Abstract
The effects of superficial temporal artery to middle cerebral artery (STA-MCA) double bypass on recurrent hemorrhage in the operated hemisphere in hemorrhagic moyamoya disease (HMD) have not been clearly demonstrated. This study evaluated the effectiveness of STA-MCA double bypass in the prevention of further hemorrhagic or ischemic events in the operated hemispheric sides in comparison to the conservatively treated non-operated sides. We retrospectively analyzed 52 hemispheres of 36 patients with adult-onset HMD treated with STA-MCA double bypass. Twenty and 16 patients underwent unilateral (unilateral group) and bilateral (bilateral group) surgery, respectively. In addition, the perioperative and long-term outcomes of the 52 operated sides and 20 non-operated sides in the unilateral group were compared. All bypass surgeries were successful, but 21% of the operated sides showed hyperperfusion as estimated by our methods. Perioperative mortality and morbidity rate were 0% and 5.6%, respectively. Concerning long-term follow-up, the annual rebleeding rate (ARR) in the unilateral and bilateral group was 2.7% and 2.6%/person-year, respectively (p = 0.256). The ARR in the operated and non-operated sides was 1.1% and 1.8%/side-year, respectively (p = 0.163). Two of 20 non-operated sides suffered from ischemic infarction during the follow-up period, while none of the 52 operated sides experienced ischemic events (p < 0.05). Although the long-term rebleeding rate in the operated hemisphere tended to be lower after STA-MCA double bypass compared with that in the non-operated hemisphere, the difference was not statistically significant. In conclusion, while STA-MCA double bypass could not clearly prevent rebleeding, it can prevent further ischemic attacks in patients with HMD.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Moyamoya disease is a progressive occlusive cerebrovascular disease of the intracranial internal carotid arteries (ICA) with extensive fine collateral vessels [26]. In moyamoya disease, characteristic clinical features may be seen including serious neurological deficits due to cerebral ischemic and/or hemorrhagic insults [13, 22, 26]. Intracranial hemorrhage is more common in adult patients and the natural history of hemorrhagic moyamoya disease (HMD) is very poor. Recurrent hemorrhagic attacks have been frequently observed (28–63%) in patients with HMD [6, 12, 18], which increase the mortality rate from 6.8 to 28.6% [12]. The Japan Adult Moyamoya (JAM) trial, which was a multicenter, prospective, randomized controlled trial, aimed to clarify whether an extracranial-intracranial bypass can prevent rebleeding in patients with HMD. This study indicated that superficial temporal artery to middle cerebral artery (STA-MCA) bypass had greater potential than conservative therapies to prevent rebleeding [16]. However, no other large long-term clinical studies have attempted to determine the effective treatments for HMD. Additionally, in most previous HMD studies, including the JAM trial, STA-MCA bypass was performed on both hemispheres and surgical outcomes were analyzed in each patient. We have treated approximately 300 patients with moyamoya disease at a single Japanese institute over 15 years. In patients with HMD, STA-MCA double bypass was basically performed only on the hemorrhagic side and the contralateral side was conservatively observed. In this paper, we present a retrospective analysis of the perioperative and long-term outcomes in adult-onset HMD treated with STA-MCA double bypass and investigate the effectiveness of surgery, focusing on the prevention of further rebleeding in the operated hemispheric sides.
Methods
Patient selection and determinants of the operative side
From 1999 to 2014, 445 sides of 294 consecutive moyamoya disease patients underwent STA-MCA bypass surgery at the Department of Neurosurgery, Tokyo Women’s Medical University, Tokyo, Japan. Thirty-six of 294 patients were adult-onset patients with HMD, whose 52 hemispheres were treated with STA-MCA double bypass. This study retrospectively investigated 52 sides of 36 HMD patients. These 36 patients satisfied the diagnostic criteria of the Ministry of Health, Labor, and Welfare, Tokyo, Japan, for moyamoya disease [24]. There were no patients with unilateral moyamoya disease in this series. Surgical treatment was performed only after informed consent was obtained. This study was approved by an institutional review committee.
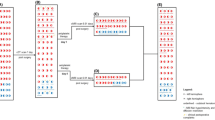
The operative side was determined as follows (Fig. 1): patients whose hemorrhagic sites were clearly detected by computed tomography (CT) and/or magnetic resonance imaging (MRI) had STA-MCA double bypass only on the hemorrhagic side (unilateral group). The patients whose hemorrhagic sites were bilateral or were not evidently detected because of massive intraventricular hemorrhage, or those with accompanying ischemic events in the non-hemorrhagic side, had bypass surgery on both sides (bilateral group). Of these 36 patients, 20 and 16 comprised the unilateral and bilateral group, respectively. As the 20 unilaterally operated patients had 20 non-operated sides, we also created a group composed of the 52 operated sides and another that included the 20 non-operated sides.
The distribution of the surgical group and operative sides. The 36 patients were divided into unilateral surgical group (20 patients) and bilateral surgical group (16 patients). In the unilateral surgical group, the sides were divided into operated side (20 sides) and contralateral non-operated side (20 sides). Adding to 32 sides of 16 bilateral group patients, total operated side was up to 52 sides
Surgical procedures and definition of hyperperfusion
STA-MCA double bypass was performed in all patients. Under general anesthesia, mainly using propofol, the parietal and frontal branches of the STA were anastomosed to the supra- and infrasylvian M4 segments of the MCA in a side-to-end fashion (Fig. 2a). We used a colored flexible cylindrical silicone rubber stent (300–400 μm in diameter and 3–4 mm in length) that helped to confirm the ostium of the transparent thin-walled recipient arteries and make precise anastomoses possible (Fig. 2b) [23]. The patency of anastomoses was confirmed by Doppler ultrasonography intraoperatively. Immediately after the bypass surgery, postoperative hyperperfusion was evaluated by measuring the resting regional cerebral blood flow (rCBF) in the bilateral MCA territories with xenon CT under continued sedation with propofol. Postoperative hyperperfusion was defined as a > 50% increase in postoperative ipsilateral rCBF compared with postoperative contralateral rCBF (method 1) or > 100% increase in corrected postoperative ipsilateral rCBF compared with preoperative ipsilateral rCBF (method 2) [11, 30]. Corrected rCBF was calculated as follows: corrected rCBF = postoperative ipsilateral rCBF × preoperative contralateral rCBF/postoperative contralateral rCBF [11]. If hyperperfusion was detected, sedation with propofol was maintained for a few to several days depending on the repeated rCBF studies. All patients received antiplatelet agents (mainly aspirin, 100 mg daily) after the bypass surgery. The patients in the unilateral group underwent STA-MCA double bypass on the hemorrhagic side alone, and those in the bilateral group underwent operations on both sides. In the bilateral surgical group, the contralateral STA-MCA double bypass was performed at least 3 months after the ipsilateral bypass.
Patient follow-up
Perioperative complications related to bypass surgery were investigated during hospitalization and perioperative mortality and morbidity rate was estimated at 30 days postoperatively. All patients were followed up at our outpatient clinic for at least 36 months (64.6 months on average). The incidence of rebleeding, cerebral infarction, and other diseases during long-term follow-up was recorded. To analyze the outcomes, the modified Rankin scale (mRS) scores were assessed before surgery, at 30 days after the surgery, and at the latest follow-up. Morbidity was defined as all permanent morbidity (e.g., any neurological or other deficit) not present before surgery. Angiographic evaluation was performed within 7 days, at 3 months, and at 1 year after the surgery by brain magnetic resonance angiography (MRA) or digital subtraction angiography (DSA) to evaluate the patency of the bypasses and changes in abnormal collaterals.
Statistical analysis
Descriptive statistics are presented as mean ± standard deviation. Statistical analyses were performed using the χ2 test and statistical significance was set as p < 0.05. Freedom from rebleeding was estimated using the Kaplan-Meier product-limit method, and the log-rank test was used to compare the difference between the two groups. The risk hazard ratio was estimated using the Cox proportional hazard model. The generalized Wilcoxon test, which places more weight on early events, was also used to assess the sensitivity of the log-rank test. All data were calculated using a commercially available software package (JMP® 11.0; SAS Institute Inc. Cary, NC).
Results
Clinical features of patients with HMD
The clinical features of the patients with HMD are summarized in Table 1. Of the 36 patients, 14 were male and 22 were female (age range, 20 to 68 years). Hypertension was observed in 12 patients and family history of moyamoya disease was noted in 3. There were 11 patients with a history of cerebral ischemic insults, such as symptomatic cerebral infarction, and/or transient ischemic attacks (TIAs), such as aphasia, dysarthria, and motor weakness. Other comorbidities such as diabetes mellitus, hyperlipidemia, and cardiovascular disease were rarely found. A favorable preoperative mRS score of 0–2 was found in 14 patients (38.9%).
Table 1 also shows a summary of the 41 episodes of intracranial hemorrhage in each group. In the bilateral group, 5 of 16 patients experienced a second hemorrhagic insult before the STA-MCA bypass. The 41 episodes included 7 putaminal, 7 thalamic, 4 caudate, 6 subcortical, 1 subarachnoid, and 16 intraventricular hemorrhages.
Perioperative outcomes
STA-MCA double bypass was successfully carried out in all 52 sides of the 36 patients. The perioperative outcomes (within 30 days of surgery) are summarized in Table 2. As for the hemodynamic study, rCBF in the operative MCA territory improved after the surgery, with a 30.1 ± 23.5% increase measured by method 1 and a 34.7 ± 30.9% increase measured by method 2. Postoperative hyperperfusion estimated by our methods was detected in 11 (21%) operated sides (Fig. 3a). Sedation was continued in these patients to maintain a stable blood pressure for several days after the surgery.
An illustrative case that demonstrated focal vasogenic edema with hyperperfusion. Xenon CT demonstrated focal hyperperfusion just after left STA-MCA double bypass (a). MRI 2 days after surgery showed high-intensity signal in FLAIR (b) and ADC (c), and a slightly high intensity on DWI (d) in the subcortical region at the anastomotic site
Perioperative transient neurological deterioration (TND) presenting with motor weakness, aphasia, and/or dysarthria was observed in 6 operated sides. Partial seizures were observed in 4 operated sides. The symptoms were reversible and disappeared within 1 week. The 7 operated sides showed focal vasogenic edema at the bypassed site on postoperative MRI. This was evidenced by high intensity at the lesion on fluid-attenuated inversion recovery (FLAIR) and apparent diffusion coefficient (ADC), and a slightly high intensity on diffusion-weighted imaging (DWI) (Fig. 3b–d). The occurrence of focal vasogenic edema is not always correlated with either postoperative hyperperfusion or TNDs. The edema gradually disappeared in all but the 4 of the cases that subsequently developed subcortical hemorrhage in the lesion. The 4 operated sides with focal hyperperfusion eventually developed subcortical hemorrhage at the bypassed site. Among the 36 patients, 2 had permanent neurological deficits due to postoperative intracerebral hemorrhage, and their mRS score deteriorated at 30 days after the surgery. The 30-day perioperative mortality and morbidity rates were 0% and 5.6%, respectively.
Long-term outcomes
The long-term outcomes are shown in Table 3. Five patients experienced rebleeding during the long-term follow-up period (mean, 64.6 months). The rebleeding rate was 13.9% (2.6%/person-year). Three patients in the unilateral group had rebleeding: 1 thalamic, 1 subcortical, and 1 intraventricular. Two patients in the bilateral group also experienced rebleeding: 1 thalamic and 1 caudate. Of the 5 patients, 2 exhibited headache only, 2 exhibited a slight deterioration of their neurological features, and 1 died of rebleeding. Concerning ischemic events, 2 patients in the unilateral group experienced ischemic infarction during the follow-up period. Both occurred in the non-operated sides, and STA-MCA double bypass was subsequently performed. The p values comparing the unilateral and bilateral group using the log-rank test were 0.258, and that comparing operated and non-operated sides was 0.029. The overall stroke occurred in 5 patients of unilateral group, and 2 patients in bilateral group (p = 0.187).
Two patients underwent ventriculoperitoneal shunt placement due to slowly progressing hydrocephalus. Three patients died, 1 each because of rebleeding, cardiovascular disease, and unknown events. The mortality rate of this series was 8.3% (1.5%/person-year). At the latest follow-up, deterioration of mRS score compared with that at discharge was observed in 8 patients, while improvements after rehabilitation were found in 5 patients. A favorable outcome (mRS 0–2) was found in 14 patients (38.9%), a similar proportion to that preoperatively.
In all patients, the patency of the STA-MCA double bypass was confirmed with brain MRA. Postoperative DSA showed excellent visualization of the MCA cortical arteries, and the abnormal collateral vessels of the operated side disappeared or were evidently diminished after STA-MCA double bypass in all patients (Fig. 4).
Rebleeding rates in each category
Table 4 summarizes the long-term rebleeding rates and Kaplan-Meier rebleeding-free curves in the unilateral and bilateral groups and in the operated and non-operated groups.
The annual rebleeding rate (ARR) in the unilateral and the bilateral groups was 2.7% and 2.6%/person-year, respectively. The p values, comparing the two groups using the log-rank test and Wilcoxon test, were 0.988 and 0.256, respectively (risk hazard ratio 0.987, 95% confidence interval 0.160–7.612; Fig. 5a). Focusing on rebleeding sites, 3 of 52 (5.8%) operated sides showed recurrence of hemorrhage, with 1 thalamic, 1 caudate, and 1 subcortical hemorrhage. In the non-operated sides, 2 of 20 (10.0%) sides showed rebleeding, consisting of 1 thalamic and 1 intraventricular hemorrhage. ARR in the operated and non-operated groups was 1.1% and 1.8%/side-year, respectively. The p values, using the log-rank test and the Wilcoxon test to compare the operated and non-operated groups, were 0.702 and 0.163, respectively (risk hazard ratio 1.425, 95% confidence interval 0.185–8.756; Fig. 5b).
Discussion
Our findings suggested that STA-MCA double bypass for adult patients with HMD had a tendency to reduce long-term rebleeding but a significant difference in ARR was not found between the operated and non-operated sides.
Previous studies have reported poor outcomes associated with the natural history of HMD [12, 15, 18]. Kobayashi et al. reported a representative ARR of 7.1%/person-year in patients with HMD treated conservatively [12]. They also showed 28.6% mortality and 21.4% good recovery rates in patients with rebleeding. Indirect bypasses, such as encephalo-duro-arterio-synangiosis, have also been described as ineffective for preventing rebleeding. The long-term prognosis after indirect bypass is similar to that of conservative treatment [1, 10, 12]. On the other hand, the JAM trial indicated that bilateral STA-MCA bypass lowered ARR to 2.7%/year in the long term, a statistically significant superiority over non-operated patients [16]. Some authors have also reported an 11–14% rebleeding rate in patients with HMD who underwent direct revascularization surgeries, which would be considerably superior to the historical general recurrence rate in patients with HMD [1, 5, 8, 31]. Although our ARR was comparable to that of previous studies, a statistically significant difference in ARR between the unilateral and bilateral groups and between the operated and the non-operated side groups was not observed. Larger patient numbers and longer follow-up would be required to evaluate the statistical significance of our findings.
It has been reported that hemodynamic stress on the abnormal collateral vessels or microaneurysms in the dilated perforating arteries may be causes of hemorrhage in HMD [4, 17]. Previous studies have indicated that a direct bypass might reduce hemodynamic stress in the cortical and moyamoya vessels [1, 22, 27]. The disappearance of microaneurysms after STA-MCA bypass has also been reported [20]. Nevertheless, the precise pathophysiology concerning rebleeding in HMD is still not fully understood. It is more important to clearly determine the cerebral hemodynamics and cerebral arterial morphological changes, not only in the cerebral cortical areas but also in the deep cerebral regions, following STA-MCA double bypass in patients with HMD than after conservative treatment. Microvascular evaluation of HMD with high-resolution imaging techniques such as 7-Tesla MRI might provide further information, including perforating arteries running through the deep cerebrum [9, 29], and would provide clues to elucidate the precise mechanism of hemorrhage in HMD.
Efficacy of STA-MCA double bypass in HMD
In our series, STA-MCA double bypass dramatically improved rCBF in the operated MCA territory. Double bypass to each supra- and infrasylvian vessel may improve rCBF in a larger area than single bypass [14, 19]. However, rebleeding still occurred despite technically successful STA-MCA bypass and apparent resolution of abnormal vessels. In contrast, STA-MCA double bypass evidently prevented ischemic attacks in patients with HMD. In our series, up to 30% of the patients had a history of ischemic attacks, including symptomatic cerebral infarction or TIAs with aphasia, dysarthria, and motor weakness. However, ischemic events did not occur in the operated hemispheric sides following STA-MCA double bypass. Interestingly, the fact that patients with HMD often suffered from ischemic insults has not been appropriately investigated. Fujii et al. reported that 59 of 455 patients with HMD had history of an ischemic episode prior to hemorrhage [2]. Kawaguchi et al. also reported that up to 36% of their conservatively treated patients experienced ischemic events during the follow-up period [10]. The efficacy of STA-MCA bypass in preventing ischemic attacks would be of significant benefit to patients with HMD.
It is significant that patients with moyamoya disease have cerebral hemodynamic compromise, and a minor increase in intracranial pressure due to hemorrhagic insults could easily cause irreversible ischemia [7, 25]. A previous study had reported that the cortical arterial pressure in moyamoya disease is < 30 mmHg, considerably lower than normal values of approximately 80 mmHg [22]. In addition, the cerebral vascular resistance of the cortical MCA (M4) is significantly lower than that of the cervical ICA [22]. From these data, we assume that the effect of STA-MCA bypass may normalize not only the CBF but also the perfusion pressure in the operated hemisphere. Therefore, we suggest that STA-MCA bypass might be effective for not only preventing further ischemic events but also reducing simultaneous ischemic complications in case of rebleeding in patients with HMD.
From this perspective, STA-MCA double bypass in the non-hemorrhagic side would be recommended when hemodynamic insufficiency is present. However, our result showed that the difference in the overall incidence of strokes between the unilateral and bilateral surgical groups was not significant. Routine “bilateral” surgery for HMD remains controversial.
Reducing perioperative complications
During the perioperative period, some patients developed serious complications. Although TNDs or transient focal vasogenic edema would not lead to permanent neurological deficit, postoperative hyperperfusion occasionally causes critical sequelae [3, 21]. In our series, the perioperative morbidity rate was 5.6%, comprised entirely of subcortical hemorrhage at the bypassed site due to hyperperfusion. This observation might be unique to STA-MCA “double” bypass as a result of increasing rCBF. Therefore, we instituted the following counter measures to prevent hyperperfusion-related complications: (1) general anesthesia is continued after bypass surgeries [30]; (2) when hyperperfusion is observed by our methods, strict blood pressure control under sedation is continued until the rCBF falls to normal; and (3) the free radical scavenger edaravone, which reduces the incidence of hyperperfusion-related TNDs, is administered [28]. These precautions may explain why only a few of our patients have developed postoperative complications related to hyperperfusion in the recent period.
Limitations
Some of this study’s limitations should be noted. First, this was a retrospective single-center study, and data collection was performed by us. The statistical power of our findings might not be strong. Second, HMD is still very rare, and the number of patients examined in our study might not be large enough to demonstrate statistical significance in each category. Finally, our data on non-operated (conservatively treated) sides were obtained with respect to the contralateral sides of the operated hemisphere, and these were not regarded as patient-based study nor truly conservatively treated hemispheres. Since STA-MCA double bypass surgery is the first-line treatment option at our center, we did not have adequate data on patients treated conservatively or with indirect bypass. This presented difficulties in comparing STA-MCA double bypass with other treatment modalities.
Conclusions
We presented the perioperative and long-term outcomes of STA-MCA double bypass for patients with adult-onset HMD. Hyperperfusion following STA-MCA double bypass is a concerning perioperative complication and should be managed by strict blood pressure control with rCBF measurements. The long-term rebleeding rate tended to be lower in the operated hemisphere; however, the difference between the operated and non-operated sides was not statistically significant. STA-MCA double bypass could dramatically change the hemodynamics in the operated cortical region and significantly prevent ischemic attacks in HMD.
Change history
04 January 2019
Table 2 of the original version of this article contained an added data. Correct Table 2 is presented here.
References
Choi WS, Lee SB, Kim DS, Huh PW, Yoo DS, Lee TG, Cho KS (2013) Thirteen-year experience of 44 patients with adult hemorrhagic moyamoya disease from a single institution: clinical analysis by management modality. J Cerebrovasc Endovasc Neurosurg 15:191–199. https://doi.org/10.7461/jcen.2013.15.3.191
Fujii K, Ikezaki K, Irikura K, Miyasaka Y, Fukui M (1997) The efficacy of bypass surgery for the patients with hemorrhagic moyamoya disease. Clin Neurol Neurosurg 99(Suppl 2):S194–S195
Fujimura M, Shimizu H, Mugikura S, Tominaga T (2009) Delayed intracerebral hemorrhage after superficial temporal artery-middle cerebral artery anastomosis in a patient with moyamoya disease: possible involvement of cerebral hyperperfusion and increased vascular permeability. Surg Neurol 71:223–227; discussion 227. https://doi.org/10.1016/j.surneu.2007.07.077
Funaki T, Takahashi JC, Yoshida K, Takagi Y, Fushimi Y, Kikuchi T, Mineharu Y, Okada T, Morimoto T, Miyamoto S (2016) Periventricular anastomosis in moyamoya disease: detecting fragile collateral vessels with MR angiography. J Neurosurg 124:1766–1772. https://doi.org/10.3171/2015.6.jns15845
Houkin K, Kamiyama H, Abe H, Takahashi A, Kuroda S (1996) Surgical therapy for adult moyamoya disease. Can surgical revascularization prevent the recurrence of intracerebral hemorrhage? Stroke 27:1342–1346
Huang Z, Ding X, Men W, Zhang D, Zhao Y, Wang R, Wang S, Zhao J (2015) Clinical features and outcomes in 154 patients with haemorrhagic moyamoya disease: comparison of conservative treatment and surgical revascularization. Neurol Res 37:886–892. https://doi.org/10.1179/1743132815y.0000000073
Iwama T, Kotani Y, Yamakawa H, Nagata I, Hashimoto N, Sakai N (2001) Cerebral ischemic complications following intracranial bleeding in patients with moyamoya disease—three case reports. Neurol Med Chir 41:450–453
Jiang H, Ni W, Xu B, Lei Y, Tian Y, Xu F, Gu Y, Mao Y (2014) Outcome in adult patients with hemorrhagic moyamoya disease after combined extracranial-intracranial bypass. J Neurosurg 121:1048–1055. https://doi.org/10.3171/2014.7.jns132434
Kang CK, Park CW, Han JY, Kim SH, Park CA, Kim KN, Hong SM, Kim YB, Lee KH, Cho ZH (2009) Imaging and analysis of lenticulostriate arteries using 7.0-Tesla magnetic resonance angiography. Magn Reson Med 61:136–144. https://doi.org/10.1002/mrm.21786
Kawaguchi S, Okuno S, Sakaki T (2000) Effect of direct arterial bypass on the prevention of future stroke in patients with the hemorrhagic variety of moyamoya disease. J Neurosurg 93:397–401. https://doi.org/10.3171/jns.2000.93.3.0397
Kawamata T, Okada Y, Kawashima A, Yoneyama T, Yamaguchi K, Ono Y, Hori T (2009) Postcarotid endarterectomy cerebral hyperperfusion can be prevented by minimizing intraoperative cerebral ischemia and strict postoperative blood pressure control under continuous sedation. Neurosurgery 64:447–453; discussion 453-444. https://doi.org/10.1227/01.neu.0000339110.73385.8a
Kobayashi E, Saeki N, Oishi H, Hirai S, Yamaura A (2000) Long-term natural history of hemorrhagic moyamoya disease in 42 patients. J Neurosurg 93:976–980. https://doi.org/10.3171/jns.2000.93.6.0976
Kuroda S, Ishikawa T, Houkin K, Nanba R, Hokari M, Iwasaki Y (2005) Incidence and clinical features of disease progression in adult moyamoya disease. Stroke 36:2148–2153. https://doi.org/10.1161/01.str.0000182256.32489.99
Kuroda S, Kawabori M, Hirata K, Shiga T, Kashiwazaki D, Houkin K, Tamaki N (2014) Clinical significance of STA-MCA double anastomosis for hemodynamic compromise in post-JET/COSS era. Acta Neurochir 156:77–83. https://doi.org/10.1007/s00701-013-1961-0
Liu X, Zhang D, Shuo W, Zhao Y, Wang R, Zhao J (2013) Long term outcome after conservative and surgical treatment of haemorrhagic moyamoya disease. J Neurol Neurosurg Psychiatry 84:258–265. https://doi.org/10.1136/jnnp-2012-302236
Miyamoto S, Yoshimoto T, Hashimoto N, Okada Y, Tsuji I, Tominaga T, Nakagawara J, Takahashi JC (2014) Effects of extracranial-intracranial bypass for patients with hemorrhagic moyamoya disease: results of the Japan Adult Moyamoya trial. Stroke 45:1415–1421. https://doi.org/10.1161/strokeaha.113.004386
Morioka M, Hamada J, Kawano T, Todaka T, Yano S, Kai Y, Ushio Y (2003) Angiographic dilatation and branch extension of the anterior choroidal and posterior communicating arteries are predictors of hemorrhage in adult moyamoya patients. Stroke 34:90–95
Morioka M, Hamada J, Todaka T, Yano S, Kai Y, Ushio Y (2003) High-risk age for rebleeding in patients with hemorrhagic moyamoya disease: long-term follow-up study. Neurosurgery 52:1049–1054 discussion 1054-1045
Morisawa H, Kawamata T, Kawashima A, Hayashi M, Yamaguchi K, Yoneyama T, Okada Y (2013) Hemodynamics and changes after STA-MCA anastomosis in moyamoya disease and atherosclerotic cerebrovascular disease measured by micro-Doppler ultrasonography. Neurosurg Rev 36:411–419. https://doi.org/10.1007/s10143-012-0441-y
Ni W, Xu F, Xu B, Liao Y, Gu Y, Song D (2012) Disappearance of aneurysms associated with moyamoya disease after STA-MCA anastomosis with encephaloduro myosynangiosis. J Clin Neurosci 19:485–487. https://doi.org/10.1016/j.jocn.2011.05.036
Ohue S, Kumon Y, Kohno K, Watanabe H, Iwata S, Ohnishi T (2008) Postoperative temporary neurological deficits in adults with moyamoya disease. Surg Neurol 69:281–286; discussion 286-287. https://doi.org/10.1016/j.surneu.2007.01.047
Okada Y, Shima T, Nishida M, Yamane K, Yamada T, Yamanaka C (1998) Effectiveness of superficial temporal artery-middle cerebral artery anastomosis in adult moyamoya disease: cerebral hemodynamics and clinical course in ischemic and hemorrhagic varieties. Stroke 29:625–630
Okada Y, Shima T, Yamane K, Yamanaka C, Kagawa R (1999) Cylindrical or T-shaped silicone rubber stents for microanastomosis—technical note. Neurol Med Chir 39:55–57 discussion 57-58
Research (2012) Guidelines for diagnosis and treatment of moyamoya disease (spontaneous occlusion of the circle of Willis). Neurol Med Chir 52:245–266
Su IC, Yang CC, Wang WH, Lee JE, Tu YK, Wang KC (2008) Acute cerebral ischemia following intraventricular hemorrhage in moyamoya disease: early perfusion computed tomography findings. J Neurosurg 109:1049–1051. https://doi.org/10.3171/jns.2008.109.12.1049
Suzuki J, Kodama N (1983) Moyamoya disease—a review. Stroke 14:104–109
Takahashi JC, Funaki T, Houkin K, Inoue T, Ogasawara K, Nakagawara J, Kuroda S, Yamada K, Miyamoto S (2016) Significance of the hemorrhagic site for recurrent bleeding: prespecified analysis in the Japan Adult Moyamoya trial. Stroke 47:37–43. https://doi.org/10.1161/strokeaha.115.010819
Uchino H, Nakayama N, Kazumata K, Kuroda S, Houkin K (2016) Edaravone reduces hyperperfusion-related neurological deficits in adult moyamoya disease: historical control study. Stroke 47:1930–1932. https://doi.org/10.1161/strokeaha.116.013304
Wrede KH, Johst S, Dammann P, Ozkan N, Monninghoff C, Kraemer M, Maderwald S, Ladd ME, Sure U, Umutlu L, Schlamann M (2014) Improved cerebral time-of-flight magnetic resonance angiography at 7 Tesla—feasibility study and preliminary results using optimized venous saturation pulses. PLoS One 9:e106697. https://doi.org/10.1371/journal.pone.0106697
Yamaguchi K, Kawamata T, Kawashima A, Hori T, Okada Y (2010) Incidence and predictive factors of cerebral hyperperfusion after extracranial-intracranial bypass for occlusive cerebrovascular diseases. Neurosurgery 67:1548–1554; discussion 1554. https://doi.org/10.1227/NEU.0b013e3181f8c554
Yoshida Y, Yoshimoto T, Shirane R, Sakurai Y (1999) Clinical course, surgical management, and long-term outcome of moyamoya patients with rebleeding after an episode of intracerebral hemorrhage: an extensive follow-up study. Stroke 30:2272–2276
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
For this type of study, formal consent is not required.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised as Table 2 contains added data.
Rights and permissions
About this article
Cite this article
Ishiguro, T., Okada, Y., Ishikawa, T. et al. Efficacy of superficial temporal artery-middle cerebral artery double bypass in patients with hemorrhagic moyamoya disease: surgical effects for operated hemispheric sides. Neurosurg Rev 42, 559–568 (2019). https://doi.org/10.1007/s10143-018-01059-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-018-01059-z