Abstract
Although low-level laser therapy (LLLT) was discovered already in the 1960s of the twentieth century, it took almost 40 years to be widely used in clinical dermatology/surgery. It has been demonstrated that LLLT is able to increase collagen production/wound stiffness and/or improve wound contraction. In this review, we investigated whether open and sutured wounds should be treated with different LLLT parameters. A PubMed search was performed to identify controlled studies with LLLT applied to wounded animals (sutured incisions—tensile strength measurement and open excisions—area measurement). Final score random effects meta-analyses were conducted. Nineteen studies were included. The overall result of the tensile strength analysis (eight studies) was significantly in favor of LLLT (SMD = 1.06, 95% CI 0.66–1.46), and better results were seen with 30–79 mW/cm2 infrared laser (SMD = 1.44, 95% CI 0.67–2.21) and 139–281 mW/cm2 red laser (SMD = 1.52, 95% CI 0.54–2.49). The overall result of the wound contraction analysis (11 studies) was significantly in favor of LLLT (SMD = 0.99, 95% CI 0.38–1.59), and the best results were seen with 53–300 mW/cm2 infrared laser (SMD = 1.18, 95% CI 0.41–1.94) and 25–90 mW/cm2 red laser (SMD = 1.6, 95% CI 0.27–2.93). Whereas 1–15 mW/cm2 red laser had a moderately positive effect on sutured wounds, 2–4 mW/cm2 red laser did not accelerate healing of open wounds. LLLT appears effective in the treatment of sutured and open wounds. Statistical heterogeneity indicates that the tensile strength development of sutured wounds is more dependent on laser power density compared to the contraction rate of open wounds.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Although low-level laser therapy (LLLT) was discovered already in the 1960s of the twentieth century [1], it took almost 40 years to be widely used in clinical dermatology/surgery [2]. In general, lasers with output powers in the range between 5 and 50 mW delivering doses of 1–4 J/cm2 have been found to be most effective in stimulating tissue repair [3]. Since a dose may only be found effective when it reaches the targeted tissue, it must be taken into consideration that the power of laser is reduced as it penetrates the tissues. Although the penetration profiles for selected lasers may differ [4], in general it can be expected that for example a near infrared laser at 810 nm lose approximately 75% of its power during skin transmission [5] and with red laser, the loss of power is even higher [6].
It is well known that the amount of granulation tissue (GT) that is formed during the proliferation phase depends on the size of the wound. Therefore, an open wound heals with extensive new tissue formation, whereas a primary wound forms no or only a negligible amount of GT [7, 8]. The GT is composed of cells (fibroblasts and endothelial cells) that are present in the normal tissue before injury, cells (immune/inflammatory cells and other bone marrow-derived cells) that are recruited to the wound-associated stroma from distal sites and non-cellular components such as the extracellular matrix (ECM) [9].
A crucial step in accelerating the healing of open wounds is wound contraction (WC) during which the surrounding skin moves centripetally and leaves a remarkably smaller scar [10]. This process includes massive ECM remodeling assured by a more compact organization of collagen fibrils and water elimination. A principal role in WC is played by α-smooth muscle acting (α-SMA) expressing fibroblasts, so-called myofibroblasts, discovered in 1971 [11]. In contrast, sutures can be removed as soon as the wound is fixed and no longer need mechanical support since early suture resection assures a better cosmetic result [12]. During the first days after injury, the ECM is highly hydrated allowing cell invasion and assures only poor wound stiffness. Later during the maturation phase, it becomes more densely composed by collagen associated with an exponential increase of wound tensile strength (TS) [7] assured by gradual replacement of collagen type III by collagen type I [13]. Unfortunately, even a year later, a scar remains weaker and functionally deficient compared to healthy skin.
LLLT has been shown to effectively modulate selected steps in skin wound healing, including generation of myofibroblasts [14], stimulation of collagen deposition [15] and keratinocyte proliferation [16], and/or reduction of oxidative stress and inflammation [17, 18]. In vitro, ex vivo, and in vivo models as well as clinical studies have shown that wound healing is promoted by different laser wavelengths/powers/doses; thus, no optimal set of parameters has yet been identified [19]. Although absorbed energy may differ for each wound type, only a few studies have been focusing on direct comparison of LLLT on the healing of two basic wound models (Fig. 1) [20]. Therefore, in this review, special attention was given to compare the efficiency of the most commonly used red and infrared LLLT on the healing of sutured incisions (focusing on TS) and open excisions (focusing on WC).
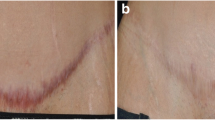
Schema of sutured incision and open excision (E epidermis, D dermis). Photographs of skin wounds (sutured incision and open excision) at the day of surgery (day 0) and following 7 days of healing (day 7). Histological picture of sutured skin incision with thin incisional gab (I) with only minimal amount of granulation tissue (area between dashed lines) surrounded by intact dermis (D) and open skin excision with extensively formed granulation tissue (GT) rich on cells and high caliber vessels. Both histological pictures show wounds at day 7 stained with hematoxylin and eosin (H+E; ×200)
Methods
Eligibility criteria
We only included controlled animal studies reported in English language with open wounds (the healing rate measured with wound size) and/or incisional sutured wounds (the healing rate measured with tensile strength) treated with LLLT.
Search strategy
A systematic PubMed search was performed throughout all years until December 31, 2017 to identify studies containing information of LLLT effect on skin wound healing, in terms of TS of sutured wounds and WC of open wounds. The search string for identifying articles contained search and MeSH terms describing open and sutured wounds, TS, WC, wound healing, and LLLT (search keywords in the category sutured wounds/incisions: “tensile strength laser therapy wound,” and “tensile strength laser therapy incision,” “tensile strength laser therapy incisional wound”; search keywords in the category open wounds/excisions: “laser therapy open wound,” “laser therapy excisional wound,” “laser therapy excision skin wound,” and “laser therapy wound contraction”; search keywords for both categories: “low level laser therapy skin wound repair animal”).
Study selection
Initially, one reviewer carefully read the titles and abstracts of the publications, identified by the search, and retrieved them in full text, unless they were clearly irrelevant. The same reviewer evaluated the full texts of all potentially eligible articles and made a careful decision to include or exclude each article, with close attention to the eligibility criteria. Subsequently, another reviewer checked the first reviewers’ work by reading the full texts of the articles initially included and excluded. Any retrieved article not meeting the eligibility criteria was excluded and had its details listed with reason for exclusion.
Data extraction and meta-analysis
The data extraction involved a two-person procedure. First, one reviewer extracted the means, variance data and time points of assessment for analysis, and then, another reviewer checked this work. Laser parameters, including mW/spot, mW/cm2, J/spot, J/session, treatment time, wavelength, distance or contact with laser probe, scanning in the middle, and/or edge of the wound, were additionally collected or calculated when possible, to investigate which ones that might impact healing rate.
All presented meta-analyses were conducted using final score random effects models, weighting the individual trial effect estimates relatively even, when heterogeneity is present. Impact from heterogeneity on the meta-analyses, i.e., inconsistency, was examined using I2 statistics [21]. The level of inconsistency was categorized as low (25%), moderate (50%), and high (75%) [22].
The standardized mean difference (SMD) was calculated as the difference between the final scores of the LLLT and control groups divided by the pooled standard deviation (SD). The SDs were extracted or estimated from other variance data, i.e., standard errors, 95% confidence intervals, p values, visually from graphs, and medians of SDs. The SMD was adjusted to Hedges’ g, using a correlation factor, as Cohens’ d can exaggerate the results, especially in analyses based on few trials and small sample sizes. The SMD was clinically interpreted as proposed by Cohen, i.e., a SMD of 0.2 represents a small, ~ 0.5 a moderate, and > 0.8 a large effect [23].
All intervention groups have been incorporated in the meta-analyses by splitting their related control groups. That is, except for the studies by Yasukawa et al. [24] and Hegde et al. [25], since they had 10 and 5 intervention groups and only 10 and 7 animals in the control groups, respectively, and consequently, some of their intervention groups were merged.
The meta-analyses were performed using the software programs Excel 2016 for Microsoft Windows and Review Manager, version 5.3 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014).
Results
Study selection
The literature search yielded 51 potentially eligible publications of which 19 were judged eligible (Table 1) and 32 judged irrelevant (Table 2). Eighteen articles had data that allowed for meta-analysis of either TS of sutured incisions or WC of open excisions.
Synthesis of results—tensile strength of sutured wounds
Data allowing for meta-analysis of TS of sutured wounds were available from eight studies with 28 comparisons (n = 378). The overall result favored LLLT over control after 7–56 days by a significant SMD of 1.06 [95% CI 0.66 to 1.46] with moderate inconsistency. Higher mW/cm2 yielded better point effect estimates and could explain some of the heterogeneity:
A subgroup of nine comparisons (n = 134) with infrared 30–79 mW/cm2 laser favored LLLT over control after 11–23 days by a significant SMD of 1.44 [95% CI 0.67 to 2.21] with moderate inconsistency.
A subgroup of three comparisons (n = 36) with 1.08 mW/cm2 pulsed infrared laser favored LLLT over control after 15 days by a non-significant SMD of 0.69 [95% CI − 1.8 to 3.19] with high inconsistency.
A subgroup of two comparisons (n = 55) with 139–281 mW/cm2 red laser favored LLLT over control after 7 days by a significant SMD of 1.52 [95% CI 0.54 to 2.49] with no inconsistency.
A subgroup of 14 comparisons (n = 153) with 1–15 mW/cm2 red laser favored LLLT over control after 7–56 days by a significant SMD of 0.73 [95% CI 0.34 to 1.12] with no inconsistency (Fig. 2).
Forest plot for a meta-analysis based on TS of sutured wounds. Synthesized results are shown as totals in the bottom of the figure. Plots on the right-hand side of the y-axis indicate that LLLT is superior. The uppercase letters after author name and publication year refer to the comparison groups in Table 1
Synthesis of results—contraction of open wounds
Data allowing for meta-analysis of contraction of open wounds were available from 11 studies with 23 comparisons (n = 583). The overall result favored LLLT over control after 6–14 days by a significant SMD of 0.99 [95% CI 0.38 to 1.59] with high inconsistency. None of the collected laser parameters could explain the heterogeneity, and thus, we subgrouped studies by wavelength and mW/cm2 as in the TS analysis. Higher mW/cm2 yielded better point effect estimates:
A subgroup of seven comparisons (n = 192) with infrared 53–300 mW/cm2 laser favored LLLT over control after 6–7 days by a significant SMD of 1.18 [95% CI 0.41 to 1.94] with high inconsistency.
A subgroup of eight comparisons (n = 285) with 25–90 mW/cm2 red laser favored LLLT over control after 7–14 days by a significant SMD of 1.6 [95% CI 0.27 to 2.93] with high inconsistency.
A subgroup of eight comparisons (n = 106) with 2–4 mW/cm2 red laser favored LLLT over control after 6–7 days by a non-significant SMD of 0.11 [95% CI − 0.68 to 0.91] with moderate inconsistency (Fig. 3).
Forest plot for a meta-analysis based on WC. Synthesized results are shown as totals in the bottom of the figure. Plots on the right-hand side of the y-axis indicate that LLLT is superior. The uppercase letters after author name and publication year refer to the comparison groups in Table 1
Synthesis of results—LLLT in diabetic animals
Data allowing for meta-analysis of TS of sutured wounds of diabetic animals only were available from three studies with six comparisons (n = 86). The overall result favored 1–79 mW/cm2 LLLT over control after 11–23 days by a significant SMD of 1.84 [95% CI 0.83 to 2.84] with moderate inconsistency (Fig. 4).
Forest plot for a meta-analysis based on TS of diabetic sutured wounds. Synthesized results are shown as totals in the bottom of the figure. Plots on the right-hand side of the y-axis indicate that LLLT is superior. The uppercase letters after author name and publication year refer to the comparison groups in Table 1
Data allowing for meta-analysis of WC of open wounds of diabetic animals were available from two studies with five comparisons (n = 132). The overall result favored control over 4–38 mW/cm2 LLLT after 7–8 days by a non-significant SMD of − 0.51 [95% CI − 1.11 to 0.09] with low inconsistency (Fig. 5).
Forest plot for a meta-analysis based on WC of diabetic sutured wounds. Synthesized results are shown as totals in the bottom of the figure. Plots on the right-hand side of the y-axis indicate that LLLT is superior. The uppercase letters after author name and publication year refer to the comparison groups in Table 1
Discussion
Our previous experimental wound therapy-related works [26,27,28,29] demonstrated that identical treatment protocols of sutured and open wounds yielded different outcomes, which may present an aspect with crucial significance for clinical practice. These findings are in line with the overall meta-analysis results of the present study, indicating that LLLT is effective in accelerating healing of open excisions and sutured incisions.
In detail, our meta-analysis indicates that both 53–300 mW/cm2 infrared laser and 25–90 mW/cm2 red laser is effective in accelerating WC, whereas less intense red laser in the range 2–4 mW/cm2 is not effective. In comparison, 30–79 mW/cm2 infrared laser and 139–281 mW/cm2 red laser seem to be effective in increasing wound TS, and even red laser with as low as 1.27–15 mW/cm2 appears moderately effective (SMD = 0.73). These discrepancies support the hypothesis that the higher amount of granulation tissue in open wounds [7, 8] absorbs more of the laser energy compared to the relatively intact superficial skin in sutured wounds.
The observed differences in the effectiveness of LLLT between healthy and diabetic models may be related to differences in their respective wound microenvironment and/or the use of different laser parameters. Imbalance in the production of growth factors, abnormal ECM function, and poor blood supply are the key factors responsible for delayed wound healing in diabetic patients [30]. Furthermore, diabetic animals have in general decreased expression of α-SMA [31], and subsequently, these wounds exert lower contraction rates [32] which may also explain the poor efficiency of LLLT in this model. The TS analysis of sutured diabetic animals yielded a very large effect estimate in favor of LLLT, whereas LLLT did not improve WC of open wounds in diabetic animals. It is plausible that this discrepancy is due to the higher mW/cm2 and/or longer wavelength applied to the sutured wounds. On the other hand, the effect of LLLT in diabetic foot ulcers (DFU) has been investigated in an interesting systematic review and meta-analysis and those results showed that LLLT has significant potential to become a portable, non-invasive, easy-to-use, and cost-effective treatment modality for DFU [33].
Since the review revealed different effects of LLLT in both models of wound healing, several underlying biological mechanisms need to be considered. In this context, it has been shown that LLLT with 635-nm diode laser (dose 0.3 J/cm2, output power 89 mW) inhibited TGF-β1/Smad3-mediated conversion of fibroblasts into myofibroblasts, and this effect involved the modulation of TRPC1 ion channels [34]. These data indicate a potential antifibrotic effect of LLLT and suggest that this therapy is a promising therapeutic tool against tissue fibrosis. On the other hand, open wounds irradiated with a higher dose of HeNe laser (dose 1 J/cm2, output power 10 mW) contained α-SMA positive cells, reduced inflammation, and better organization of collagen fibrils [35]. Similarly, combined red (685 nm) and blue (470 nm) therapy (both power densities 8 mW/cm2 and daily doses 3.36 J/cm2) of sutured skin incisions led to improved formation of cross-linked collagen fibers [36]. The abovementioned data are very interesting, since very low doses, and not high doses, are able to block selected pathways which led to very specific effects of treatment.
Recent progress in wound healing research has also highlighted the significance of the ECM as an essential part of the niche. Important molecules involved in ECM remodeling are matrix metalloproteinases (MMPs), a family of calcium-dependent, zinc-containing endopeptidases that are negatively regulated by their inhibitors (TIMPs). In particular, MMP-2 enables endothelial cell migration during angiogenesis and facilitates re-epithelization and fibroblast growth. On the other hand, MMP-2 also digests several structural glycoproteins (including collagen type III) and gelatins [37]. Collagen type III plays an important role in wound stiffness during the early stages of wound healing [7, 13], thus may represent additional mechanisms of wound-type specific differences of treatment. In this context, LLLT has been shown to improve open wound healing by enhancing neocollagenesis, neoangiogenesis, and MMP-2 expression [38]. However, upregulation of MMP-2 has led to a decrease of wound TS in an aged-associated rat model of skin incision [39]. From this point of view, it would be interesting to test this LLLT protocol on the biomechanical parameters of skin incisions.
LLLT parameter/wound-type-dependent effects—red lasers
The first paper comparing both incisions and excisions was published already in 1983. In this work, no significant differences were observed in healing between laser-treated (tested doses 1.1 and 2.2 J/cm2) and untreated control wounds. Conversely, rat skin incisions exposed to 2.2 J/cm2 demonstrated significant increase in wound TS over controls [40]. These results have been partially confirmed later, since LLLT at 632.8 nm with a output power of 1.56 mW and a dose of 1.22 J/cm2 has also resulted in improvement in the wound TS [41]. Nevertheless, no differences were found between control and laser treated (wavelength 632.8 nm; output power 4 mW) groups in the WC of open and TS of closed wounds [42]. Rats received a treatment of 1, 2, or 4 J/cm2 in the case of excisions and 2 J/cm2 for skin incisions. In addition, by comparing two parameter settings of HeNe laser, it has been shown that power density of 281 mW/cm2 with a dose of 4.21 J/cm2 is more effective than 139 mW/cm2 and 2.09 J/cm2 [24]. Furthermore, LLLT produced a better effect when treatment was applied every other day compared to daily treatment.
The first pivotal paper describing inverse relationship between wavelength and power density was published in 2004 [43]. LLLT at 670 and 685 nm (dose 10 J/cm2; delivered by 2, 15, or 25 mW) has been found more effective when combining higher intensity with shorter wavelength or lower intensity with higher wavelength, using an open wound model. Motivated by this study, we have tested red LLLT, by the means of both basic wound healing models. We have also shown that the LLLT effect depends upon dosing (dose 5 J/cm2; tested power densities 1, 5, 15, and 40 mW/cm2), wavelength (635 vs. 670 nm), and wound type (incision vs. excision) [26,27,28]. In detail, open wounds were effectively stimulated by applying higher intensities with both 635 and 670 nm wavelengths [26, 27], which corresponds to the paper published in 2004. However, the model of sutured incisions revealed that LLLT at 635 and 670 nm was more effective in increasing wound TS when higher intensity was combined with shorter wavelength and/or lower intensity was combined with longer wavelength [28]. Later realized in vivo experiments reinforced the data, demonstrating positive effects of LLLT on wound stiffness. Laser irradiation at 635 nm of both energy densities, 1 and 3 J/cm2 (delivered by 50 mW), increased wound TS [44]. Similarly, LLLT at 660 nm energy densities of 1 and 5 J/cm2, but not 10 J/cm2, enhanced wound TS in a rat incisional wound model [45]. Interestingly, wound treatment at 660 nm (tested doses 3 and 30 J/cm2, power density 25.47 mW/cm2) revealed that the higher tested energy density was more effective in modifying the morphology of the scar tissue (increased collagen and glycosaminoglycan content as well as elevated density of blood vessels) and led to a faster course of healing [46]. LLLT at 670 nm (tested doses 4 and 8 J/cm2, power density: not provided) reduced inflammation and increased collagen deposition and the presence of myofibroblasts [47]. Wound contraction was faster following therapy with the lower tested dose.
LLLT parameter/wound-type-dependent effects—infrared lasers
Other examples of wound-type-specific effects have been demonstrated using infrared lasers. The 808 nm (5 J/cm2 delivered with different power densities 100, 200, and 300 mW/cm2) wavelength has been found more effective when energy was delivered at the lowest tested power density. This has been verified by increased GT formation, collagen deposition, and faster rate of wound contraction in an open wound healing model [48]. However, LLLT at similar parameters (809 nm; tested energy densities 1 and 3 J/cm2; delivered by 50 mW) did not have any positive effects on wound TS [44]. Other examples of the wound-type-specific LLLT effects may be reflected in our experiment conducted with diabetic rats. In this study, treatment with an infrared 810-nm laser with a power density of 30 mW/cm2 and daily dose of 0.9 J/cm2 reverse wound impairment mediated by diabetes induction in open wounds, but was not that effective in increasing wound TS of sutured incisions [49]. A single irradiation at 830 nm produced a slightly better result by applying the dose of 1.3 J/cm2 when compared to the dose of 3 J/cm2 (power density 53 mW/cm2) on open wounds in rats [50]. Our theory regarding a parameter/wound-type efficiency of LLLT was supported by other study where treatment of full-thickness incisions with a laser radiation at 830 nm (dose 5.0 J/cm2; power density 79 mW/cm2) resulted in significantly enhanced cutaneous wound TS in healthy and diabetic mice [51]. On the other hand, a direct comparison of two laser doses, 30 and 90 J/cm2, at 830 nm (power density 73 mW/cm2) revealed that the higher dose was more beneficial to the healing process via modulating the morphology and oxidative status of open wounds [52]. In another study, healthy and diabetic rats were submitted to a treatment with either 0.03 or 0.2 J/cm2 dose by a pulsed infrared 890-nm laser with 80 Hz frequency [53]. Interestingly, a lower LLLT dose significantly decreased wound TS in healthy rats, whereas a higher dose significantly increased the maximum load of wounds in both healthy and diabetic animals.
Conclusion
In summary, our review has resulted in two (clinical and experimental) important observations. Firstly, LLLT appears effective in treatment of sutured and open wounds. Laser power density could explain some of the statistical heterogeneity in the TS analysis, but not in the WC analysis. This indicates that TS development of sutured wounds is more dependent on laser mW/cm2, compared to the contraction rate of open wounds. Thus, it is plausible that optimized treatment of the two wound types require different laser parameters. More comprehensive subgroup analyses with other laser parameters, e.g., dose and irradiation protocol, were prohibited due to poorly reported treatment parameters. However, we may conclude that laser doses around 5 J/cm2 can accelerate wound healing. Secondly, common use of both basic wound models (sutured incision and open excision) should become the gold standard in all further experimental studies comparing the efficiency of specific treatment protocols/approaches. However, our results should be interpreted with caution, as only one literature database was used and due to statistical heterogeneity, which could be a product of other LLLT parameters than power density and wavelength. Finally, a direct extrapolation from data seen in animal studies to the human clinical situation is not possible due to the inter-species variability. Since the general molecular regulation of wound healing should be similar, further clinical LLLT investigations are encouraged, with comprehensive reporting of LLLT parameters.
References
Mester E, Szende B, Tota JG (1969) Effect of low intensity laser radiation, repeatedly administered over a long period, on the skin and inner organs of mice. Radiobiol Radiother (Berl) 10(3):371–377
Avci P, Gupta A, Sadasivam M, Vecchio D, Pam Z, Pam N, Hamblin MR (2013) Low-level laser (light) therapy (LLLT) in skin: stimulating, healing, restoring. Semin Cutan Med Surg 32(1):41–52
Mester A (2013) Laser biostimulation. Photomed Laser Surg 31(6):237–239
Bordvik DH, Haslerud S, Naterstad IF, Lopes-Martins RAB, Leal Junior ECP, Bjordal JM, Joensen J (2017) Penetration time profiles for two class 3B lasers in in situ human Achilles at rest and stretched. Photomed Laser Surg 35(10):546–554
Anders JJ, Wu X (2016) Comparison of light penetration of continuous wave 810 nm and superpulsed 904 nm wavelength light in anesthetized rats. Photomed Laser Surg 34(9):418–424
Lee LK, Whitehurst C, Pantelides ML, Moore JV (1995) In situ comparison of 665 nm and 633 nm wavelength light penetration in the human prostate gland. Photochem Photobiol 62(5):882–886
Gal P, Toporcer T, Vidinsky B, Mokry M, Novotny M, Kilik R, Smetana K Jr, Gal T, Sabo J (2006) Early changes in the tensile strength and morphology of primary sutured skin wounds in rats. Folia Biol (Praha) 52(4):109–115
Gal P, Kilik R, Mokry M, Vidinsky B, Vasilenko T, Mozes S, Bobrov N, Tomori Z, Bober J, Lenhardt L (2008) Simple method of open skin wound healing model in corticosteroid-treated and diabetic rats: standardization of semi-quantitative and quantitative histological assessments. Vet Med-Czech 53(12):652–659
Motegi SI, Ishikawa O (2017) Mesenchymal stem cells: the roles and functions in cutaneous wound healing and tumor growth. J Dermatol Sci 86(2):83–89
Ehrlich HP, Hunt TK (2012) Collagen organization critical role in wound contraction. Adv Wound Care (New Rochelle) 1(1):3–9
Gabbiani G, Ryan GB, Majne G (1971) Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia 27(5):549–550
Burkitt HG, Quick CRG, Gatt D (1990) Essential surgery: problems, diagnosis and management. Churchill Livingstone, Edinburgh 771 s. p
Sabol F, Dancakova L, Gal P, Vasilenko T, Novotny M, Smetana K, Lenhardt L (2012) Immunohistological changes in skin wounds during the early periods of healing in a rat model. Vet Med-Czech 57(2):77–82
de Loura Santana C, Silva Dde F, Deana AM, Prates RA, Souza AP, Gomes MT, de Azevedo Sampaio BP, Shibuya JF, Bussadori SK, Mesquita-Ferrari RA, Fernandes KP, Franca CM (2015) Tissue responses to postoperative laser therapy in diabetic rats submitted to excisional wounds. PLoS One 10(4):e0122042
De Castro IC, Rocha CA, Gomes Henriques AC, Cavalcanti de Sousa AP, Lisboa MV, Sotero Dda R, Pinheiro AL, Cury PR, Santos JN (2014) Do laser and led phototherapies influence mast cells and myofibroblasts to produce collagen? Lasers Med Sci 29(4):1405–1410
Sperandio FF, Simoes A, Correa L, Aranha AC, Giudice FS, Hamblin MR, Sousa SC (2015) Low-level laser irradiation promotes the proliferation and maturation of keratinocytes during epithelial wound repair. J Biophotonics 8(10):795–803
de Melo Rambo CS, Silva JA Jr, Serra AJ, Ligeiro AP, Vieira RP, Albertini R, Leal-Junior EC, de Tarso Camillo de Carvalho P (2014) Comparative analysis of low-level laser therapy (660 nm) on inflammatory biomarker expression during the skin wound-repair process in young and aged rats. Lasers Med Sci 29(5):1723–1733
Tatmatsu-Rocha JC, Ferraresi C, Hamblin MR, Damasceno Maia F, do Nascimento NR, Driusso P, Parizotto NA (2016) Low-level laser therapy (904nm) can increase collagen and reduce oxidative and nitrosative stress in diabetic wounded mouse skin. J Photochem Photobiol B 164:96–102
Kuffler DP (2016) Photobiomodulation in promoting wound healing: a review. Regen Med 11(1):107–122
Davidson JM (1998) Animal models for wound repair. Arch Dermatol Res 290(Suppl):S1–S11
Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21(11):1539–1558
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327(7414):557–560
Higgins JPT, Green S, Cochrane Collaboration (2008) Cochrane handbook for systematic reviews of interventions. Wiley-Blackwell, Chichester, Hoboken, p xxi 649 p
Yasukawa A, Hrui H, Koyama Y, Nagai M, Takakuda K (2007) The effect of low reactive-level laser therapy (LLLT) with helium-neon laser on operative wound healing in a rat model. J Vet Med Sci 69(8):799–806
Hegde VN, Prabhu V, Rao SB, Chandra S, Kumar P, Satyamoorthy K, Mahato KK (2011) Effect of laser dose and treatment schedule on excision wound healing in diabetic mice. Photochem Photobiol 87(6):1433–1441
Gal P, Mokry M, Vidinsky B, Kilik R, Depta F, Harakalova M, Longauer F, Mozes S, Sabo J (2009) Effect of equal daily doses achieved by different power densities of low-level laser therapy at 635 nm on open skin wound healing in normal and corticosteroid-treated rats. Lasers Med Sci 24(4):539–547
Lacjakova K, Bobrov N, Polakova M, Slezak M, Vidova M, Vasilenko T, Novotny M, Longauer F, Lenhardt L, Bober J, Levkut M, Sabol F, Gal P (2010) Effects of equal daily doses delivered by different power densities of low-level laser therapy at 670 nm on open skin wound healing in normal and corticosteroid-treated rats: a brief report. Lasers Med Sci 25(5):761–766
Vasilenko T, Slezak M, Kovac I, Bottkova Z, Jakubco J, Kostelnikova M, Tomori Z, Gal P (2010) The effect of equal daily dose achieved by different power densities of low-level laser therapy at 635 and 670 nm on wound tensile strength in rats: a short report. Photomed Laser Surg 28(2):281–283
Novotny M, Vasilenko T, Varinska L, Smetana K Jr, Szabo P, Sarissky M, Dvorankova B, Mojzis J, Bobrov N, Toporcerova S, Sabol F, Matthews BJ, Gal P (2011) ER-alpha agonist induces conversion of fibroblasts into myofibroblasts, while ER-beta agonist increases ECM production and wound tensile strength of healing skin wounds in ovariectomised rats. Exp Dermatol 20(9):703–708
Sorg H, Tilkorn DJ, Hager S, Hauser J, Mirastschijski U (2017) Skin wound healing: an update on the current knowledge and concepts. Eur Surg Res 58(1–2):81–94
Darby IA, Bisucci T, Hewitson TD, MacLellan DG (1997) Apoptosis is increased in a model of diabetes-impaired wound healing in genetically diabetic mice. Int J Biochem Cell Biol 29(1):191–200
Kao HK, Chen B, Murphy GF, Li Q, Orgill DP, Guo L (2011) Peripheral blood fibrocytes: enhancement of wound healing by cell proliferation, re-epithelialization, contraction, and angiogenesis. Ann Surg 254(6):1066–1074
Tchanque-Fossuo CN, Ho D, Dahle SE, Koo E, Li CS, Isseroff RR, Jagdeo J (2016) A systematic review of low-level light therapy for treatment of diabetic foot ulcer. Wound Repair Regen 24(2):418–426
Sassoli C, Chellini F, Squecco R, Tani A, Idrizaj E, Nosi D, Giannelli M, Zecchi-Orlandini S (2016) Low intensity 635 nm diode laser irradiation inhibits fibroblast-myofibroblast transition reducing TRPC1 channel expression/activity: new perspectives for tissue fibrosis treatment. Lasers Surg Med 48(3):318–332
de Araujo CEN, Ribeiro MS, Favaro R, Zezell DM, Zorn TMT (2007) Ultrastructural and autoradiographical analysis show a faster skin repair in He-Ne laser-treated wounds. J Photoch Photobio B 86(2):87–96
Figurova M, Ledecky V, Karasova M, Hluchy M, Trbolova A, Capik I, Hornak S, Reichel P, Bjordal JM, Gal P (2016) Histological assessment of a combined low-level laser/light-emitting diode therapy (685 nm/470 nm) for sutured skin incisions in a porcine model: a short report. Photomed Laser Surg 34(2):53–55
Xue M, Jackson CJ (2015) Extracellular matrix reorganization during wound healing and its impact on abnormal scarring. Adv Wound Care (New Rochelle) 4(3):119–136
de Medeiros ML, Araujo-Filho I, da Silva EM, de Sousa Queiroz WS, Soares CD, de Carvalho MG, Maciel MA (2017) Effect of low-level laser therapy on angiogenesis and matrix metalloproteinase-2 immunoexpression in wound repair. Lasers Med Sci 32(1):35–43
Ballas CB, Davidson JM (2001) Delayed wound healing in aged rats is associated with increased collagen gel remodeling and contraction by skin fibroblasts, not with differences in apoptotic or myofibroblast cell populations. Wound Repair Regen 9(3):223–237
Surinchak JS, Alago ML, Bellamy RF, Stuck BE, Belkin M (1983) Effects of low-level energy lasers on the healing of full-thickness skin defects. Lasers Surg Med 2(3):267–274
Lyons RF, Abergel RP, White RA, Dwyer RM, Castel JC, Uitto J (1987) Biostimulation of wound healing in vivo by a helium-neon laser. Ann Plast Surg 18(1):47–50
Allendorf JD, Bessler M, Huang J, Kayton ML, Laird D, Nowygrod R, Treat MR (1997) Helium-neon laser irradiation at fluences of 1, 2, and 4 J/cm2 failed to accelerate wound healing as assessed by both wound contracture rate and tensile strength. Lasers Surg Med 20(3):340–345
do Nascimento PM, Pinheiro AL, Salgado MA, Ramalho LM (2004) A preliminary report on the effect of laser therapy on the healing of cutaneous surgical wounds as a consequence of an inversely proportional relationship between wavelength and intensity: histological study in rats. Photomed Laser Surg 22(6):513–518
Solmaz H, Ulgen Y, Gulsoy M (2017) Photobiomodulation of wound healing via visible and infrared laser irradiation. Lasers Med Sci 32(4):903–910
Suzuki R, Takakuda K (2016) Wound healing efficacy of a 660-nm diode laser in a rat incisional wound model. Lasers Med Sci 31(8):1683–1689
Novaes RD, Goncalves RV, Cupertino MC, Araujo BM, Rezende RM, Santos EC, Leite JP, Matta SL (2014) The energy density of laser light differentially modulates the skin morphological reorganization in a murine model of healing by secondary intention. Int J Exp Pathol 95(2):138–146
Medrado AR, Pugliese LS, Reis SR, Andrade ZA (2003) Influence of low level laser therapy on wound healing and its biological action upon myofibroblasts. Lasers Surg Med 32(3):239–244
Lau P, Bidin N, Krishnan G, AnaybBaleg SM, Sum MB, Bakhtiar H, Nassir Z, Hamid A (2015) Photobiostimulation effect on diabetic wound at different power density of near infrared laser. J Photochem Photobiol B 151:201–207
Dancakova L, Vasilenko T, Kovac I, Jakubcova K, Holly M, Revajova V, Sabol F, Tomori Z, Iversen M, Gal P, Bjordal JM (2014) Low-level laser therapy with 810 nm wavelength improves skin wound healing in rats with streptozotocin-induced diabetes. Photomed Laser Surg 32(4):198–204
Rezende SB, Ribeiro MS, Nunez SC, Garcia VG, Maldonado EP (2007) Effects of a single near-infrared laser treatment on cutaneous wound healing: biometrical and histological study in rats. J Photochem Photobiol B 87(3):145–153
Stadler I, Lanzafame RJ, Evans R, Narayan V, Dailey B, Buehner N, Naim JO (2001) 830-nm irradiation increases the wound tensile strength in a diabetic murine model. Lasers Surg Med 28(3):220–226
Goncalves RV, Novaes RD, Cupertino Mdo C, Moraes B, Leite JP, Peluzio Mdo C, Pinto MV, da Matta SL (2013) Time-dependent effects of low-level laser therapy on the morphology and oxidative response in the skin wound healing in rats. Lasers Med Sci 28(2):383–390
Dadpay M, Sharifian Z, Bayat M, Bayat M, Dabbagh A (2012) Effects of pulsed infra-red low level-laser irradiation on open skin wound healing of healthy and streptozotocin-induced diabetic rats by biomechanical evaluation. J Photochem Photobiol B 111:1–8
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Not applicable.
Rights and permissions
About this article
Cite this article
Gál, P., Stausholm, M.B., Kováč, I. et al. Should open excisions and sutured incisions be treated differently? A review and meta-analysis of animal wound models following low-level laser therapy. Lasers Med Sci 33, 1351–1362 (2018). https://doi.org/10.1007/s10103-018-2496-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-018-2496-7