Abstract
Moyamoya disease (MMD) is a chronic, occlusive cerebrovascular disease with unknown etiology characterized by progressive stenosis at the terminal portion of the internal carotid artery and the abnormal vascular network formation at the base of the brain. Superficial temporal artery-middle cerebral artery (STA-MCA) bypass is a preferred surgical procedure for ischemic-onset MMD patients by improving cerebral blood flow. Recent evidence further indicates that flow-augmentation bypass has a potential role for preventing re-bleeding in hemorrhagic-onset MMD patients. Based on such cumulative evidence, there is a worldwide increase in the number of MMD patients undergoing bypass surgery, thus thorough understanding of the basic pathology of MMD including peri-operative hemodynamics is critical for avoiding surgical complications. The author sought to demonstrate the standard surgical procedure of STA-MCA bypass with indirect pial synangiosis for adult MMD patients and its pitfall in the early postoperative period, introducing the characteristic peri-operative hemodynamic condition of adult MMD after surgery, such as local cerebral hyperperfusion and intrinsic hemodynamic ischemia caused by watershed shift phenomenon.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Moyamoya disease
- Flow-augmentation bypass
- Revascularization surgery
- Surgical complication
- Cerebral hyperperfusion
9.1 Introduction
Moyamoya disease (MMD) is a chronic, occlusive cerebrovascular disease with unknown etiology characterized by progressive stenosis at the terminal portion of the internal carotid artery (ICA) and the abnormal vascular network formation at the base of the brain [1]. MMD is known to have a characteristic nature to convert the vascular supply for the cerebral tissue from intracranial/internal carotid (IC) system to extracranial/external carotid (EC) system, the so called IC-EC conversion system [2, 3], which is well documented by Suzuki’s angiographic staging in the initial report of MMD [1]. The Suzuki’s angiographic staging may not reflect the severity of MMD but it well describes the exact self-compensatory pathophysiology of this entity, including its long-term temporal profile [3]. Insufficiency of this “IC-EC conversion system” could clinically lead to cerebral ischemia and/or intracranial hemorrhage from the fragile vascular networks, the so called moyamoya vessels.
While considering the basic pathology of MMD as a gradual conversion of the vascular supply from IC to EC system, extracranial-intracranial bypass such as superficial temporal artery-middle cerebral artery (STA-MCA) bypass may have a perfect concept to complement the “IC-EC conversion system” of MMD [3]. In fact, STA-MCA bypass has been established as a preferred surgical procedure for ischemic-onset MMD patients by improving cerebral hemodynamics [2,3,4]. Furthermore, recent evidence indicates that STA-MCA bypass has a potential role for preventing re-bleeding in hemorrhagic-onset MMD patients [5, 6]. In this chapter, the author sought to demonstrate the standard surgical procedure of STA-MCA bypass with indirect pial synangiosis for adult MMD and its pitfall during the early postoperative period, introducing the characteristic peri-operative hemodynamic condition of adult MMD patients after surgery, such as local cerebral hyperperfusion and intrinsic hemodynamic ischemia by watershed shift phenomenon, defined as a paradoxical cerebral blood flow (CBF) decrease at the adjacent cerebral cortex near the site of the anastomosis.
9.2 Surgical Indication of STA-MCA Bypass for MMD
9.2.1 Concept of Surgical Revascularization for MMD
Concept of the revascularization surgery for MMD includes not only the vascular reconstruction by STA-MCA anastomosis, but also the consolidation for the future neovascularization by indirect pial synangiosis [2, 3]. Surgical procedures of indirect pial synangiosis include lots of variations such as encephalo-duro-arterio-synangiosis (EDAS), encephalo-myo-synangiosis, encephalo-duro-myo-synangiosis (EDMS), and multiple burr hole surgery [4, 7, 8]. The concept of the revascularization surgery, either direct or indirect bypass procedure, is to facilitate the physiological conversion of the vascular supply for the brain from IC system to the EC system [2, 4]. Now it is well-known that the STA-MCA bypass not only prevents recurrent stroke by improving CBF in ischemic MMD patients, but also could ameliorate the hemodynamic stress to the vulnerable collateral anastomosis including choroidal anastomosis, and thus reduce the risk of re-bleeding from the affected vessels in hemorrhagic-onset patients with adult MMD [5, 6, 9, 10].
9.2.2 Best Surgical Indication of STA-MCA Bypass for MMD
Current surgical indication for MMD in our institute is highlighted in Table 9.1. STA-MCA bypass has been reported a preferred surgical procedure for the ischemic-onset MMD patients, which provides long-term effect of stroke prevention and the improvement of the outcome of cognitive impairment [2, 4, 7, 8]. Although there is no multicenter randomized control trial to evaluate the efficacy of STA-MCA bypass for ischemic-onset MMD patients, multiple meta-analyses indicated the effectiveness of STA-MCA bypass for MMD patients with ischemic symptoms [11, 12]. Indirect revascularization procedure such as EDAS is also recommended for pediatric MMD, but simultaneous use of direct bypass procedure has been considered as an essential item for adult patients [4, 7]. As for the hemorrhagic-onset MMD patients, there had been a controversy whether STA-MCA bypass could have a potential role for preventing re-bleeding, but the Japan Adult Moyamoya (JAM) Trial, a randomized controlled trial which investigated the impact of STA-MCA bypass for preventing re-bleeding in hemorrhagic MMD patients, strongly suggested the efficacy of STA-MCA bypass for preventing re-bleeding in hemorrhagic-onset MMD patients [5, 6]. The inclusion criteria of JAM trial represented as follows. (1) Adult patient (aged from 16 to 65 years old), (2) STA-MCA bypass within 1–12 months after the onset of hemorrhage, (3) independent activity of daily living (modified Rankin Scale of 0–2), and (4) absence of major cortical damage [5]. The initial report of JAM trial indicated that the annual re-bleeding tares of the surgically treated group (2.7%) was significantly lower than that in non-surgical group (7.6%/year, p = 0.042) [5]. Moreover, the second report of JAM trial with the pre-specified subgroup analysis further suggested that annual re-bleeding rate of the MMD patients with posterior hemorrhage was as high as 17.1% per year, and STA-MCA bypass significantly reduced the risk of re-bleeding in patients with posterior hemorrhage (p = 0.001) [6]. Taken together, ischemic-onset MMD and/or adult MMD patients with posterior hemorrhage may have a best surgical indication for STA-MCA bypass on the affected hemisphere.
9.2.3 Controversy Issues of the Surgical Indication for MMD
STA-MCA bypass for asymptomatic MMD patients is currently not recommended because the natural history of this patient population is undetermined [4, 7]. To address this critical question, Asymptomatic Moyamoya Registry (AMORE); a multicenter observational study is currently undertaken in Japan to clarify the natural history of asymptomatic patients with MMD [13]. Alternatively, more recent supplemental analysis of JAM trial indicated that the development of choroidal anastomosis, which is defined as dilatation and extension of choroidal collateral toward the medullary arteries, was significantly associated with posterior hemorrhage in MMD patients [9], and choroidal anastomosis is a strong indicator for hemorrhagic presentation [10]. Choroidal anastomosis was also found to be more prominent in hemorrhagic-onset MMD patients as compared to ischemic-onset patients [14]. More surprisingly, another supplemental analysis of JAM trial examined the effect of choroidal anastomosis on de novo hemorrhage in adult MMD, and the authors found that annual de novo bleeding risk of the non-hemorrhagic/choroidal anastomosis-positive hemisphere was as high as 5.8% per year [15]. These results raise the question whether asymptomatic hemispheres with choroidal anastomosis should be treated by STA-MCA bypass for reducing the substantial risk of de novo hemorrhage. This issue should be solved by future prospective multicenter study.
9.3 Surgical Procedure of STA-MCA Bypass for Adult MMD
9.3.1 Standard Procedure of Direct/Indirect Combined Revascularization
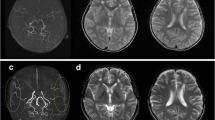
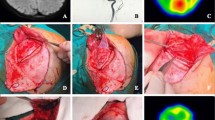
Intra-operative finding of the representative case of an adult patient with hemorrhagic-onset MMD is shown (Fig. 9.1). Under general anesthesia, the head is positioned at 80° by three-point fixture. Skin incision is usually made along with the donor STA approximately 8–10 cm in length, a skin flap is inverted by L-shape when I use of parietal STA as donor or by question-marked incision (frontal STA as donor). Craniotomy is performed around the Sylvian fissure end, and the stump of the STA is prepared as semi-fish mouth shape with 1.6 mm (Fig. 9.1). Then the stump of STA is anastomosed by 10-0 or 11-0 nylon monofilament suture to the M4 segment of the MCA, approximately 0.6 mm in diameter (Fig. 9.1). After reperfusion, the patency of STA-MCA anastomosis is confirmed by intra-operative indocyanine green (ICG) video-angiography and Doppler ultrasonography (arrows in Fig. 9.1). The STA-MCA bypass is followed by indirect pial synangiosis with EDMS. The inner layer of the free bone flap is routinely drilled out for avoiding postoperative compression of the brain surface by the temporal muscle pedicle, and a wide bone window is made on the side of EDMS flap insertion [16]. The temporal muscle is usually split out into two layers. The bone flap is fixed both by two pieces of titanium plates (ThinFlap®, SHINOBI®) and a bio-absorbable plate; LactoSorb® (82% Poly-L-Lactic Acid and 18% Poly-Glycolic Acid). The author has been employing this combined revascularization procedure since 2004, with long-term favorable outcomes. The bypass could be visualized by standard MR angiography of 1.5 or 3 T (arrow in Fig. 9.2) without affecting brain parenchyma. Serial CBF measurement by 123I-IMP SPECT indicated typical temporal profile of postoperative hemodynamic change, as characterized by the relatively localized CBF increase near the site of the anastomosis on postoperative day (POD) 1 (arrow in Fig. 9.2) and subsequent favorable distribution of CBF in a wider territory of the ipsilateral ACA/MCA territories on POD 7 (Fig. 9.2).
(a) Representative pre-operative finding of magnetic resonance (MR) angiography (a) and MR imaging with fluid attenuated inversion recovery (b) of a 38-year-old woman with hemorrhagic-onset MMD. Intra-operative view of direct/indirect combined revascularization. Surgical view before (c), during (d), and after left STA-MCA bypass (e, f). Indocyanine green video-angiography demonstrated apparently patent bypass with favorable distribution of bypass flow (arrow in f)
Postoperative MR angiography demonstrated that the right STA-MCA bypass was patent (arrow in a). Serial CBF measurement by N-isopropyl-p-[123I] iodoamphetamine single-photon emission computed tomography (123I-IMP SPECT) (b) indicating typical temporal profile of postoperative hemodynamic change, as characterized by the relatively localized CBF increase near the site of the anastomosis on POD 1 (arrow) and subsequent distribution of CBF in a wider territory of the ipsilateral MCA territory
9.4 Intrinsic Peri-Operative Hemodynamics and Optimal Peri-Operative Management
9.4.1 Ischemic Complication of Combined Revascularization Procedure for MMD
Surgical complications of the STA-MCA bypass for MMD generally include peri-operative cerebral infarction and cerebral hyperperfusion (CHP) syndrome (Table 9.2). Peri-operative ischemic stroke could be caused by various factors such as intra-operative hypotension and/or hypocapnia by inadequate general anesthesia, anemia, and surgical procedure [4, 7]. Peri-operative ischemic complications are categorized into three distinct mechanisms; thrombo-embolism at the site of the microvascular anastomosis [17], intrinsic hemodynamic ischemia by “watershed shift phenomenon” [18,19,20], and mechanical compression of the brain surface by swollen temporal muscle pedicle used for additional indirect pial synangiosis [21]. Among them, “watershed shift phenomenon” is a characteristic pathophysiological condition after STA-MCA bypass for MMD, which is initially proposed by Professor Heros [22]. Watershed shift ischemia is defined as a paradoxical CBF decrease at the adjacent cortex near the site of local CHP [18, 19]. Possible mechanism underling watershed shift phenomenon is that retrograde blood supply from STA-MCA bypass could conflict with the anterograde CBF from more proximal MCA, resulting in the transient CBF decrease at the cortex supplied by the adjacent branch of MCA [20]. Clinical outcome of watershed shift ischemia is generally favorable but its simultaneous occurrence with CHP could make the peri-operative pathology more complex and difficult, since the management of each condition is contradictory [18, 19]. To avoid ischemic complications, it is essential to maintain proper peri-operative hydration and blood pressure control with prompt hemoglobin concentration maintenance [4, 7]. Efficacy of the anti-platelet agent during the peri-operative period is controversial among MMD patients, but recent multiple studies strongly suggested the advantage of aspirin administration to reduce the risk of peri-operative complication after STA-MCA bypass for MMD [23, 24].
9.4.2 Significance of Transient Local Cerebral Hyperperfusion (CHP) in Adult MMD Patients
CHP syndrome is one of the most serious complications after STA-MCA bypass for MMD [16, 25,26,27,28,29,30]. Excessive local increase in CBF at the site of the STA-MCA bypass is known to result in local hyperemia associated with vasogenic edema and/or delayed intracerebral hemorrhage in MMD, especially in adult patients. Most common clinical presentation of local CHP is transient neurological deteriorations without causing permanent neurological deficit in most cases [26, 28], but it could also lead to epileptic seizure and/or delayed intracerebral hemorrhage in a rare occasion [29]. Blood pressure dependent worsening of the focal neurological sign has a diagnostic value for CHP syndrome in adult MMD. Although the CHP syndrome after low flow bypass including STA-MCA anastomosis was considered relatively rare until the early 2000, we have reported for the first time that the incidence of CHP syndrome after STA-MCA bypass was significantly higher in MMD patients than that in atherosclerotic occlusive cerebrovascular disease patients undergoing same STA-MCA bypass procedure [25]. Representative finding of local CHP is shown in Fig. 9.3. 123I-IMP SPECT 1 day after the left STA-MCA bypass with indirect pial synangiosis revealed intense focal increase of CBF at the site of the anastomosis (arrows in Fig. 9.3). Under strict blood pressure control with the administration of minocycline hydrochloride and edaravone, a free radial scavenger, the patient remained asymptomatic for 7 days, when local CHP was ameliorated by 123I-IMP SPECT.
Prognosis of local CHP is generally favorable, but it could again lead to delayed intracerebral hemorrhage and/or intractable seizure in a rare occasion. Kameyama and colleagues reported the significance of quantitative CBF analysis in the early postoperative period of STA-MCA bypass for adult MMD patients. They indicated that pathological threshold of postoperative local CBF increase ratio was 184.5% for CHP syndrome and 241.3% for hemorrhagic CHP syndrome [31]. Based on these findings, quantitative CBF analysis at the site of the anastomosis could provide critical information to perform prompt peri-operative management for adult MMD patients. Regarding the prediction of CHP after STA-MCA bypass for MMD, multiple risk factors were recently reported. The indicators for postoperative CHP phenomenon or CHP syndrome are as follows; adult-onset or elderly patient’s age [28, 30], onset of hemorrhagic [16, 28], operation on the dominant hemisphere [32, 33], decreased CBF before surgery [31] or increased cerebral blood volume before surgery [30], diameter mismatch between donor and recipient arteries [33], poorer intra-operative distribution pattern by ICG video-angiography or by brain surface thermography [34, 35], and lower total protein levels or higher hematocrit concentration in the pre-operative blood examination [36]. Regarding the involvement of genetic background, Tashiro et al. found that MMD patients with the RNF213 gene polymorphism c.14576G>A [37, 38], have significantly higher risk for prolonged/delayed CHP [39]. More recently, Nishizawa and colleagues attempted to predict CHP after STA-MCA bypass by three-dimensional time-of-flight (3D-TOF) MR angiography in adult MMD patients, indicating that the signal intensity of the intracranial major arteries, including the decreased signal intensity of the peripheral cortical arteries on pre-operative 3D-TOF MR angiography could identify adult MMD patients at higher risk for CHP after direct revascularization surgery [40]. Based on these observations, it is essential to manage adult MMD patients with higher risk of CHP under more strict blood pressure to avoid deleterious effect of local CHP during the early postoperative period.
9.4.3 Limitation of the Current Peri-Operative Management Strategy
Beneficial effect of the blood pressure lowering is established to counteract with the deleterious impact of CHP phenomenon [41], but the excessive blood pressure decrease has a substantial risk for peri-operative ischemic stroke at the remote area, either ipsilateral or contralateral hemisphere, from STA-MCA bypass [33]. To manage the complex hemodynamic condition in the early postoperative period in MMD patients, the author and colleagues previously reported that moderate but prophylactic blood pressure lowering between 110 and 130 mmHg of the systolic blood pressure could significantly reduce the risk of CHP syndrome, without increasing the incidence of ischemic complication, after STA-MCA for MMD patients [18, 33, 41]. Alternatively, the prophylactic use of pharmacological agents was reported to be beneficial to prevent CHP syndrome in the previous literatures. Minocycline hydrochloride and/or edaravone (a free radical scavenger) significantly reduced the incident of CHP syndrome in MMD patients [33, 42]. The author and colleagues introduced minocycline hydrochloride, a neuro-protective antibiotic, to prevent both CHP syndrome and cerebral ischemia at the remote area [33]. Nevertheless, concomitant manifestation of local CHP with cerebral ischemia at the remote area, such as contralateral hemisphere and/or adjacent cortex affected by “watershed shift phenomenon,” is still a major issue that can result in peri-operative neurological deterioration [18]. Therefore, mechanism underlying peri-operative pathologies including CHP after revascularization surgery should be further investigated in the future study.
9.5 Conclusion
STA-MCA bypass has a potential role for preventing stroke recurrence and/or re-bleeding in patients with MMD. Long-term outcome of STA-MCA bypass for MMD is generally favorable, but thorough understanding of the unique pathophysiological condition of MMD and prompt peri-operative management is essential to avoid surgical complications including CHP syndrome and concomitant cerebral ischemia.
References
Suzuki J, Takaku A. Cerebrovascular ‘moyamoya’ disease. Disease showing abnormal net-like vessels in base of brain. Arch Neurol. 1969;20:288–99.
Fujimura M, Tominaga T. Lessons learned from moyamoya disease: outcome of direct/indirect revascularization surgery for 150 affected hemispheres. Neurol Med Chir (Tokyo). 2012;52:327–32.
Fujimura M, Tominaga T. Current status of revascularization surgery for moyamoya disease: special consideration for its ‘internal carotid-external carotid (IC-EC) conversion’ as the physiological reorganization system. Tohoku J Exp Med. 2015;236:45–53.
Research Committee on the Pathology and Treatment of Spontaneous Occlusion of the Circle of Willis, Health Labour Sciences Research Grant for Research on Measures for Intractable Diseases. Guidelines for diagnosis and treatment of moyamoya disease (Spontaneous Occlusion of the Circle of Willis). Neurol Med Chir (Tokyo). 2012;52:245–66.
Miyamoto S, Yoshimoto T, Hashimoto N, Okada Y, Tsuji I, Tominaga T, et al. Effects of extracranial-intracranial bypass for patients with hemorrhagic moyamoya disease: results of the Japan Adult Moyamoya Trial. Stroke. 2014;45:1415–21.
Takahashi JC, Funaki T, Houkin K, Inoue T, Ogasawara K, Nakagawara J, et al. Significance of the hemorrhagic site for recurrent bleeding: prespecified analysis in the Japan Adult Moyamoya Trial. Stroke. 2016;47:37–43.
Tominaga T, Suzuki N, Miyamoto S, Koizumi A, Kuroda S, Takahashi JC, et al. Recommendations for the management of moyamoya disease: A statement from Research Committee on Spontaneous Occlusion of the Circle of Willis (Moyamoya Disease) [2nd edition]. Surg Cereb Stroke. 2018;46:136–40. (In Japanese)
Cho WS, Kim JE, Kim CH, Ban SP, Kang HS, Son YJ, et al. Long-term outcomes after combined revascularization surgery in adult moyamoya disease. Stroke. 2014;45:3025–31.
Funaki T, Takahashi JC, Houkin K, Kuroda S, Takeuchi S, Fujimura M, et al. Angiographic features of hemorrhagic moyamoya disease with high recurrence risk: a supplementary analysis of the Japan Adult Moyamoya Trial. J Neurosurg. 2018;128:777–84.
Funaki T, Takahashi JC, Houkin K, Kuroda S, Takeuchi S, Fujimura M, et al. High rebleeding risk associated with choroidal collateral vessels in hemorrhagic moyamoya disease: analysis of a nonsurgical cohort in the Japan Adult Moyamoya Trial. J Neurosurg. 2019;130(2):337–673. https://doi.org/10.3171/2017.9.JNS17576.
Jeon JP, Kim JE, Cho WS, Bang JS, Son YJ, Oh CW. Meta-analysis of the surgical outcomes of symptomatic moyamoya disease in adults. J Neurosurg. 2018;128:793–9.
Qian C, Yu X, Li J, Chen J, Wang L, Chen G. The efficacy of surgical treatment for the secondary prevention of stroke in symptomatic moyamoya disease: a meta-analysis. Medicine (Baltimore). 2015;94:e2218.
Kuroda S, AMORE Study Group. Asymptomatic moyamoya disease: literature review and ongoing AMORE study. Neurol Med Chir (Tokyo). 2015;55:194–8.
Funaki T, Takahashi JC, Houkin K, Kuroda S, Fujimura M, Tomata Y, et al. Effect of choroidal collateral vessels on de novo hemorrhage in moyamoya disease: analysis of nonhemorrhagic hemispheres in the Japan Adult Moyamoya Trial. J Neurosurg. 2019;132:408–14. https://doi.org/10.3171/2018.10.JNS181139.
Fujimura M, Funaki T, Houkin K, Takahashi JC, Kuroda S, Tomata Y, et al. Intrinsic development of choroidal and thalamic collaterals in hemorrhagic-onset moyamoya disease: case-control study of the Japan Adult Moyamoya Trial. J Neurosurg. 2019;130:1453–9. https://doi.org/10.3171/2017.11.JNS171990.
Tokairin K, Kazumata K, Uchino H, Ito M, Ono K, Tatezawa R, et al. Post-operative intracerebral hemorrhage after combined revascularization surgery in moyamoya disease: profiles and clinical associations. World Neurosurg. 2018;120:e593–600. https://doi.org/10.1016/j.wneu.2018.08.132.
Fujimura M, Kaneta T, Tominaga T. Efficacy of superficial temporal artery-middle cerebral artery anastomosis with routine postoperative cerebral blood flow measurement during the acute stage in childhood moyamoya disease. Childs Nerv Syst. 2008;24:827–32.
Tu XC, Fujimura M, Rashad S, Mugikura S, Sakata H, Niizuma K, et al. Uneven cerebral hemodynamic change as a cause of neurological deterioration in the acute stage after direct revascularization for moyamoya disease: cerebral hyperperfusion and remote ischemia caused by the ‘watershed shift’. Neurosurg Rev. 2017;40:507–12.
Tashiro R, Fujimura M, Kameyama M, Mugikura S, Endo H, Takeuchi Y, et al. Incidence and risk factors of the watershed shift phenomenon after superficial temporal artery-middle cerebral artery anastomosis for adult moyamoya disease. Cerebrovasc Dis. 2019;47:178–87.
Hayashi T, Shirane R, Fujimura M, Tominaga T. Postoperative neurological deterioration in pediatric moyamoya disease. Watershed shift and hyperperfusion. J Neurosurg Pediatr. 2010;6:73–81.
Fujimura M, Kaneta T, Shimizu H, Tominaga T. Cerebral ischemia owing to compression of the brain by swollen temporal muscle used for encephalo-myo-synangiosis in moyamoya disease. Neurosurg Rev. 2009;32:245–9.
Heros RC, Scott RM, Kistler JP, Ackerman RH, Conner ES. Temporary neurological deterioration after extracranial-intracranial bypass. Neurosurgery. 1984;15:178–85.
Schubert GA, Biermann P, Weiss C, Seiz M, Vajkoczy P, Schmiedek P, et al. Risk profile in extracranial/intracranial bypass surgery—the role of antiplatelet agents, disease pathology, and surgical technique in 168 direct revascularization procedures. World Neurosurg. 2014;82:672–7.
Zhao Y, Zhang Q, Zhang D, Zhao Y. The effect of aspirin in the postoperative management of adult ischemic moyamoya disease. World Neurosurg. 2017;105:728–31.
Fujimura M, Shimizu H, Inoue T, Mugikura S, Saito A, Tominaga T. Significance of focal cerebral hyperperfusion as a cause of transient neurologic deterioration after EC-IC bypass for moyamoya disease: comparative study with non-moyamoya patients using n-isopropyl-p-[(123)i]iodoamphetamine single-photon emission computed tomography. Neurosurgery. 2011;68:957–65.
Fujimura M, Kaneta T, Mugikura S, Shimizu H, Tominaga T. Temporary neurologic deterioration due to cerebral hyperperfusion after superficial temporal artery-middle cerebral artery anastomosis in patients with adult-onset moyamoya disease. Surg Neurol. 2007;67:273–82.
Kim JE, Oh CW, Kwon OK, Park SQ, Kim SE, Kim YK. Transient hyperperfusion after superficial temporal artery/middle cerebral artery bypass surgery as a possible cause of postoperative transient neurological deterioration. Cerebrovasc Dis. 2008;25:580–6.
Fujimura M, Mugikura S, Kaneta T, Shimizu H, Tominaga T. Incidence and risk factors for symptomatic cerebral hyperperfusion after superficial temporal artery-middle cerebral artery anastomosis in patients with moyamoya disease. Surg Neurol. 2009;71:442–7.
Fujimura M, Shimizu H, Mugikura S, Tominaga T. Delayed intracerebral hemorrhage after superficial temporal artery-middle cerebral artery anastomosis in a patient with moyamoya disease: possible involvement of cerebral hyperperfusion and increased vascular permeability. Surg Neurol. 2009;71:223–7.
Uchino H, Kuroda S, Hirata K, Shiga T, Houkin K, Tamaki N. Predictors and clinical features of postoperative hyperperfusion after surgical revascularization for moyamoya disease: a serial single photon emission CT/positron emission tomography study. Stroke. 2012;43:2610–6.
Kameyama M, Fujimura M, Tashiro R, Sato K, Endo H, Niizuma K, et al. Significance of quantitative cerebral blood flow measurement in the acute stage after revascularization surgery for adult moyamoya disease: implication for the pathological threshold of local cerebral hyperperfusion. Cerebrovasc Dis. 2019;48:217–25.
Hwang JW, Yang HM, Lee H, Lee HK, Jeon YT, Kim JE, et al. Predictive factors of symptomatic cerebral hyperperfusion after superficial temporal artery-middle cerebral artery anastomosis in adult patients with moyamoya disease. Br J Anesth. 2013;110:773–9.
Fujimura M, Niizuma K, Inoue T, Sato K, Endo H, Shimizu H, et al. Minocycline prevents focal neurologic deterioration due to cerebral hyperperfusion after extracranial-intracranial bypass for moyamoya disease. Neurosurgery. 2014;74:163–70.
Horie N, Fukuda Y, Izumo T, Hayashi K, Suyama K, Nagata I. Indocyanine green videoangiography for assessment of postoperaive hyperperfusion in moyamoya disease. Acta Neurochir. 2014;156:919–26.
Nakagawa A, Fujimura M, Arafune T, Sakuma I, Tominaga T. Clinical implications of intraoperative infrared brain surface monitoring during superficial temporal artery-middle cerebral artery anastomosis in patients with moyamoya disease. J Neurosurg. 2009;111:1158–64.
Katsuki M, Fujimura M, Tashiro R, Tomata Y, Nishizawa T, Tominaga T. Pre-operative higher hematocrit and lower total protein levels are independent risk factors for cerebral hyperperfusion syndrome after superficial temporal artery-middle cerebral artery anastomosis with pial synangiosis in adult moyamoya disease patients—case-control study. Neurosurg Rev. 2021;44(4):2191–200. https://doi.org/10.1007/s10143-020-01395-z.
Kamada F, Aoki Y, Narisawa A, Abe Y, Komatsuzaki S, Kikuchi A, et al. A genome-wide association study identifies RNF213 as the first moyamoya disease gene. J Hum Genet. 2011;56:34–40.
Liu W, Morito D, Takashima S, Minoehary Y, Kobayashi H, Hitomi T, et al. Identification of RNF213 as a susceptibility gene for moyamoya disease and its possible role in vascular development. PLoS One. 2011;6:e22452.
Tashiro R, Fujimura M, Katsuki M, Nishizawa T, Tomata Y, Niizuma K, et al. Prolonged/delayed cerebral hyperperfusion in adult moyamoya disease patients with RNF213 gene polymorphism c.14576G>A (rs112735431) after superficial temporal artery-middle cerebral artery anastomosis. J Neurosurg. 2020;23:1–8. https://doi.org/10.3171/2020.6.JNS201037. Online ahead of print
Nishizawa T, Fujimura M, Katsuki M, Mugikura S, Tashiro R, Sato K, et al. Prediction of cerebral hyperperfusion after superficial temporal artery-middle cerebral artery anastomosis by three-dimensional-time-of-flight magnetic resonance angiography in adult patients with moyamoya disease. Cerebrovasc Dis. 2020;49:396–403.
Fujimura M, Inoue T, Shimizu H, Saito A, Mugikura S, Tominaga T. Efficacy of prophylactic blood pressure lowering according to a standardized postoperative management protocol to prevent symptomatic cerebral hyperperfusion after direct revascularization surgery for moyamoya disease. Cerebrovasc Dis. 2012;33:436–45.
Uchino H, Nakayama N, Kazumata K, Kuroda S, Houkin K. Edaravone reduces hyperperfusion-related neurological deficits in adult moyamoya disease: historical control study. Stroke. 2016;47:1930–2.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Ethics declarations
Funding
This study was supported by JSPS KAKENHI grant number 20K09362 (Miki Fujimura).
Conflicts of interest
The authors have nothing to declare.
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Fujimura, M. (2022). Moyamoya Disease-Standards and Advances in Revascularization Procedure and Peri-operative Management. In: Kato, Y., Ansari, A. (eds) Cerebrovascular Surgery. Advances and Technical Standards in Neurosurgery, vol 44. Springer, Cham. https://doi.org/10.1007/978-3-030-87649-4_9
Download citation
DOI: https://doi.org/10.1007/978-3-030-87649-4_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-87648-7
Online ISBN: 978-3-030-87649-4
eBook Packages: MedicineMedicine (R0)