Abstract
The systemic inflammatory response index (SIRI) is a well-known marker of systemic inflammation reflecting the body’s inflammatory/immune state. The study aimed to evaluate the relationship between the SIRI on admission and aneurysmal subarachnoid hemorrhage (aSAH)-associated pneumonia and compare with other currently used bio-markers. We reviewed 562 successive patients with aneurysmal SAH who underwent endovascular treatment between January 2019 and September 2021. ASAH-associated pneumonia was diagnosed using the modified Centers for Disease Control and Prevention criteria. The SIRI on admission was calculated as monocyte count × neutrophil count / lymphocyte count. Multiple logistic regression models were used for data analysis. A total of 158 (28.11%) patients developed aSAH-associated pneumonia. Using the Multiple logistic regression analysis, a notable dose–response association was found between the elevated SIRI (fourth quartile) and aSAH-associated pneumonia (adjusted odds ratio = 6.759; 95% confidence interval [CI], 3.280–13.930; p < 0.001 [p for trend < 0.001]). The SIRI (0.701, 95% CI: 0.653–0.749) presented a higher area under the curve (AUC) than systemic immune- inflammation index (SII) (0.669, 95% CI: 0.620–0.718) (p = 0.089); neutrophil-to-lymphocyte ratio (NLR) (0.665, 95% CI: 0.616–0.714) (p = 0.035) and platelet-lymphocyte ratio (PLR) (0.587, 95% CI: 0.534–0.641) (p < 0.001). A higher SIRI on admission was associated with aSAH-associated pneumonia, which may guide further clinical trials of prophylactic antibiotic therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aneurysmal subarachnoid hemorrhage (aSAH)-associated pneumonia accounts for approximately 13.2–29.4% of patients with aSAH [1,2,3,4], which worsens patient prognosis and prolongs the duration of hospitalization [5, 6]. Endovascular treatment is currently considered an effective therapeutic option for aneurysmal subarachnoid hemorrhage, which is associated with less injury compared to surgical clipping. We hypothesized that there might be differences in the inflammatory status between these two treatments. And a previous study by Li R et al. showed variations in the risk of pneumonia formation between the two treatments [7]. However, the risk factors and predictors of pneumonia after aSAH treated with endovascular coiling have not yet been reported.
Previous studies have shown that subarachnoid hemorrhage rapidly activates an inflammatory cascade [8, 9], leading to leukocytosis, platelet aggregation, and lymphocyte apoptosis [10, 11]. There is mounting evidence indicating that aSAH-associated immunosuppression may contribute to the development of infections [9] and potentially influence outcomes in patients with aSAH. Therefore, it is necessary to identify bio-markers that can represent the body’s inflammatory response to predict the occurrence of aSAH-associated pneumonia. However, only lactate dehydrogenase and IL-6 have been reported as independent risk factors for post-stroke prognosis [2, 12, 13].
The systemic inflammatory response index (SIRI) is a well-known marker of systemic inflammation where the immune state of the body can be calculated by quantifying the absolute count of neutrophils, monocytes, and lymphocytes in the peripheral blood of patients. To date, SIRI has been widely used in tumor research [14, 15], and is performed immediately after admission. However, the predictive value of the SIRI for aSAH after endovascular treatment-associated pneumonia remains unclear.
Therefore, this study intends to evaluate the relationship between the SIRI and pneumonia after aneurysmal subarachnoid hemorrhage by endovascular treatment and compare it with other biomarkers.
Methods
Study design and patients
From January 2019 to September 2021, aSAH patients who received endovascular treatment at three large centers in China were recruited (Beijing Tiantan Hospital; Beijing Tongren Hospital; The Third Xiangya Hospital, Central South University). Written informed consent was obtained from all the patients. This study was reviewed and approved by the hospital review committees, and complied with the principles of the Declaration of Helsinki.
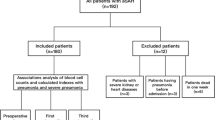
Considering the impact of systemic inflammation, and the dynamic changes in inflammation over time, we enrolled patients with aSAH aged 18 years or older, and had a time interval of symptom onset to hospital admission of less than 3 days and excluded those with additional aneurysms ( e.g., blood bubble, dissection, fusiform, infectious, cerebrovascular or Moyamoya malformation), incomplete data, history of neurosurgical interventions or those who had cancer, inflammatory diseases, autoimmune diseases, or admission accompanied by infectious disease. The diagnosis of aneurysm by digital subtraction angiography (DSA), computed tomographic angiography (CTA), and magnetic resonance angiography (MRA), and SAH by computed tomographic(CT).
Data collection
Baseline data were evaluated, including modified Fisher grades, general information (age, sex, weight, height, body temperature, respiratory rate, heart rate), past medical history (hypertension, hyperlipidemia, diabetes, previous cerebral ischemia/cerebral hemorrhage), personal history (smoking and drinking history), and occurrence of aSAH-associated pneumonia. The absolute neutrophil, lymphocyte, and monocyte counts were calculated from blood samples collected from all patients on admission. Blood samples was analyzed in the hospital within 6 h using an autoanalyzer (Mindray Hematology Analyzer BC-6900 series; Mindray Corporation, Shenzhen, China).
Definition of variables
We endorsed the diagnostic criteria for aSAH-associated pneumonia based on the modified Centers for Disease Control and Prevention criteria, excluding patients diagnosed with pneumonia before admission [16]. The IA morphological features, including aneurysm size, neck width, maximum height, maximum width, aspect ratio, size ratio, parent vessel diameter, shape, the Hunt-Hess scale, and the modified Fisher grades that were obtained by two readers with 5 years of experience in vascular neuroimaging, respectively. Assuming a large deviation in the morphologic parameters of IA, the final norm was determined by a third reader with 10 years of experience in vascular neuroimaging. The definition of deviations were as follows: (1) inconsonant interpretation of the aneurysmal shape, location, and the modified Fisher grades for categorical variables; and (2) the difference of data measurement is greater than 1 mm, for the continuous variables, such as aneurysm size, neck width, maximum height, maximum width, and parent vessel diameter.
SIRI is calculated as monocyte count × neutrophil count / lymphocyte count[17]; Systemic immune- inflammation index (SII) is calculated as platelet count × neutrophil count / lymphocyte count[18]; neutrophil-to-lymphocyte ratio (NLR) is calculated as neutrophil count / lymphocyte count[19]; platelet-lymphocyte ratio (PLR) is calculated as platelet count / lymphocyte count[20].
Statistical analysis
Continuous variables were presented as medians with interquartile range or means ± standard deviations, whereas categorical data were expressed as proportions. Baseline variables were compared between the χ2 test or Fisher`s exact test for categorical variables and the Mann–Whitney U test for continuous variables with a normal or skewed distribution. We used two models, no variable adjustment was performed in the first model, and all potential confounders were included in the multivariate logistic regression analysis in the two model. A nomogram was developed based on the results of the multivariable logistic analysis to predict the occurrence of pneumonia. The predictive performance of the nomogram was evaluated using receiver operating characteristic (ROC) analysis, C-index, and calibration curve. To assess the ability of SIRI, SII, NLR, and PLR to predict aSAH-associated pneumonia, we evaluated and calculated the area under the curve (AUC) and receiver operating characteristic (ROC) curves, which were also used to determine the optimal cut-off point using Youden index. Differences were evaluated for statistical significance using the DeLong test. Adjusted odds ratios (ORs) and their 95% confidence intervals (CIs) were calculated. In addition, the dose–response relationship between SIRI, SII, NLR, PLR, and aSAH-associated pneumonia was evaluated using Restricted cubic spline (RCS) combined with a logistic regression model, with four knots (at the 25th,50th,75th, and 95th percentiles) and the OR of all potential covariates was adjusted (Model 2).
All statistical analyses were performed using the R software version 3.6.3 (R Foundation for Statistical Computing, Vienna, Austria). A two-tailed p value < 0.05 was measured statistically significant.
Results
In total, 562 consecutive patients (median age: 58 years (range 49–66), women: 362 [64.4%]) were ultimately enrolled in this study (Fig. 1). The median with interquartile ranges [Median(IQR)] for each biomarker sample collected are as follows: SIRI,3.71 (1.93–6.29); SII, 2080.02 (1153.63–3384.03); NLR, 9.86 (5.49–14.92); PLR, 200.40 (149.23–288.52), respectively. Of those, 158 (28.11%) patients developed aSAH-associated pneumonia.
The patients were divided into two groups based on the occurrence of aSAH-associated pneumonia are shown in Table 1. The pneumonia group presented with higher modified fisher scale (p < 0.001) and higher Hunt-Hess scale (p < 0.001), higher rates of hypertension (p < 0.001), diabetes (p = 0.008), hyperlipidemia (p = 0.004), previous stroke (p < 0.001), previous or current smoking (p = 0.022), previous or current drinking (p = 0.035), SR (p = 0.038) in Table 1. We observed that the meaningfully association between posterior circulation aneurysms and the development of pneumonia (p = 0.001), while there was no significant correlation between aneurysms in other locations and pneumonia. Patients in the pneumonia group also had higher C-reactive protein (CRP) (p < 0.001), lymphocytes (p = 0.008), neutrophils (p < 0.001), monocytes (p < 0.001), and leukocytes (p < 0.001) in Table 1. SIRI, SII, NLR, and PLR of the pneumonia group are higher than that of the non-pneumonia group, respectively (5.58, 3.32–10.20 vs 3.19, 1.64–5.49, p < 0.001; 2941.94, 1732.85–4270.80 vs 1854.80, 942.43–2873.32, p < 0.001; 12.24, 8.76–18.49 vs 8.75, 4.52–13.74, p < 0.001; 244.89, 156.29–327.81 vs 192.25, 146.15–273.05, p = 0.001; Table 1).
The relationship between SIRI, SII, NLR, PLR, and aSAH-associated pneumonia compared to CRP, leucocyte, and neutrophil
Table 2 shows the association between SIRI, SII, NLR, and PLR quartiles and aSAH-associated pneumonia. Compared with the lowest quartile, patients in the second, third, and fourth SIRI, SII, NLR, and PLR quartiles were associated with an increased risk of the developing aSAH-associated pneumonia (p < 0.001). SIRI (adjusted OR:2.229, 95%CI: 1.064–4.670; 2.383,95% CI: 1.145–4.956; 6.759, 95% CI: 3.280–13.930, respectively), SII (adjusted OR: 2.016, 95% CI: 0.99–4.102; 2.132, 95% CI: 1.062–4.281; 5.910, 95% CI: 2.964–11.786; respectively), NLR (adjusted OR: 0.873, 95% CI: 0.428–1.781; 1.934, 95% CI: 0.989–3.785; 3.733, 95% CI: 1.938–7.191; respectively),PLR (adjusted OR: 1.191, 95% CI: 0.619–2.288; 2.366, 95% CI: 1.255–4.462; 2.141, 95% CI: 1.162–3.943; respectively) factors increase the risk of developing aSAH-associated pneumonia.
Figure 2 shows the non-linear association between the SIRI, SII, NLR, and PLR and the risk of high aSAH-associated pneumonia with restricted cubic splines (RCS). We found that the elevated SIRI was connected with a higher risk of aSAH-associated pneumonia.
Adjusted odds ratios of blood-based biomarkers of SIRI (A), SII (B), NLR (C), and PLR (D) with aSAH-associated pneumonia. SIRI = systemic inflammatory response index; SII = systemic immune inflammation index; NLR = neutrophil-to-lymphocyte ratio; PLR = platelet-to-lymphocyte ratio. Red line indicates adjusted odds ratio, and blue lines indicate the 95% confidence interval bands. The other vertical dashed lines indicate the first, second, and third quartiles of SIRI, SII, NLR, and PLR. The data were fitted using a logistic regression model of restricted cubic splines with 4 knots (25th, 50th, 75th, and 95th percentiles) for SIRI, SII, NLR, PLR, adjusting for potential covariates as model 2. The lowest 5% and highest 5% of patients are not shown in the figures because of small sample sizes
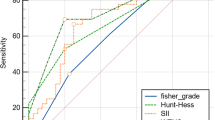
When comparing SIRI with PLR, SII, NLR CRP, leucocyte, and neutrophil of the predictive power, the SIRI (0.701, 95% CI: 0.653–0.749) presented a higher (AUC) than SII (0.669, 95% CI: 0.620–0.718), NLR (0.665, 95% CI: 0.616–0.714), PLR (0.587, 95% CI: 0.534–0.641), CRP (0.617, 95% CI: 0.576–0.658), leucocyte (0.691,95% CI:0.651–0.729), neutrophil(0.695, 95% CI: 0.655–0.733) ( Figs. 3, 4; Table 3). The optimal cut-off value for SIRI of aSAH-associated pneumonia was 4.703, which predicted aSAH-associated pneumonia with a sensitivity of 70.8, a specificity of 60.1 (Table 3). The SIRI improve the predictive ability of CRP, leucocyte, and neutrophil, (CRP + SIRI) (0.722, 95% CI: 0.683–0.759), (leucocyte + SIRI) (0.709, 95% CI: 0.670–0.746), (neutrophil + SIRI)(0.710, 95% CI: 0.671–0.747) (Fig. 4). Delong’s test showed that compared with SII (p = 0.089), leukocyte (p = 0.538), and neutrophil counts (p = 0.657), SIRI exhibited similar predictive abilities without statistically significant differences (Figs. 3, 4). Moreover, SIRI demonstrated superior predictive ability compared to NLR (p = 0.035), PLR (p < 0.001), and CRP (p = 0.019) individually (Figs. 3, 4). In the combined predictive models, the predictive abilities of SIRI + CRP (p = 0.171), SIRI + leukocyte count (p = 0.373), and SIRI + neutrophil count (p = 0.295) were comparable to that of SIRI alone, without statistically significant differences (Fig. 4). All potential significant factors associated with pneumonia occurrence were incorporated into the nomogram (Fig. 5A) to predict the probability of developing pneumonia. The calibration curves (Fig. 5B) revealed a strong agreement between the predicted and observed probabilities. Additionally, the C-index was 0.827, and the ROC curve (Fig. 5C) demonstrated excellent discrimination with an AUC of 0.827 (95% CI 0.789–0.865) (p < 0.001) for the nomogram in predicting pneumonia.
Comparison for the Predictive Ability of Blood-based Biomarkers for aSAH-associated Pneumonia. aDeLong et al.; ROC = receiver operating characteristic. SIRI (0.701 [0.653–0.749]); SII (0.669 [0.620–0.718]); NLR (0.665 [0.616–0.714]); PLR (0.587 [0.534–0.641]). Furthermore, the difference between SIRI and NLR (P = 0.035); SIRI and PLR (P < 0.001); SII and PLR (P < 0.001); NLR and PLR (P < 0.001) have statistical significance
Utility of nomogram for predicting pneumonia after aneurysmal subarachnoid hemorrhage by endovascular treatment. Points were age, modified Fisher score, hypertension, diabetes, hyperlipidemia, previous stroke, heart disease, previous or current smoking, previous or current drinking, location, SR and SIRI, MFS = modified Fisher score
Discussions
Here, we show that a higher SIRI is associated with aSAH-associated pneumonia. Pneumonia is a common and frequent complication in patients with stroke in the first 90 days, with a peak incidence on the third day [21]. Poor functional outcome even death was correlated with aSAH-associated pneumonia [22], so the prevention of pneumonia is very important. Previous studies have shown that higher SIRI is closely associated with the severity and prognosis of stroke [23]. We investigated whether SIRI can predict aSAH-associated pneumonia and evaluated its predictive power compared with SII, NLR, and PLR. Our study demonstrated that SIRI, SII, NLR, and PLR on admission were independently correlated with aSAH-associated pneumonia. ROC curve analysis and nomogram elucidated that SIRI on admission have a well predictive ability.
In several studies, an association between inflammation and the development of pneumonia has been reported. And SIRI, as an inflammatory biomarker, we found for the first time that it can be used to predict the occurrence of pneumonia. There are several convincing reasons for the close relationship between the SIRI and aSAH-associated pneumonia. The first explanation might be immunodepression after aneurysmal subarachnoid hemorrhage [9].The initial ruptured aneurysm deposits blood in the subarachnoid space [24]. Over time, the breakdown and degradation of red blood cells leads to the deposition of hemoglobin. Methemoglobin, heme, and chlorinated heme produced by the breakdown of red blood cells may lead to the activation of TLR4, an inflammatory cascade signal that damages neurons [24,25,26,27,28,29]. Due to immunosuppression, neutrophils are demarginated and stimulated by Inflammatory factors; however, lymphocytes undergo apoptosis, with a shift from pro-inflammatory Th-1–type response to an anti-inflammatory Th-2–type response; this outcome increases the susceptibility [30, 31], which may increases the probability of pneumonia following subarachnoid hemorrhage [32, 33]. Moreover, it has been reported that monocytes could be target cells in immunosuppression; monocyte deactivation, with decreased antigen presentation capacity and depressed secretion of proinflammatory cytokines, increased the risk for pneumonia. The second explanation might be brain injury on hospital admission. In the relationship between the acute brain injury and the occurrence of pneumonia, inflammation potentially assumes a significant role. Studies have shown that [34] following acute brain injury, there is a subsequent activation of the body’s inflammatory response, triggering the activation of immune cells and the release of inflammatory factors. This cascading process has the potential to exert a notable influence on the inflammatory response within the pulmonary system, subsequently impacting the susceptibility to infection. For example, within several hours after brain injury, T cell inactivation is seen. These variations could play important roles in acute brain injury and precede pneumonia. In addition, alterations in immune system after brain injury that could develop infectious complications as follows: decrease of monocyte function; decrease of T cell functions (decreased response to mitogenic activation and decreased IL-2 production); and decrease of B cell function. However, further studies are required to confirm these hypotheses.
Our study also suggests a relation between older age (p < 0.001) and aSAH-associated pneumonia. Moreover, the proportion of hypertension, diabetes, hyperlipidemia, previous stroke, heart disease, previous or current smoking, previous or current drinking were significantly higher in the pneumonia group. Interestingly, we also found that the SIRI was higher when the patients were older. Previous studies have shown that patients with underlying diseases have a high inflammatory load [35] this suggested that SIRI may be a mediating effect between advanced age and pneumonia. However, further research is needed to confirm. As in previous studies, patients with aSAH-associated pneumonia had poorer outcomes during hospitalization and discharge than patients without aSAH-associated pneumonia [22, 35]. Although studies have shown that prophylactic use of antibiotics in stroke patients cannot improve their functional outcome [36] and antibiotics are not recommended for patients with elevated SIRI, enhanced respiratory management can be performed. During the patient’s hospitalization, we observed a total of eight deaths. Of these, two patients did not develop pneumonia during hospitalization, as previously reported [37], which reminds us of the importance of early detection of pneumonia.
Strength and limitations
This study firstly showed that the SIRI on admission can predict aSAH-associated pneumonia. The strengths of this study are as follows. First, patients with aneurysmal subarachnoid hemorrhage all were treated with endovascular treatment, the mainstream treatment now, avoided the bias caused by the clipping of treatment Second, several necessary parameters for SIRI required on admission, could be obtained quickly.
Our study had several limitations. The reasons for the limited predictive ability of inflammatory biomarkers may include the following: (1) The occurrence of pneumonia is a complex process influenced by various factors, including pathogen infection, immune status, and the body’s resistance. While inflammatory markers can reflect the body’s inflammatory state, they may not encompass all possible factors comprehensively. (2) Sufficient sample size is required for the establishment of predictive models, and our sample size may be relatively small. (3) The lack of external validation studies on the predictive ability of inflammatory biomarkers may limit the assessment of their accuracy and stability. This is a retrospective study that restricts the generalization of the findings to some extent. Prospective multicenter studies should be considered in the future. The peak incidence of stroke-associated pneumonia usually occurred three to seven days after admission [21], and our study showed that the peak incidence of the occurrence time of pneumonia was mainly concentrated between 2–4 days after admission, with a maximum value of 16 days and a minimum value of 2 days, the SIRI on admission could not dynamically reveal the patient’s inflammatory state at that time. Furthermore, our database was insufficient for collecting the specific distribution of lymphocyte subtypes (such as T cells or B cells).
Conclusions
In conclusion, SIRI is a reliable predictor for pneumonia after aneurysmal subarachnoid hemorrhage by endovascular treatment, which may provide insights into further clinicalpractices of prophylactic antibiotic therapy. Nevertheless, further prospective multicenter studies are needed to verify the association between SIRI and pneumonia after aneurysmal subarachnoid hemorrhage by endovascular treatment.
Data availability
We enrolled patients at three centers (Beijing Tiantan Hospital, Capital Medical University; Beijing Tongren Hospital, Capital Medical University; The Third Xiangya Hospital, Central South University) from January 2019 to September 2021. If necessary, we can provide the data set to the editor.
References
Zheng S, Wang H, Chen G, Shangguan H, Yu L, Lin Z et al (2021) Higher serum levels of lactate dehydrogenase before microsurgery predict poor outcome of aneurysmal subarachnoid hemorrhage. Front Neurol 12:720574. https://doi.org/10.3389/fneur.2021.720574
Ding C, Peng L, Lin Y, Yu L, Wang D, Kang D (2019) Elevated lactate dehydrogenase level predicts postoperative pneumonia in patients with aneurysmal subarachnoid hemorrhage. World neurosurgery 129:e821–e830. https://doi.org/10.1016/j.wneu.2019.06.041
Alaraj A, Hussein A, Esfahani D, Amin-Hanjani S, Aletich V, Charbel F (2017) Reducing length of stay in aneurysmal subarachnoid hemorrhage: A three year institutional experience. J Clin Neurosci: Off J Neurosurg Soc Australasia 42:66–70. https://doi.org/10.1016/j.jocn.2017.03.049
Levine J, Kofke A, Cen L, Chen Z, Faerber J, Elliott J et al (2010) Red blood cell transfusion is associated with infection and extracerebral complications after subarachnoid hemorrhage. Neurosurgery 66:312–8; discussion 8. https://doi.org/10.1227/01.Neu.0000363747.47587.6c
Frontera J, Fernandez A, Schmidt J, Claassen J, Wartenberg K, Badjatia N et al (2008) Impact of nosocomial infectious complications after subarachnoid hemorrhage. Neurosurgery 62:80–7; discussion 7. https://doi.org/10.1227/01.Neu.0000311064.18368.Ea
Giede-Jeppe A, Reichl J, Sprügel M, Lücking H, Hoelter P, Eyüpoglu I et al (2019) Neutrophil-to-lymphocyte ratio as an independent predictor for unfavorable functional outcome in aneurysmal subarachnoid hemorrhage. J Neurosurg 132:400–407. https://doi.org/10.3171/2018.9.Jns181975
Li R, Lin F, Chen Y, Lu J, Han H, Yan D et al (2021) In-hospital complication-related risk factors for discharge and 90-day outcomes in patients with aneurysmal subarachnoid hemorrhage after surgical clipping and endovascular coiling: a propensity score-matched analysis. J Neurosurg 1–12. https://doi.org/10.3171/2021.10.Jns211484
Chaudhry S, Kahlert U, Kinfe T, Lamprecht A, Niemelä M, Hänggi D et al (2020) Elevated systemic IL-10 Levels indicate immunodepression leading to nosocomial infections after aneurysmal subarachnoid hemorrhage (SAH) in patients. Int J Mol Sci 21. https://doi.org/10.3390/ijms21051569
Sarrafzadeh A, Schlenk F, Meisel A, Dreier J, Vajkoczy P, Meisel C (2011) Immunodepression after aneurysmal subarachnoid hemorrhage. Stroke 42:53–58. https://doi.org/10.1161/strokeaha.110.594705
Gibson P, Cuthbertson B, Croal B, Rae D, El-Shafei H, Gibson G et al (2010) Usefulness of neutrophil/lymphocyte ratio as predictor of new-onset atrial fibrillation after coronary artery bypass grafting. Am J Cardiol 105:186–191. https://doi.org/10.1016/j.amjcard.2009.09.007
Tao C, Wang J, Hu X, Ma J, Li H, You C (2017) Clinical value of neutrophil to lymphocyte and platelet to lymphocyte ratio after aneurysmal subarachnoid hemorrhage. Neurocrit Care 26:393–401. https://doi.org/10.1007/s12028-016-0332-0
Chaudhry S, Stoffel-Wagner B, Kinfe T, Güresir E, Vatter H, Dietrich D et al (2017) Elevated systemic IL-6 Levels in patients with aneurysmal subarachnoid hemorrhage is an unspecific marker for Post-SAH complications. Int J Mol Sci 18. https://doi.org/10.3390/ijms18122580
Warusevitane A, Karunatilake D, Sim J, Smith C, Roffe C (2016) Early Diagnosis of pneumonia in severe stroke: clinical features and the diagnostic role of C-Reactive protein. PloS one 11:e0150269. https://doi.org/10.1371/journal.pone.0150269
Chen Y, Sun J, Hu D, Zhang J, Xu Y, Feng H et al (2021) Predictive Value of pretreatment lymphocyte-to-monocyte ratio and platelet-to-lymphocyte ratio in the survival of nasopharyngeal carcinoma patients. Cancer Manag Res 13:8767–8779. https://doi.org/10.2147/cmar.S338394
Yang H, Wang K, Li B, Li S, Li Y, Yuan L (2021) The prognostic role of blood inflammatory biomarkers and eGFR mutation status in stage iiia/n2 non-small cell lung cancer patients treated with trimodality therapy. Front Oncol 11:707041. https://doi.org/10.3389/fonc.2021.707041
Smith C, Kishore A, Vail A, Chamorro A, Garau J, Hopkins S et al (2015) Diagnosis of stroke-associated pneumonia: recommendations from the pneumonia in Stroke Consensus Group. Stroke 46:2335–2340. https://doi.org/10.1161/strokeaha.115.009617
Qi Q, Zhuang L, Shen Y, Geng Y, Yu S, Chen H et al (2016) A novel systemic inflammation response index (SIRI) for predicting the survival of patients with pancreatic cancer after chemotherapy. Cancer 122:2158–2167. https://doi.org/10.1002/cncr.30057
Hu B, Yang X, Xu Y, Sun Y, Sun C, Guo W et al (2014) Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res: Off J Am Assoc Cancer Res 20:6212–6222. https://doi.org/10.1158/1078-0432.Ccr-14-0442
Peng L, Wang Y, Liu F, Qiu X, Zhang X, Fang C et al (2020) Peripheral blood markers predictive of outcome and immune-related adverse events in advanced non-small cell lung cancer treated with PD-1 inhibitors. Cancer Immunol Immunother : CII 69:1813–1822. https://doi.org/10.1007/s00262-020-02585-w
Dommershuijsen L, Ruiter R, Erler N, Rizopoulos D, Ikram M, Ikram M (2022) Peripheral Immune cell numbers and C-Reactive protein in parkinson’s disease: results from a population-based study. J Parkinson’s Dis 12:667–678. https://doi.org/10.3233/jpd-212914
de Jonge J, van de Beek D, Lyden P, Brady M, Bath P, van der Worp H (2021) Temporal profile of pneumonia after stroke. Stroke STROKEAHA120032787. https://doi.org/10.1161/strokeaha.120.032787
Rass V, Gaasch M, Kofler M, Schiefecker A, Ianosi B, Rhomberg P et al (2018) Systemic inflammatory response syndrome as predictor of poor outcome in nontraumatic subarachnoid hemorrhage patients. Crit Care Med 46:e1152–e1159. https://doi.org/10.1097/ccm.0000000000003429
Zhang Y, Xing Z, Zhou K, Jiang S (2021) The predictive role of systemic inflammation response index (SIRI) in the prognosis of stroke patients. Clin Interv Aging 16:1997–2007. https://doi.org/10.2147/cia.S339221
Lucke-Wold B, Logsdon A, Manoranjan B, Turner R, McConnell E, Vates G et al (2016) Aneurysmal Subarachnoid hemorrhage and neuroinflammation: a comprehensive review. Int J Mol Sci 17:497. https://doi.org/10.3390/ijms17040497
Pradilla G, Chaichana K, Hoang S, Huang J, Tamargo R (2010) Inflammation and cerebral vasospasm after subarachnoid hemorrhage. Neurosurg Clin N Am 21:365–379. https://doi.org/10.1016/j.nec.2009.10.008
Muhammad S, Hänggi D (2021) Inflammation and anti-inflammatory targets after aneurysmal subarachnoid hemorrhage. Int J Mol Sci 22. https://doi.org/10.3390/ijms22147355
Atangana E, Schneider U, Blecharz K, Magrini S, Wagner J, Nieminen-Kelhä M et al (2017) Intravascular inflammation triggers intracerebral activated microglia and contributes to secondary brain injury after experimental subarachnoid hemorrhage (eSAH). Transl Stroke Res 8:144–156. https://doi.org/10.1007/s12975-016-0485-3
de Oliveira MA, Macdonald R (2018) Neuroinflammation as a target for intervention in subarachnoid hemorrhage. Front Neurol 9:292. https://doi.org/10.3389/fneur.2018.00292
Frontera J, Provencio J, Sehba F, McIntyre T, Nowacki A, Gordon E et al (2017) The Role of Platelet Activation and Inflammation in Early Brain Injury Following Subarachnoid Hemorrhage. Neurocrit Care 26:48–57. https://doi.org/10.1007/s12028-016-0292-4
Morga R, Dziedzic T, Moskala M, Slowik A, Pera J (2020) Clinical Relevance of changes in peripheral blood cells after intracranial aneurysm rupture. J Stroke Cerebrovasc Dis: Off J Natl Stroke Assoc 29:105293. https://doi.org/10.1016/j.jstrokecerebrovasdis.2020.105293
Chamorro Á, Meisel A, Planas A, Urra X, van de Beek D, Veltkamp R (2012) The immunology of acute stroke. Nat Rev Neurol 8:401–410. https://doi.org/10.1038/nrneurol.2012.98
Prass K, Meisel C, Höflich C, Braun J, Halle E, Wolf T et al (2003) Stroke-induced immunodeficiency promotes spontaneous bacterial infections and is mediated by sympathetic activation reversal by poststroke T helper cell type 1-like immunostimulation. J Exp Med 198:725–736. https://doi.org/10.1084/jem.20021098
Tam A, Ilodigwe D, Mocco J, Mayer S, Kassell N, Ruefenacht D et al (2010) Impact of systemic inflammatory response syndrome on vasospasm, cerebral infarction, and outcome after subarachnoid hemorrhage: exploratory analysis of CONSCIOUS-1 database. Neurocrit Care 13:182–189. https://doi.org/10.1007/s12028-010-9402-x
Lauzier D, Jayaraman K, Yuan J, Diwan D, Vellimana A, Osbun J et al (2023) Early Brain injury after subarachnoid hemorrhage: incidence and mechanisms. Stroke 54:1426–1440. https://doi.org/10.1161/strokeaha.122.040072
Finlayson O, Kapral M, Hall R, Asllani E, Selchen D, Saposnik G (2011) Risk factors, inpatient care, and outcomes of pneumonia after ischemic stroke. Neurology 77:1338–1345. https://doi.org/10.1212/WNL.0b013e31823152b1
Westendorp W, Vermeij J, Zock E, Hooijenga I, Kruyt N, Bosboom H et al (2015) The Preventive Antibiotics in Stroke Study (PASS): a pragmatic randomised open-label masked endpoint clinical trial. Lancet (London, England) 385:1519–1526. https://doi.org/10.1016/s0140-6736(14)62456-9
Nam K, Kim T, Lee J, Kwon H, Lee Y, Ko S et al (2018) High neutrophil-to-lymphocyte ratio predicts stroke-associated pneumonia. Stroke 49:1886–1892. https://doi.org/10.1161/strokeaha.118.021228
Acknowledgements
None.
Funding
This study was supported by Natural Science Foundation of China (81771233, 82171290), Research and Promotion Program of Appropriate Techniques for Intervention of Chinese High-risk Stroke People(GN-2020R0007), BTH Coordinated Development-Beijing Science and Technology Planning Project(Z181100009618035), Beijing Municipal Administration of Hospitals’ Ascent Plan (DFL20190501), and Beijing Natural Science Foundation (19L2013; 22G10396).
Author information
Authors and Affiliations
Contributions
Yongkai Qin , Aihua Liu , Baorui Zhang and Zhongxue Wu conceived the study concept. Yongkai Qin ,Lang Liu, Shangfeng Zhao and Wei Wang participated in the design of the study. Yongkai Qin, Lang Liu, Mingyang Han, Siyuan Dong, Songfeng Zhao, Yan Miao, Shenkun Tang collected data. Siyuan Dong, Yongkai Qin, Mingyang Han, Shangfeng Zhao and Wei Wang analyzed and interpreted the data. Yongkai Qin, Lang Liu ,Baorui Zhang, and Aihua Liu drafted and edited the manuscript. Aihua Liu, Baorui Zhang, Yongkai Qin, and Lang Liu had full access to all the data in the study and takes responsibility for the data and the accuracy of the data analysis. All the authors approved the final manuscript.
Corresponding authors
Ethics declarations
Ethical approval
Informed consent was obtained for all participants. This study was approved by the centers review board (Beijing Tiantan Hospital, Capital Medical University; Beijing Tongren Hospital, Capital Medical University; The Third Xiangya Hospital, Central South University) and was in accordance with the principles of the Declaration of Helsinki.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Qin, Y., Liu, L., Zhao, S. et al. Blood inflammatory biomarkers predict in-hospital pneumonia after endovascular treatment of aneurysm in patients with aneurysmal subarachoid hemorrhage. Neurosurg Rev 46, 171 (2023). https://doi.org/10.1007/s10143-023-02082-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10143-023-02082-5