasan D, Ortega-Gutierrez S (2017) Brain multimodality monitoring: a new tool in neurocritical care of comatose patients. Crit Care Res Pract 2017:6097265. https://doi.org/10.1155/2017/6097265
Vergouwen MD, Frijns CJ, Roos YB, Rinkel GJ, Baas F, Vermeulen M (2004) Plasminogen activator inhibitor-1 4G allele in the 4G/5G promoter polymorphism increases the occurrence of cerebral ischemia after aneurysmal subarachnoid hemorrhage. Stroke 35:1280–1283. https://doi.org/10.1161/01.STR.0000128707.48644.7e
Vergouwen MD, Vermeulen M, van Gijn J, Rinkel GJ, Wijdicks EF, Muizelaar JP, Mendelow AD, Juvela S, Yonas H, Terbrugge KG, Macdonald RL, Diringer MN, Broderick JP, Dreier JP, Roos YB (2010) Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: proposal of a multidisciplinary research group. Stroke 41:2391–2395. https://doi.org/10.1161/STROKEAHA.110.589275
Washington CW, Zipfel GJ, Participants in the International Multi-disciplinary Consensus Conference on the Critical Care Management of Subarachnoid H (2011) Detection and monitoring of vasospasm and delayed cerebral ischemia: a review and assessment of the literature. Neurocrit Care 15:312–317. https://doi.org/10.1007/s12028-011-9594-8
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
RJ, DP, MDO, PD, KHW, and US are the members of the Editorial Board of Neurosurgical Review.
Ethical approval
Ethical approval is not required for this type of study.
Informed consent
Informed consent is not required for this type of study.
Electronic supplementary material
ESM 1
(DOCX 147 kb)
Rights and permissions
About this article
Cite this article
Jabbarli, R., Pierscianek, D., Darkwah Oppong, M. et al. Laboratory biomarkers of delayed cerebral ischemia after subarachnoid hemorrhage: a systematic review. Neurosurg Rev 43, 825–833 (2020). https://doi.org/10.1007/s10143-018-1037-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-018-1037-y