Abstract
Optimal parameters of low-level laser therapy (LLLT) for wound healing are still discussed. Hence, our study was aimed to compare effects of different power densities of LLLT at 635 nm in rats. Four, round, full-thickness, skin wounds were made on the backs of 48 rats that were divided into two groups (non-steroid laser-treated and steroid laser-treated). Three wounds were stimulated daily with a diode laser (daily dose 5 J/cm2) each with different power density (1 mW/cm2, 5 mW/cm2, and 15 mW/cm2), whereas the fourth wound served as a control. Two days, 6 days, and 14 days after surgery, eight animals from each group were killed and samples were removed for histological evaluation. In the non-steroid laser-treated rats, significant acceleration of epithelization and collagen synthesis 2 days and 6 days after surgery was observed in stimulated wounds. In steroid laser-treated rats, 2 days and 14 days after surgery, a decreased leucocyte/macrophage ratio and a reduction in the area of granulation tissue were recorded, respectively. In conclusion, LLLT, by the method we used, improved wound healing in the non-steroid laser-treated rats, but it was useless after corticosteroid treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite the use of many promising physical methods, such as vacuum assisted closure (VAC) [1], magnetic fields [2], light emitting diodes (LEDs) [3] or low-level laser therapy (LLLT) [4], delayed wound healing is still a significant problem in clinical practice. In general, it is well known that long-term application of corticosteroids is responsible for poor wound healing. Accordingly, in steroid-treated animals and humans, inhibition of the inflammatory phase, decrease of the wound strength, wound contracture, and inhibited epithelization and fibroblastic proliferation have been found [5–7].
LLLT belongs to modern experimental approaches used in wound healing therapy. In numerous clinical and experimental studies it has been proven that low-energy laser light reduces pain, positively influences inflammatory, proliferative and maturation phases of wound healing, and increases wound tensile strength [8–14]. Even if the importance of LLLT has been stressed by these results, this method has not yet been generally accepted for use in clinical practice. Nevertheless, several researchers have shown that LLLT may have adverse effects on wound healing [15–20], although one of the reasons for its ineffectiveness may be associated with the use of extremely low doses [21].
At the present time there is no general agreement about the exact way how LLLT influences wound healing. Nevertheless, it has been well documented that the helium–neon (HeNe) laser, using a dose of 4 J/cm2, accelerates wound closure and increases collagen deposition [22, 23]. Furthermore, in a previous study, we demonstrated a positive effect of HeNe laser irradiation at a dose of 3 J/cm2 on the healing of primary sutured skin wounds [24]. On the other hand, Kana et al. revealed a deceleration in healing by increasing the dose to 20 J/cm2 on open skin wounds [22]. Similarly, in wounded fibroblasts exposed to 5 J/cm2 of HeNe laser irradiation, increased cell proliferation, migration, and viability have been observed, whereas higher doses, 10 J/cm2 and 16 J/cm2, have been found to be inhibitory [25, 26].
It was found that longer wavelengths are associated with a greater depth of penetration [27]. Hence, it may be hypothesized that different wavelengths of laser light stimulate wound healing variously. Accordingly, in an experiment in which the effects of 665 nm, 675 nm, and 810 nm laser light on the proliferation of fibroblasts and endothelial cells were compared, it was found that the highest proliferation activity was observed in 665 nm light-stimulated cultures, while 810 nm inhibited the proliferation of fibroblasts [28]. Since most of the target cells of LLLT are located in the epidermis and hair follicles (epidermal stem cells), as well as in the upper parts of the dermis (fibroblast, macrophages, endothelial cells), we selected a red laser to be tested in our study instead of an infra-red one [29, 30].
In the experiment we tested a diode laser that produces radiation at 635 nm, comparable to that of a HeNe laser (632.8 nm); thus, similar effects might be expected if analogous doses were to be used. However, the optimal power density is still unknown. Therefore, the aim of our study was to compare the influence of different power densities, achieving equal daily doses, on skin wound healing in non-steroid laser-treated and steroid laser-treated rats, using an excisional model and histological evaluation.
Materials and methods
Animal model
This experiment was approved by the Ethics Committee of the Faculty of Medicine of P. J. Šafárik University and by the State Veterinary Administration of the Slovak Republic.
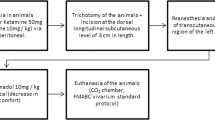
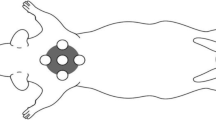
Ten-months-old male Sprague-Dawley rats (n = 48), weighing 500–550 g, were included in the experiment and randomly divided into two groups of 24 animals, i.e., non-steroid laser-treated group (N) and steroid laser-treated group (S). The rats were subjected to general anaesthesia [ketamine 40 mg/kg (Narkamon a.u.v., Spofa, Prague, Czech Republic), xylazine 15 mg/kg (Rometar a.u.v., Spofa, Prague, Czech Republic), tramadol 5 mg/kg (Tramadol-K, Krka, Novo Mesto, Slovenia)], and four, round, full-thickness, skin wounds, 4 mm in diameter, were made on the back of each rat. The wounds were placed at the corners of a quadrangle with 5 cm sides.
Steroid-treated animals were given a bolus dose of 20 mg/kg methyl-prednisolone (Depo-Medrol, Pharmacia and Upjohn, Puurs, Belgium), intramuscularly, shortly before surgery, whereas animals from the non-steroid group remained untreated.
Low level laser therapy
We irradiated three wounds on each rat daily (for a maximum of 6 days) with a commercially available gallium–aluminum–arsenium (GaAlAs) diode laser (Maestro/CCM, Medicom Praha, Prague, Czech Republic; λ = 635 nm; shape of beam oval, r1 = 5 mm, r2 = 2.5 mm; time of treatment at 15 mW/cm2 = 5 min 33 s, at 5 mW/cm2 = 16 min 40 s, and at 1 mW/cm2 = 83 min 20 s; probe distance to wound 10 cm) to administer the total daily dose of 5 J/cm2, while the fourth wound was not irradiated and served as a control. One of the laser-treated wounds was irradiated at 1 mW/cm2 power density, the second at 5 mW/cm2 and the third at 15 mW/cm2. The positions of the laser-treated and control wounds were rotated within the groups. During treatment, the rats were restrained in a Plexiglas cage with an oval opening over each currently stimulated wound, and the other wounds were protected from reflected laser light.
Histopathological evaluation
Eight animals from each group were killed by ether inhalation 2 days, 6 days or 14 days after surgery. The tissue specimens were processed routinely for light microscopy (fixing, dehydrating, embedding, sectioning, staining with hematoxylin and eosin (HE, basic staining) and van Gieson’s stain (VG, non-specific collagen staining)).
We used a semi-quantitative method to evaluate the following histological structures/processes, i.e., polymorphonuclear leukocytes (PMNLs), re-epithelization, fibroblasts, new vessels, and collagen synthesis. The sections were evaluated in a blind manner, according to the scale 0, 1, 2, 3, 4 (Table 1).
We used QuickPHOTO MICRO 2.2 (Promicra, Prague, Czech Republic) software to quantify the following parameters: (1) To determine the inflammatory phase, we calculated the ratio of the number of PMNLs to the number of tissue macrophages (TMs) in specimens removed from animals killed 2 days after surgery. The numbers of PMNLs and TMs were counted in one high-resolution field from each section. (2) The length of the epithelial sheet was morphometrically evaluated in sections removed from animals killed 6 days after surgery and expressed as the percentage covering the whole wound surface. (3) To determine the effect of LLLT on granulation tissue (GT) formation, we measured the area of GT sections made from animals killed 6 days and 14 days after surgery.
Statistical analysis
For each evaluated parameter mean values ± standard deviations (SDs) were calculated. Data obtained from the semi-quantitative evaluation were compared, using the non-parametric Kruskal–Wallis test. Analysis of variance (ANOVA), followed by Tukey–Kramer’s multiple comparison test, was used for the comparison of the PMNL/TM ratios and the GT areas. Significance was accepted at P < 0.05.
Results
During the post-surgery period, the animals remained healthy, with no clinical evidence of infection. The results of our histological investigation are summarized in Figs. 1, 2, 3 and 4.
Morphometric analysis of epithelization (expressed as percentage) of absolute re-epithelization) 6 days after surgery and granulation tissue (GT) area (in square millimeters) 6 days and 14 days after surgery; cell counting from one high-resolution field (×1,000), PMNL/TM ratio 2 days after surgery (data are presented as means ± SDs). *P < 0.05, **P < 0.01) N non-steroid laser-treated group, S steroid laser-treated group
Day 2
Non-steroid laser-treated group
Formation of the demarcation line was the most typical histological change in this time period. There were no significant differences in the numbers of the most common acute inflammatory cells (PMNLs) between wounds (Fig. 1). In contrast, significant difference in the process of re-epithelization was shown (Figs. 1 and 5). In comparison with non-stimulated wounds, in wounds stimulated by 15 mW/cm2 accelerated migration and proliferation of keratinocytes was observed [0 mW/cm2 and 15 mW/cm2, respectively (P < 0.05)]. Comparable numbers of new vessels and fibroblasts near the excisions were recorded (Fig. 2). At the edges of the excisions, significant differences in the creation of new collagen fibers was recorded between control and stimulated wounds [0 mW/cm2 vs 5 mW/cm2 (P < 0.01); 0 mW/cm2 vs 15 mW/cm2 (P < 0.01); 1 mW/cm2 vs 5 mW/cm2 (P < 0.01); 1 mW/cm2 vs 15 mW/cm2 (P < 0.01] (Fig. 3).
Skin wounds 2 days after surgery in non-steroid laser-treated animals. a Control wound (a thickness of cut edges of the epidermis, b demarcation line separating necrosis from the vital tissue); b 1 mW/cm2 LLLT (a the beginning of epithelial cell migration, b demarcation line); c 5 mW/cm2 LLLT (a migration of epithelial cells, b demarcation line); d 15 mW/cm2 LLLT (a most prominent migration of epithelial cells, b demarcation line, c hair follicle). HE; scale bar represents 500 µm
Steroid laser-treated group
In general, the wound healing process in steroid laser-treated animals was slowed (Figs. 1, 2 and 3). Migration of keratinocytes and creation of new collagen fibers had not started. The ratio of PMNL/TM was significantly higher in non-stimulated wounds than in all stimulated wounds [PMNL/TM: 0 mW/cm2 vs 1 mW/cm2 (P < 0.01); 0 mW/cm2 vs 5 mW/cm2 (P < 0.01), 0 mW/cm2 vs 15 mW/cm2 (P < 0.01)]; thus, the inflammatory phase was accelerated after LLLT (Fig. 4).
Day 6
Non-steroid laser-treated group
The histological analysis in this group demonstrated a remission of the inflammatory process in all wounds (Fig. 1). The keratinocytes had migrated beneath the scab and mostly completely bridged the excisions. However, different progress of re-epithelization between certain stimulated and non-stimulated wound was shown [0 mW/cm2 vs 15 mW/cm2; 1 mW/cm2 vs 15 mW/cm2 (P < 0.01)] (Fig. 1). Results of semi-quantitative analysis were confirmed by morphometrical analysis (0 mW/cm2 vs 15 mW/cm2 [P < 0.01); 1 mW/cm2 vs 15 mW/cm2 (P < 0.05)] (Fig. 4). This time period showed a typical histological picture of the proliferative phase, with expressive representation of fibroblasts and new vessels in all wounds, with no significant differences between wounds (Fig. 2). On the other hand, a greater number of new collagen fibers was seen in wounds stimulated by laser radiation at higher power densities [0 mW/cm2 vs 5 mW/cm2 (P < 0.01); 0 mW/cm2 vs 15 mW/cm2 (P < 0.01); 1 mW/cm2 vs 5 mW/cm2 (P < 0.05); 1 mW/cm2 vs 15 mW/cm2 (P < 0.01)] (Figs. 3 and 6). No significant differences between wounds were observed when the GT area was measured (Fig. 4).
Skin wounds 6 days after surgery in non-steroid laser-treated animals. a Control wound (a granulation tissue without a significant quantity of collagen, b striated muscle layer, c subcutaneous tissue); b 1 mW/cm2 LLLT (a granulation tissue located in the middle of the wound, without collagen, b granulation tissue located near normal dermis contains new collagen, c subcutaneous tissue); c 5 mW/cm2 LLLT (a upper part of granulation tissue without a significant quantity of collagen, b lower part of granulation tissue rich in new collagen and vessels, c striated muscle layer); d 15 mW/cm2 LLLT (a whole granulation tissue contains new collagen, b subcutaneous tissue). VG stain; scale bar represents 500 µm)
Steroid laser-treated group
The typical steroid-induced impairment of wound healing characterized this group. However, no significant differences in the evaluated histological parameters between the stimulated and non-stimulated wounds were observed (Figs. 1, 2, 3 and 4).
Day 14
Non-steroid laser-treated group
For this time period remodeling and reorganization of extracellular matrix (ECM) was characterized; thus, the scar was formed (Fig. 3). In addition, a mild regress of vessels in the granulation tissue was shown in this group (Fig. 2). However, significant histological differences between wounds were not seen, either in the semi-quantitatively evaluated structures or in those quantitatively evaluated (Figs. 2, 3 and 4).
Steroid laser-treated group
With regard to the impaired process of wounding in the steroid group, the decrease in the number of vessels and the increase in collagen fibers was not apparent by any significant differences between wounds (Figs. 2 and 3). Nevertheless, in this group, the scars of the laser-treated wounds were significantly reduced [0 mW/cm2 vs 5 mW/cm2 (P < 0.05); 0 mW/cm2 vs 15 mW/cm2 (P < 0.05)] (Figs. 4 and 7).
Discussion
The effectiveness of the various wavelengths and power densities of laser radiation on wound healing has still not been fully clarified. From a comparison of the effects of different wavelengths (670 nm vs 685 nm) and intensities (2 mW, 15 mW, and 25 mW), achieving a total dose of 10 J/cm2, LLLT has been found to be more effective if higher intensity is combined with shorter wavelength or lower intensity with longer wavelength [31]. Nevertheless, from assessment of the effects of different wavelengths, i.e., 633 nm, 670 nm, 820 nm, on redox changes in HeLa cells, it has been found that cytochrome c oxidase becomes more oxidized, due to irradiation at each wavelength used [32]. These results support the suggestion that the mechanism of LLLT at the cellular level is generally based on the increase of oxidative metabolism in mitochondria.
Results from our investigation point to an inverse relationship between power density and the course of the inflammatory phase. Whereas the lowest power density slightly increased wound infiltration in the control rats with inflammatory cells 6 days after surgery, both higher power densities acted in a slightly anti-inflammatory manner during all the time intervals examined. This trend is in agreement with results from a study published by do Nascimento and co-workers [31], as well as from our previous study where we demonstrated that high doses of LLLT are able to accelerate wound healing by reducing inflammation without compromising the proliferation of fibroblasts and keratinocytes [13]. On the other hand, laser stimulation significantly accelerated the inflammatory phase only in the steroid laser-treated group. However, this effect was observed rather by the power density-independent decrease in PMNL/TM values than by the absolute amount of inflammatory cell infiltration.
By evaluating the effect of LLLT on both the proliferative phase of dermis repair and epidermis regeneration, we observed that epithelization and collagen synthesis were significantly accelerated only in non-steroid laser-treated animals in a power density-dependent manner. In addition, in this group, a typical picture of the proliferative phase with expressive representation of fibroblasts and new vessels in all wounds, without significant differences between wounds, has been shown. In contrast, in steroid laser-treated rats, a significant negative impact by methyl-prednisolone on the formation of the granulation tissue was demonstrated, with no significant differences between stimulated and control wounds. Hence, in our experiment, it was demonstrated that LLLT significantly accelerated the proliferative phase only in non-steroid laser-treated animals. However, another study has shown that LLLT at 904 nm and 33 J/cm2 stimulated both the proliferative phase and granulation tissue formation in both non-steroid laser-treated and steroid laser-treated animals as well [6]. From this point of view, the exact effect of LLLT on the proliferation phase in steroid laser-treated rats remains to be answered.
In addition, scarring is a well-known side effect of skin laser treatment [33]. Hædersdal and co-workers investigated whether scarring after the acute inflammatory reaction following laser therapy could be reduced by administration of methyl-prednisolone and indomethacin. However, the results from their investigation point out that the use of the drug in the prophylaxis against scarring was not beneficial. On the other hand, our results demonstrate that LLLT, by our method, is able to accelerate wound healing without enlarging the area of the scar tissue. Furthermore, in contrast to non-steroid laser-treated rats, in steroid laser-treated rats the low-level laser therapy resulted in a statistically significant reduction in the granulation tissue 14 days after the rats had been wounded, which may indicate reduced scar formation. An explanation for this effect may be that laser-induced wound contraction by myofibroblasts [34] combined with methyl-prednisolone caused inhibition of GT formation [35]. Since, in non-steroid laser-treated rats, no effect was observed, it may be hypothesized that co-action of myofibroblasts and medication leading to GT reduction is inevitable for scar reduction.
Previously, evidence suggesting a possible systemic effect of LLLT was described [11, 36]. On the other hand, in numerous previous investigations in which control and laser-treated wounds were made on the same animal, a significantly positive effect of treatment has been observed [13, 24, 37–39]. Furthermore, results from our investigation, in which one control and three laser-treated wounds were made on the same animal, demonstrate differences between different power densities of LLLT. From this point of view, it can be hypothesized that the dominant effect of LLLT on skin wound healing is local. Therefore, the question of a systemic effect of laser therapy remains open and requires further research.
In conclusion, our results extend and reinforce the theory of the positive effect of LLLT on wound healing in normal conditions, but not after corticosteroid treatment. Moreover, our study demonstrates that LLLT at 635 nm effectively stimulates wound healing by using higher power densities. Since, by the use of the highest power density the shortest time is needed to achieve the daily dose of 5 J/cm2, it can be suggested that the use of tested laser at 15 mW/cm2 might be the optimal power density for further preclinical investigations.
References
Toporcer T, Radoňak J (2006) Vacuum assisted wound closure—overview of lesson and applications. Cas Lek Cesk 145:702–707
Milgram J, Shahar R, Levin-Harrus T, Kass P (2004) The effect of short, high intensity magnetic field pulses on the healing of skin wounds in rats. Bioelectromagnetics 25:271–277. doi:10.1002/bem.10194
Whelan HT, Smits RL Jr, Buchman EV, Whelan NT, Turner SG, Margolis DA et al (2001) Effect of NASA light-emitting diode irradiation on wound healing. J Clin Laser Med Surg 19:305–314. doi:10.1089/104454701753342758
Elwakil TF (2007) An in-vivo experimental evaluation of He-Ne laser photostimulation in healing Achilles tendons. Lasers Med Sci 22:53–59. doi:10.1007/s10103–006–0423–9
Anstead GM (1998) Steroids, retinoids and wound healing. Adv Wound Care 11:277–285
Pessoa ES, Melhado RM, Theodoro LH, Garcia VG (2004) A histologic assessment of the influence of low-intensity laser therapy on wound healing in steroid-treated animals. Photomed Laser Surg 22:199–204. doi:10.1089/1549541041438533
Rogers CC, Hanaway M, Alloway RR, Alexander JW, Boardman RE, Trofe J et al (2005) Corticosteroid avoidance ameliorates lymphocele formation and wound healing complications associated with sirolimus therapy. Transplant Proc 37:795–797. doi:10.1016/j.transproceed.2004.12.076
Iijima K, Shimovama N, Shimovama M, Yamamoto T, Shimizu T, Mizuguchi T (1989) Effect of repeated irradiation of low-power He-Ne laser in pain relief from postherpetic neuralgia. Clin J Pain 5:271–274
Kreisler MB, Haj HA, Noroozi N, Willershausen B (2004) Efficacy of low level laser therapy in reducing postoperative pain after endodontic surgery—a randomized double blind clinical study. Int J Oral Maxillofac Surg 33:38–41 doi:10.1054/ijom.2002.0449
Nakaji S, Shiroto C, Yodono M, Umeda T, Liu Q (2005) Retrospective study of adjunctive diode laser therapy for pain attenuation in 662 patients: detailed analysis by questionnaire. Photomed Laser Surg 23:60–65. doi:10.1089/pho.2005.23.60
Schindl A, Heinze G, Schindl M, Pernerstorfer-Schon H, Schindl L (2002) Systemic effects of low-intensity laser irradiation on skin microcirculation in patients with diabetic microangiopathy. Microvasc Res 64:240–246. doi:10.1006/mvre.2002.2429
Toida M, Watanabe F, Goto K, Shibata T (2003) Usefulness of low-level laser for control of painful stomatitis in patients with hand-foot-and-mouth disease. J Clin Laser Med Surg 21:363–367 doi:10.1089/104454703322650176
Gál P, Vidinský B, Toporcer T, Mokrý M, Mozeš Š, Longauer F et al (2006) Histological assessment of the effect of laser irradiation on skin wound healing in rats. Photomed Laser Surg 24:480–488. doi:10.1089/pho.2006.24.480
Stadler I, Lanzafame RJ, Evans R, Narayan V, Dailey B, Buehner N et al (2001) 830 nm irradiation increases the wound tensile strength in a diabetic murine model. Lasers Surg Med 28:220–226. doi:10.1002/lsm.1042
Damante CA, Greghi SL, Sant’Ana AC, Passanezi E, Taga R (2004) Histomorphometric study of the healing of human oral mucosa after gingivoplasty and low-level laser therapy. Lasers Surg Med 35:377–384 doi:10.1002/lsm.20111
Lagan KM, Clements BA, McDonough S, Baxter GD (2001) Low intensity laser therapy (830 nm) in the management of minor postsurgical wounds: a controlled clinical study. Lasers Surg Med 28:27–32. doi:10.1002/1096–9101(2001)28:1<27::AID-LSM1013>3.0.CO;2–4
Lundeberg T, Malm M (1991) Low-power HeNe laser treatment of venous leg ulcers. Ann Plast Surg 27:537–539 doi:10.1097/00000637–199112000–00004
Núñez SC, Nogueira GE, Ribeiro MS, Garcez AS, Lage-Marques JL (2004) He-Ne laser effects on blood microcirculation during wound healing: a method of in vivo study through laser Doppler flowmetry. Lasers Surg Med 35:363–368. doi:10.1002/lsm.20109
Petersen SL, Botes C, Olivier A, Guthrie AJ (1999) The effect of low level laser therapy (LLLT) on wound healing in horses. Equine Vet J 31:228–231
Schlager A, Oehler K, Huebner KU, Schmuth M, Spoetl L (2000) Healing of burns after treatment with 670 nm low-power laser light. Plast Reconstr Surg 105:1635–1639. doi:10.1097/00006534–200004050–00006
Tuner J, Hode L (1999) Low-level laser therapy—clinical practice and scientific background. Spjutvägen, Prima Books
Kana JS, Hutschenreiter G, Haina D, Waidelich W (1981) Effect of low-power density laser radiation on healing of open skin wounds in rats. Arch Surg 116:293–296
Bisht D, Gupta SC, Misra V, Mital VP, Sharma P (1994) Effect of low intensity laser radiation on healing of open skin wounds in rats. Indian J Med Res 100:43–46
Gál P, Kilík R, Špaková T, Pataky F, Sabo J, Pomfy M et al (2005) He-Ne laser irradiation accelerates inflammatory phase and epithelization of skin wound healing in rats. Biologia 60:691–696
Hawkins DH, Abrahamse H (2006) The role of laser fluence in cell viability, proliferation, and membrane integrity of wounded human skin fibroblasts following helium-neon laser irradiation. Lasers Surg Med 38:74–83. doi:10.1002/lsm.20271
Houreld NN, Abrahamse H (2007) Laser light influences cellular viability and proliferation in diabetic-wounded fibroblast cells in a dose- and wavelength-dependent manner. Lasers Med Sci 23:11–18. doi:10.1007/s10103–007–0445-y
Langer H, Lange W (1992) Comparison of transmission and absorption of HeNe laser and infrared light in human tissue. AKU 20:19–24
Moore P, Ridgway TD, Higbee RG, Howard EW, Lucroy MD (2005) Effect of wavelength on low-intensity laser irradiation-stimulated cell proliferation in vitro. Lasers Surg Med 36:8–12. doi:10.1002/lsm.20117
Schindl A, Schindl M, Pernerstorfer-Schon H, Schindl L (2000) Low intensity laser therapy: a review. J Investig Med 48:312–326
Motlik J, Klima J, Dvorankova B, Smetana K Jr (2007) Porcine epidermal stem cells as a biomedical model for wound healing and normal/malignant epithelial cell propagation. Theriogenology 67:105–111. doi:10.1016/j.theriogenology.2006.09.018
do Nascimento PM, Pinheiro AL, Salgado MA, Ramalho LM (2004) A preliminary report on the effect of laser therapy on the healing of cutaneous surgical wounds as a consequence of an inversely proportional relationship between wavelength and intensity: histological study in rats. Photomed Laser Surg 22:513–518. doi:10.1089/pho.2004.22.513
Karu TI, Afanasyeva NI, Kolyakov SF, Pyatibrat LV, Welser L (2001) Changes in absorbance of monolayer of living cells induced by laser radiation at 633, 670 and 820 nm. IEEE J Quantum Electron 7:982–988. doi:10.1109/2944.983303
Hædersdal M, Poulsen T, Wulf HC (1993) Laser induced wounds and scarring modified by antiinflammatory drugs: a murine model. Lasers Surg Med 13:55–61. doi:10.1002/lsm.1900130111
de Araújo CE, Ribeiro MS, Favaro R, Zezell DM, Zorn TM (2007) Ultrastructural and autoradiographical analysis show a faster skin repair in He-Ne laser-treated wounds. J Photochem Photobiol B 86:87–96. doi:10.1016/j.jphotobiol.2006.08.006
Salmela K (1981) Comparison of the effects of methylprednisolone and hydrocortisone on granulation tissue development. An experimental study in rat. Scand J Plast Reconstr Surg 15:87–91. doi:10.3109/02844318109103419
Rochkind S, Rousso M, Nissan M, Villarreal M, Barr-Nea L, Rees DG (1989) Systemic effects of low-power laser irradiation on the peripheral and central nervous system, cutaneous wounds, and burns. Lasers Surg Med 9:174–182. doi:10.1002/lsm.1900090214
Reddy GK (2003) Comparison of the photostimulatory effects of visible He-Ne and infrared Ga-As lasers on healing impaired diabetic rat wounds. Lasers Surg Med 33:344–351. doi:10.1002/lsm.10227
Kawalec JS, Hetherington VJ, Pfennigwerth TC, Dockery DS, Dolce M (2004) Effect of a diode laser on wound healing by using diabetic and nondiabetic mice. J Foot Ankle Surg 43:214–220. doi:10.1053/j.jfas.2004.05.004
Ribeiro MS, Silva DF, Maldonado EP, de Rossi W, Zezell DM (2002) Effects of 1047-nm neodymium laser radiation on skin wound healing. J Clin Laser Med Surg 20:37–40. doi:10.1089/104454702753474995
Acknowledgments
We thank Magdaléna Majnušová for preparing the histological sections. Also, we thank Marek Antol, Martin Novotný, Lenka Kostičová, and Eva Kožejová for their useful technical assistance. In addition, we thank Faiza Hussain for editorial help in preparing this manuscript. This study was partially supported by the Slovak Grant Agency of the Ministry of Education and Slovak Academy of Sciences (VEGA, 1/3361/06 and 1/4228/07). We are grateful to U.S. Steel Košice, Slovak Republic, for buying us the Olympus BX51 microscope.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gál, P., Mokrý, M., Vidinský, B. et al. Effect of equal daily doses achieved by different power densities of low-level laser therapy at 635 nm on open skin wound healing in normal and corticosteroid-treated rats. Lasers Med Sci 24, 539–547 (2009). https://doi.org/10.1007/s10103-008-0604-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-008-0604-9