Abstract
Using a 445-nm semiconductor laser for tissue incision, an effective cut is expected due to the special absorption properties of blue laser light in soft tissues. The aim of the present study was the histological evaluation of tissue samples after incision with a 445-nm diode laser. Forty soft tissue specimens were obtained from pork oral mucosa and mounted on a motorized linear translation stage. The handpiece of a high-frequency surgery device, a 970-nm semiconductor laser, and a 445-nm semiconductor laser were connected to the slide, allowing a constant linear movement (2 mm/s) and the same distance of the working tip to the soft tissue’s surface. Four incisions were made each: (I) 970-nm laser with conditioned fiber tip, contact mode at 3-W cw; (II–III): 445-nm laser with non-conditioned fiber tip, contact mode at 2-W cw, and non-contact mode (1 mm) at 2 W; and (IV): high-frequency surgery device with straight working tip, 90° angulation, contact mode at 50 W. Histological analysis was performed after H&E staining of the embedded specimens at 35-fold magnification. The comparison of the incision depths showed a significant difference depending on the laser wavelength and the selected laser parameters. The highest incision depth was achieved with the 445-nm laser contact mode (median depth 0.61 mm, min 0.26, max 1.17, interquartile range 0.58) (p < 0.05) with the lowest amount of soft tissue denaturation (p < 0.05). The lowest incision depth was measured for the high-frequency surgical device (median depth 0.36 mm, min 0.12, max 1.12, interquartile range 0.23) (p < 0.05). Using a 445-nm semiconductor laser, a higher cutting efficiency can be expected when compared with a 970-nm diode laser and high-frequency surgery. Even the 445-nm laser application in non-contact mode shows clinically acceptable incision depths without signs of extensive soft tissue denaturation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Most surgical interventions afford cutting of soft tissues. Therefore, the aim of the cutting procedure can be tissue removal and subsequent surgical pathology analysis. Moreover, it can be necessary to get rid of excess tissue fragments or use a cutting device to gain access to underlying structures. Soft tissue incisions are mostly performed using a surgical scalpel blade. However, also techniques employing electrosurgery, radiosurgery, and lasers with different wavelengths are available [1,2,3]. High-frequency electrical energy can form electric arcs between the electrode and tissue, resulting in surgical incisions with coagulated zones at the incision edges. Laser devices use the ablation properties of the respective wavelength to generate defined cutting lines and hemostasis. Therefore, various kinds of interaction with the tissue such as photochemical, thermal, or ablative effects can be observed [4]. Different laser devices such as CO2 and Nd:YAG (neodymium-doped yttrium aluminum garnet) lasers have been described for soft tissue dental surgery procedures [5]. In addition, semiconductor Iaser devices with wavelengths of 810 to 980 nm can be used for coagulation, cutting, or ablation of oral soft tissue [6,7,8]. In general, semiconductor Iasers are used in an increasing number of fields in research, industry, medicine, and to no small extent the consumer sector due to their compact structure, high optoelectric efficiency, and reliability. Recently, a blue-light semiconductor laser device with a wavelength of 445 nm was introduced for dental applications such as bacterial reduction, coagulation, and cutting of soft tissues [9, 10]. Compared to conventional red light laser systems, one of the most important advantages of blue Iaser light is that, due to its shorter wavelength and the higher absorption coefficient in the target chromophores hemoglobin and melanin, it penetrates less deeply into tissue and is scattered less [11]. Consequently, due to this low-penetration depth, the risk of accidental injuries in deeper layers should be reduced and the laser light can be guided more precisely. Moreover, the lower laser energy absorption in surrounding tissues from scattering is expected to generate less thermal side effects. Soft tissues contain high percentages of water, hemoglobin, melanin, Iipids, and proteins. The absorption maximum for blood cells is in the range of approximately 430 nm [12, 13], which results in a high energy input and can thus cause rapid coagulation. For surgical procedures, blue Iaser allows clean cutting with little bleeding and without major side effects in adjacent tissues due to the special absorption properties in the tissue components [14].
Preliminary clinical observations make the 445-nm laser technology a promising tool for a variety of applications such as surgical incision and drainage, frenectomy, fibroma excision, gingivectomy, gingivoplasty, or implant exposure. However, there are only a few studies that have systematically analyzed structural changes in oral epithelium after diode laser incision [15]. Moreover, there is only limited information about blue-light laser cutting properties. Thus, the aim of the present study was to assess the efficiency of soft tissue incision with a novel 445 nm semiconductor laser, testing the hypothesis of a blue-light laser being more efficient than conventional devices without increasing thermal side effects.
Materials and methods
For the purpose of this study, 20 lower jaws of pig cadavers were obtained directly after slaughter. Forty rectangular soft tissue specimens (two specimens from one jaw each) were prepared from pork oral mucosa and mounted on a self-constructed motorized linear translation stage (Fig. 1). The handpiece of a high-frequency surgery device (Systematica®1062, Kavo, Biberach, Germany), a 970-nm semiconductor laser (SiroLaser Advance, Dentsply Sirona, Bensheim, Germany), and a 445-nm semiconductor laser (SiroLaser Blue, Dentsply Sirona, Benheim, Germany) were connected to the slide, allowing a constant linear movement (2 mm/s) and the same distance of the working tip to the soft tissue’s surface.
Four incisions were made with a 90° angulation of the respective working tip to the specimens’ surface each: (I) 970-nm laser with 320-μm conditioned fiber tip, contact mode at 3-W cw; (II–III): 445-nm laser with non-conditioned 320-μm fiber tip, contact mode at 2-W cw, and non-contact mode (1 mm) at 2 W; and (IV): high-frequency surgery device with straight working tip, contact mode at 50 W. The output power of the 970-nm laser device was set to 3 W; the power of the 445-nm laser was set to 2 W for all measurements according to the manufacturer’s recommendation for soft tissue incisions with the devices under study, resulting in an effective output power of 2.93 ± 0.05 and 2.05 ± 0.04 W, respectively. Therefore, the effective power was measured at the fiber tip employing a digital optical power and energy meter (PM100D, Thorlabs, Dachau, Germany) and a thermal power sensor (S314C, Thorlabs, Dachau, Germany).
Incision depth
Directly after performing all incisions in one specimen, soft tissue incision depths were assessed employing the three-dimensional scanning property of a digital microscope (VHX-5000, Keyence, Neu-Isenburg, Germany) and the respective surface analysis software (Keyence). Therefore, the tissue was carefully dried with gauze fabric and compressed air. Scanning was performed at a magnification of ×20 with 16-bit high dynamic range resolution and partial illumination with one fourth of the ring light turned on to enhance the surface texture of the specimen. Incision depth was measured between the lowest point and the average distance to the right and left marginal ridge (Fig. 2).
Histological processing
Soft tissue specimens were fixed in neutral, buffered formalin for 24 h followed by stepwise (2 h each in 70, 90, and 100% ethanol) dehydration. Thereafter, specimens were incubated in a clearing solution (Roti®-Histol, #6640.1, Carl Roth, Karlsruhe, Germany) for 2 h and embedded in liquid paraffin. Subsequently, paraffin blocks were created for each sample, cut into 3-μm thick slices, and positioned on microscope slides. These specimens were incubated in a clearing agent (Roti®-Histol) for 10 min, followed by stepwise rehydration (5 min each in 100, 90, and 70% ethanol) and incubation in a hematoxylin staining solution (#1.09249.0500, Merck, Darmstadt, Germany) for 10 min. Slides were then washed with water for 10 min, followed by an incubation for 5 min in an eosin staining solution (#1-15935.0010, Merck) and subsequent rinsing process with water. Again, stepwise dehydration was performed in 70 (1 min), 90 (2 min), and 100% (3 min) ethanol, respectively, followed by incubation (10 min) in the clearing agent (Roti®-Histol) and covering with coverslips after application of Corbit balsam (Hecht, Kiel, Germany). Histological analysis of the embedded specimens was performed with a light microscope at 35-fold magnification.
Denaturation
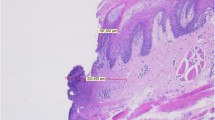
Evaluating the signs of tissue denaturation histologically, the following characteristics were observed in the specimens: coagulation with cell swelling as sign of denaturation of cytoplasmic proteins and disintegration of cell organelles, colliquation as degeneration to a liquid state with loss of cell structures, and carbonization caused by overheating of the tissue (Fig. 3). These characteristics merged into each other and therefore could not be distinguished properly. Thus, two parameters were used to assess the amount of denaturation with respect to the different study groups: (I) “maximum denaturation depth (MDD)” was defined as the denaturation’s largest width measured perpendicularly to the inner incision line and (II) “denaturation area (DA)” was defined as total extent of the denaturated tissue measured circularly around the inner incision line. All histological specimens were digitally photographed at 300 dpi to perform planimetric assessments with the Regener surface analysis software (Ottosoftware, Magdeburg, Germany). A calibration scale on the stage of the microscope was used to calibrate the software and measure distances and areas of the histologic images.
Statistical analysis
A power analysis was performed prior to the study. Therefore, the effect size was set to 0.5 according to Cohen [16]. For an alpha error of 0.05 and a power of 0.8, a sample size of at least 35 specimens in each group was calculated. Normal distribution of the values was assessed with the Shapiro-Wilk test. Since not all data were normally distributed, values for incision depth and denaturation were analyzed with a non-parametric test (Kruskal-Wallis) and Mann-Whitney pairwise comparisons. Differences were considered as statistically significant at p < 0.05. The sequentially rejective Bonferroni correction of the critical p value was used when multiple statistical tests were performed simultaneously on a single data set. Box plot diagrams show the median, first and third quartiles, and minimum and maximum values (whiskers). Values of more than 1.5 to three times the interquartile range are specified as outliers and marked as data points. Values more than three times the interquartile range are specified as far outliers and marked as asterisks.
Results
Incision depth
The comparison of the incision depths showed a significant difference depending on the treatment group. Laser wavelength and selected laser parameters had a statistically significant impact on the results. Highest incision depth was achieved with the 445-nm laser in contact mode (group II) (median depth 0.61 mm, min 0.26, max 1.17, interquartile range 0.58) (p < 0.05) (Fig. 4). The lowest incision depth was measured for the high-frequency surgical device (group IV) (median depth 0.36 mm, min 0.12, max 1.12, interquartile range 0.23) (p < 0.05). Values for the 445-nm laser in non-contact mode (median depth 0.44 mm, min 0.13, max 0.68, interquartile range 0.17) were not statistically significantly different to groups II and IV (p > 0.05 each). The 970-nm laser device (median depth 0.36 mm, min 0.16, max 0.93, interquartile range 0.29) showed a significantly lower incision depth than the 445-nm laser in contact mode (p < 0.05).
Denaturation
Assessing the histologically visible denaturation depths, highest values could be found for the 970-nm laser (median depth 0.062 mm, min 0.027, max 0.182, interquartile range 0.030) (p < 0.05) (Table 1). Lowest values were observed for the high-frequency device (median depth 0.039 mm, min 0.009, max 0.108, interquartile range 0.036) (p < 0.05). No difference could be found for the DAs with respect to the different devices under study (p > 0.05).
However, calculating the denaturation depths with respect to the observed incision depths, the risk-benefit ratio for the procedures under study can be described more precisely. Denaturation depths related to 1-mm incision depth respectively showed statistically significant highest values for the 445-nm laser in non-contact mode (median depth 0.134 mm, min 0.048, max 0.316, interquartile range 0.057) and lowest values for the 445-nm laser in contact mode (median depth 0.089 mm, min 0.052, max 0.146, interquartile range 0.052) (p < 0.05) (Table 2). Similarly, the analysis of the observed DAs showed different results when calculating the absolute values related to 1-mm incision depth, respectively. Lowest values could be found in the 445-nm contact mode group (median value 0.061 mm2, min 0.022, max 0.201, interquartile range 0.098). Statistically significant highest values could be observed in the high-frequency group (median value 0.192 mm2, min 0.004, max 1.303, interquartile range 0.251) (p < 0.05).
Discussion
The present study showed that incision depths and denaturation of soft tissue depend on the respective cutting device under study. Both 445- and 970-nm laser devices were used in the present study. It could be demonstrated that a blue-light laser device can be operated with low power settings to perform surgical incisions effectively [17]. Therefore, the 445-nm laser was used with a power setting of 2 W and the 970-nm device with 3 W, according to the manufacturer’s instructions. The cutting speed is one important factor for the efficiency of laser devices [18]. The authors describe an increase of incision depth and total thermal damage width with decreasing speed using a 1940-nm thulium fiber laser for intraoral surgery. In the present study, all incisions were performed with a constant speed, allowing an intraexperimental comparison of the devices under study. Another study assessed morphological changes after using five different laser wavelengths (450, 532, 808, 1064, and 1340 nm) based on the quality of the cut and tissue damage [19]. The authors observed the best quality of the cut and the lowest temperature increase on the specimens with the shortest laser wavelength (450 nm). The present study did not assess temperature changes. However, the MDD in the 445-nm contact mode group was significantly lower than for the 970-nm laser. Assuming that denaturation occurred primarily because of heat generation, these results are in accordance with the previously mentioned findings.
Assessing the amount of denaturation, the results could have been influenced by the formalin fixation and histological processing. Formalin acts by diffusing through the tissue and binding to amino groups, precipitating the formation of an extensive network of cross-linked proteins and nucleic acids, which can cause histological changes, such as cell shrinkage and distortion [20]. Therefore, the amount of shrinkage has to be considered. Evaluating post-resection mucosal margin shrinkage in oral cancer, the mean shrinkage from the in situ to the post-resection margin status was 22.7% [21]. Another study describes a mean shrinkage of 17.0% in the length of cutaneous surgical specimens after 24 h of fixation in 10% buffered formalin [22]. In the present study, the amount of shrinkage was not assessed, as the histological findings were used for an intraexperimental comparison and shrinkage artifacts could be expected to the same extent in all experimental groups.
Employing laser devices for excision of soft tissue specimens, histological examination of the obtained tissue should not be adversely affected. Thus, an appropriate incision procedure should avoid thermally induced damages of the soft tissues’ margins. A previous study evaluated Nd:YAG laser irradiation versus traditional scalpel incision in specimens from human oral mucosa [3]. Nd:YAG laser incisions induced serious thermal effects in small specimens with mean sizes less than 7 mm, independently from the frequency and power employed. Another study assessed the effects of Er:YAG (erbium-doped yttrium aluminum garnet) laser irradiation on oral soft tissues and demonstrated that the Er:YAG laser can be safely used in oral biopsy investigations while ensuring a successful histological evaluation [2]. Comparing conventional surgery and CO2 laser irradiation on intraoral soft tissue pathologies and evaluating the collateral thermal damage, it could be shown that the CO2 laser is an effective instrument for soft tissue excisional biopsies with minimal intraoperative and post-operative complications [23]. A retrospective study evaluating an 808-nm semiconductor laser aimed to establish if physical damage induced by the diode laser could affect the histopathological diagnosis and to evaluate the damage caused to the resection margins [24]. The results show that the 808-nm diode laser is a valid therapeutic instrument for excising oral lesions larger than 3 mm in diameter but induces serious thermal effects in small lesions with a mean size below 3 mm. However, the author suggests that the specimens taken have in vivo a diameter of at least 5 mm in order to have a reliable reading of the histological sample. An ex-vivo study assessed laser-assisted surgery performed on a bovine tongue with different wavelengths [25]. The authors describe a low influence on thermal increase using an Er:YAG laser device. The best quality of incision was obtained with a 3-W CO2 laser and 3-W diode laser. CO2 and diode lasers revealed a good histological quality. Therefore, a 808-nm infrared diode laser was used in continuous wave mode with a 320-μm fiber in contact mode. In the present study, a 970-nm infrared laser (3 W) and a 445-nm blue-light laser (2 W) were used with the same fiber diameters, showing significantly lowest thermal changes for the blue-light laser device. Moreover, a case presentation employing a 445-nm blue-light laser used for excision of an oral fibroma included a histological examination of the tissue [9]. Histological evaluation did not show severe thermal damages and allowed a clear diagnosis of the excised tissue after hematoxylin and eosin (H&E) and periodic acid-Schiff (PAS) staining. Another study assessed the thermal increase induced by four different laser wavelengths (infrared diode, Nd:YAG, Er:YAG, KTP) during implant uncovering in an animal model [19]. The study showed that laser utilization with the recommended parameters gives no risks of dangerous thermal elevation to the tissues and implants. Consequently, a risk estimation of the blue-light diode laser evaluated in the present study should come to the result that also the blue wavelength gives no risks of dangerous thermal increase in soft tissues, as the amount of histologically observable denaturation is smaller than in tissues treated with an infrared laser.
The present study aimed to perform an intraexperimental comparison of cutting efficiency and histological changes using different energy transmitting devices for soft tissue incision. The hypothesis of a 445-nm blue-light laser being more efficient than conventional devices without increasing thermal side effects could be proven. Using the blue-light semiconductor laser, a higher cutting efficiency was observed when compared with a 970-nm diode laser and high-frequency surgery. Even the 445-nm laser application in non-contact mode showed clinically acceptable incision depths without signs of extensive soft tissue denaturation. Further studies have to assess the impact of the novel blue-light laser device on cutting efficiency and thermal side effects when operated in pulsed mode.
References
Hasar ZB, Ozmeric N, Ozdemir B, Gökmenoğlu C, Baris E, Altan G, Kahraman S (2016) Comparison of radiofrequency and electrocautery with conventional scalpel incisions. J Oral Maxillofac Surg 74:2136–2141
Romeo U, Libotte F, Palaia G, Del Vecchio A, Tenore G, Visca P, Nammour S, Polimeni A (2012) Histological in vitro evaluation of the effects of Er:YAG laser on oral soft tissues. Lasers Med Sci 27:749–753
Vescovi P, Corcione L, Meleti M, Merigo E, Fornaini C, Manfredi M, Bonanini M, Govoni P, Rocca JP, Nammour S (2010) Nd:YAG laser versus traditional scalpel. A preliminary histological analysis of specimens from the human oral mucosa. Lasers Med Sci 25:685–691
Niemz MH (1996) Laser-tissue interactions—fundamentals and applications, 3rd edn. Springer, Berlin
Pick RM, Colvard MD (1993) Current status of lasers in soft tissue dental surgery. J Periodontol 64:589–602
Amaral MB, de Ávila JM, Abreu MH, Mesquita RA (2015) Diode laser surgery versus scalpel surgery in the treatment of fibrous hyperplasia: a randomized clinical trial. Int J Oral Maxillofac Surg 44:1383–1389
Derikvand N, Chinipardaz Z, Ghasemi S, Chiniforush N (2016) The versatility of 980 nm diode laser in dentistry: a case series. J Lasers Med Sci 7:205–208
Romanos GE, Gutknecht N, Dieter S, Schwarz F, Crespi R, Sculean A (2009) Laser wavelengths and oral implantology. Lasers Med Sci 24:961–970
Braun A, Berthold M, Frankenberger R (2015) The 445-nm semiconductor laser in dentistry—introduction of a new wavelength. Quintessenz 66:205–211
Fornaini C, Rocca JP, Merigo E (2016) 450 nm diode laser: a new help in oral surgery. World J Clin Cases 4:253–257
Wilson SW (2014) Medical and aesthetic lasers: semiconductor diode laser advances enable medical applications. BioOptics World 7:21–25
Beard P (2011) Biomedical photoacoustic imaging. Interface Focus 1:602–631
Naqvi KR (2014) Screening hypochromism (sieve effect) in red blood cells a quantitative analysis. BiomedOpt Express 5:1290–1295
Reichelt J, Winter J, Meister J, Frentzen M, Kraus D (2017) A novel blue light laser system for surgical applications in dentistry: evaluation of specific laser-tissue interactions in monolayer cultures. Clin Oral Investig 21:985–994
Azevedo A-S, Monteiro L-S, Ferreira F, Delgado M-L, Garcês F, Carreira S, Martins M, Suarez-Quintanilla J (2016) In vitro histological evaluation of the surgical margins made by different laser wavelengths in tongue tissues. J Clin Exp Dent 8:e388–e396
Cohen J (1988) Statistical power analysis for the behavioral sciences, 2nd edn. Lawrence Erlbaum Associates, Hillsdale, New Jersey
Fornaini C, Merigo E, Rocca JP, Lagori G, Raybaud H, Selleri S, Cucinotta A (2016) 450 nm blue laser and oral surgery: preliminary ex vivo study. J Contemp Dent Pract 17:795–800
Guney M, Tunc B, Gulsoy M (2014) Investigating the ablation efficiency of a 1940-nm thulium fibre laser for intraoral surgery. Int J Oral Maxillofac Surg 43:1015–1021
Fornaini C, Merigo E, Vescovi P, Bonanini M, Antonietti W, Leoci L, Lagori G, Meleti M (2015) Different laser wavelengths comparison in the second-stage implant surgery: an ex vivo study. Lasers Med Sci 30:1631–1639
Boonstra H, Oosterhuis JW, Oosterhuis AM, Fleuren GJ (1983) Cervical tissue shrinkage by formaldehyde fixation, paraffin wax embedding, section cutting and mounting. Virchows Arch A Pathol Anat Histopathol 402:195–201
Mistry RC, Qureshi SS, Kumaran C (2005) Post-resection mucosal margin shrinkage in oral cancer: quantification and significance. J Surg Oncol 91:131–133
Blasco-Morente G1, Garrido-Colmenero C, Pérez-López I, Carretero-García S, Martín-Castro A, Arias-Santiago S, Tercedor-Sánchez J (2015) Study of shrinkage of cutaneous surgical specimens. J Cutan Pathol 42:253–257
Tuncer I, Ozçakir-Tomruk C, Sencift K, Cöloğlu S (2010) Comparison of conventional surgery and CO2 laser on intraoral soft tissue pathologies and evaluation of the collateral thermal damage. Photomed Laser Surg 28:75–79
Angiero F, Parma L, Crippa R, Benedicenti S (2012) Diode laser (808 nm) applied to oral soft tissue lesions: a retrospective study to assess histopathological diagnosis and evaluate physical damage. Lasers Med Sci 27:383–388
Merigo E, Clini F, Fornaini C, Oppici A, Paties C, Zangrandi A, Fontana M, Rocca JP, Meleti M, Manfredi M, Cella L, Vescovi P (2013) Laser-assisted surgery with different wavelengths: a preliminary ex vivo study on thermal increase and histological evaluation. Lasers Med Sci 28:497–504
Acknowledgements
We acknowledge Dentsply Sirona for providing the semiconductor laser device and Robert Mandic and the staff of the Interdisciplinary Head & Neck Oncology Lab of the Departments of Otorhinolaryngology, Head & Neck Surgery, and Oral & Maxillofacial Surgery of the University of Marburg/University Hospital Giessen and Marburg (Campus Marburg) for preparing the histological samples.
Funding information
This study has been self-funded by the authors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Braun, A., Kettner, M., Berthold, M. et al. Efficiency of soft tissue incision with a novel 445-nm semiconductor laser. Lasers Med Sci 33, 27–33 (2018). https://doi.org/10.1007/s10103-017-2320-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-017-2320-9