Abstract
In oral pathology, laser devices can provide important advantages, especially in the treatment of certain lesions. However, there is controversy about the use of some wavelengths in the analysis of suspected dysplastic or neoplastic lesions, raising doubt about the laser’s suitability for use in biopsy procedures. In recent studies, the KTP and diode lasers have been used in biopsy procedures without histological artefacts. The aim of this in vitro study was to evaluate the exact extent of peripheral thermal damage to oral soft tissues caused by an Er:YAG laser (λ 2,940 nm) without water cooling. The study was performed on five swine cadaver tongues. Nine samples from each tongue were taken by the same operator using the Er:YAG laser with increasing energies (from 60 to 150 mJ) and fluencies (from 21 to 53 J/cm2). In addition to the laser samples, a specimen obtained using a scalpel was used as control. The samples were placed in 10% formalin solution and were examined by optical microscopy by two blinded pathologists who assigned a thermal damage score (from 0 to 3) to each sample. The Er:YAG laser produced less damage at 80 and 100 mJ and 28 and 35 J/cm2 (intermediate parameters). Although in some samples thermal damage was minimally visible, in all samples histological evaluation was clearly possible. The study demonstrated that the Er:YAG laser can be safely used in oral biopsy investigations while ensuring a successful histological evaluation, which is fundamental to correct clinical management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since their introduction into both general medicine and dentistry lasers have brought many enhancements to clinical and surgical procedures [1–8]. Currently, many different laser devices are available for dental use. They can be classified according to the wavelength, the active medium, the power level or the biological effects they generate [8]. The clinical experience gathered during past decades shows several advantages in using a laser rather than the scalpel during soft-tissue surgery, including a high degree of decontamination of the surgical area, minimal postoperative bleeding, particularly with the diode laser, the Nd:YAG laser and the potassium-titanyl-phosphate (KTP) laser, and significantly less postoperative pain and inflammation [8, 9].
The Er:YAG laser emits infrared light with a wavelength of 2,940 nm. The wavelength of the Er:YAG laser is more strongly absorbed by water than those of the diode, KTP and Nd:YAG lasers due to the atomic resonances. Furthermore, the Er:YAG laser is a solid-state laser whose lasing medium is an erbium crystal doped with yttrium aluminium garnet. The Er:YAG laser light can effectively cut soft and hard tissues, so it can be used in all fields of dentistry. Specifically, its use in performing oral biopsies has been described.
A biopsy is a surgical procedure performed to reach a clear diagnosis of a lesion [10]. So it is fundamental to keep readable and safe margins in each sample to permit correct histological visualization. However, while the use of lasers in oral biopsy procedures brings many advantages both to the surgery and in terms of patient comfort, it may induce changes in the tissue cellular structure [11]. In fact, laser irradiation may create peripheral thermal damage, that can interfere with histological diagnosis, leading to problems in evaluation of dysplastic or neoplastic infiltrating lesions [11] where the peripheral cellular layers are very important in the evaluation of their infiltrating potential [12, 13].
There are two different kinds of biopsy:
-
1.
Incisional biopsy, performed by taking one or more parts of a lesion.
-
2.
Excisional biopsy, performed by the removal of the whole lesion.
Thermal damage is always present in irradiated tissues due to the photothermal effects. At the point of incidence of a laser beam, an increase in temperature to over 100°C occurs, with tissue vaporization. Around this area, the increase in temperature exceeds 50°C. Thus, reducing peripheral damage is fundamental in oral pathology.
Given the considerable debate about the reliability of laser biopsies, the effects of the use of different lasers and wavelengths (diode 808 nm and 980 nm, Nd:YAG 1,064 nm and KTP 532 nm [14, 15]) have recently been investigated. The results showed that it was always possible to acquire a histological diagnosis although, in a few cases, especially with the Nd:YAG laser, peripheral damage was considerable and clearly seen. According to these findings, the aim of this in vitro study was to evaluate the histological effects of the Er:YAG laser with gradually increasing settings for power, fluence and irradiance, without water cooling in tissue samples obtained during biopsy procedures.
Materials and methods
The study was performed in vitro using five cadaver swine tongues that have histological and physiological characteristics similar to those of the human tongue. An Er:YAG laser (λ 2,940 nm, Delight; Sweden & Martina, Carrare, Italy) was used without a cooling system to obtain nine mucosal specimens from each tongue applying increasing energies from 60 mJ to 150 mJ, with an optical fibre of 600 µm. Thus, various groups of samples were obtained with the laser settings as shown in Table 1.
The samples were taken by the same expert oral surgeon to avoid any error resulting from interindividual differences in ability. When the operator applied the laser, he always used specific protective glasses. A control specimen was taken using a Bard-Parker no. 15 scalpel. A second operator then placed the samples in sterile test tubes containing 10% buffered formalin solution. The specimens were fixed and embedded in paraffin and then sliced into 3-μm sections and stained with haematoxylin-eosin. The samples were divided into five groups (groups 1–5) and were sent for histological analysis, that was performed using an optical microscope (Primo Star, Zeiss, Oberkochen, Germany) at a magnification of ×10 with a micrometric lens and applying the conversion factor relative to the magnification (0.035 mm/70 pins, corresponding to 5 μm).
Finally, histological analysis was performed by two blinded pathologists who assigned a score from 0 to 3 to each sample indicating the degree of peripheral thermal damage where 0 indicated no damage (no more than one cellular column damaged), 1 little damage (two to four cellular columns damaged), 2 moderate damage (five to eight cellular columns damaged) and 3 severe damage (more than eight cellular columns damaged). Thus, all samples received a thermal damage score, and the arithmetic mean score in each group was taken as the average thermal damage score (ATDS). This qualitative method was chosen because of the lack of appropriate micrometric software. To reduce subjective evaluation and personal bias the examiners standardized their approach using a tissue sample.
Results
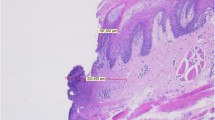
Most groups showed clear and readable cut margins, with no significant peripheral thermal damage (Table 2, Fig. 1). In particular, specimens of group 1, obtained with a fluence of 21 J/cm² and an energy of 60 mJ, showed little peripheral thermal damage with clear and safe margins, with modifications confined to the lamina propria and muscle tissue (ATDS 1.44; Fig. 2). Specimens of group 2, obtained with a fluence of 28 J/cm² and an energy of 80 mJ, showed a very thin layer of cauterization (ATDS 1.1; Fig. 3). Specimens of group 3, obtained with a fluence of 35 J/cm² and an energy of 100 mJ, showed limited signs of thermal damage both in the epithelium and the lamina propria, although the subepithelial chorion was free from thermal alteration (ATDS 1.22; Fig. 4). Specimens of group 4, obtained with a fluence of 21 J/cm² and an energy of 130 mJ, showed moderate damage localized to the epithelial margins (ATDS 1.77; Fig. 5). Specimens of group 5, obtained with a fluence of 53 J/cm² and an energy of 150 mJ, showed significant signs of peripheral thermal damage in the epithelium (ATDS 2.44; Fig. 6). Obviously, the control specimen showed no thermal damage to the cut edges (Fig. 7).
Discussion
The Er:YAG laser is a solid-state laser in which the active medium is a crystal of erbium doped with yttrium aluminium garnet. The wavelength emitted by the Er:YAG laser is 2,940 μm which shows excellent absorption by hydroxyapatite and water, allowing "cold ablation" and the cutting of soft tissues without coagulation effects. This is a significant difference compared to other lasers, such as the KTP and diode lasers, in which a high affinity for haemoglobin leads to coagulation during cutting actions. The wavelength emitted by the Er:YAG laser (2,940 nm) is such that the depth of penetration in water is extremely small (less than 1 μm) and thus cutting can be extremely precise [16].
In oral surgery it is extremely important to preserve the integrity of the specimen, especially in suspicious lesions, to keep the edges of the specimen readable and intact. Consequently, all devices that have thermal or traumatic effects on the surrounding tissues may compromise a safe and clear histological evaluation. Thus, in performing a biopsy, it is fundamental to maintain the safety of the cut margins and to permit the evaluation of both marginal infiltration and malignant transformation.
The cutting ability of the Er:YAG laser has rarely been analysed in terms of its effects on tissue histology in oral pathological procedures. In an in vitro study evaluating the use of the Er:YAG in urology, at defined power and fluence settings, the laser was shown to be able to incise urethral and ureteral tissues with minimal thermal and mechanical side effects, with low levels of thermal peripheral damage [17]. Stübinger et al. evaluated the thermal damage caused by the Er:YAG laser in hard tissue. The study showed no signs of charred tissue or wound healing disturbances during osteotomy, demonstrating that the device is safe for use during hard tissue maxillofacial surgery [18]. Another study by Romeo et al. demonstrated that the Er:YAG laser can be used safely in hard tissue in contrast to traditional cutting systems which cause peripheral bone damage. Moreover, the Er:YAG laser showed higher cutting ability and precision than a Piezosurgery device and surgical burs [19].
In this in vitro study, the Er:YAG laser showed interesting results over the entire range of power settings applied. Peripheral damage in all specimens was definitely under 1 mm, even at energies of 150 mJ. Furthermore, the best results were obtained at intermediate power settings (80–100 mJ; groups 2 and 3). With these power values, the tissue showed thermal damage confined in the epithelium of just a few microns depth. In group 1,although the low power settings could induce limited peripheral damage, the incision was less sharp and accurate, inducing the clinician to focus on the same cellular layers with greater damage to the sample edges. In groups 4 and 5, the peripheral damage to the cut margin was larger and wider due to the greater energy used, although it was less than 1 mm in all specimens.
It is clear that the choice of qualitative evaluation is less objective than a micrometric evaluation. However, this bias was taken into account by the standardization of the approach of the two pathologists. So, according to our in vitro experience, we can conclude that oral soft tissue lesions should be separated into two groups, depending on their dysplastic potential:
-
1.
Clinically nonsuspicious lesions (e.g. fibroma, angioma, mucocele etc.), for which laser treatment is basic and fundamental in the resolution of the pathology.
-
2.
Supicious dysplastic or neoplastic lesions (e.g. leucoplakia, lichen planus, carcinoma, melanoma etc.), in which peripheral thermal damage may compromise the evaluation of the real extent of the lesion, which may adversely affect diagnosis, treatment and prognosis. So it may be appropriate to enlarge the surgical incision by least about 0.5 mm in order to avoid any doubt during histological diagnosis.
Our results do not suggest that suspicious lesions should not be treated with a laser device, but lead us to recommend that in suspicious dysplastic lesions, the biopsy incision should be larger than the traditional scalpel incision in order to avoid tissue artefacts caused by the thermal effects of the laser, which might seriously compromise the diagnosis. Modern technologies provide fundamental advantages in oral surgical practice in making several treatments easier and safer, enhancing patient compliance, reducing postoperative problems and reducing the healing period. In particular, this study showed that the Er:YAG laser can be safely used during oral biopsy procedures, with controlled power settings and fluence. However, the results of the study may have been influenced by the operators’ skill in performing the laser-assisted surgical procedures.
References
Bende T, Seiler T, Wollensak J (1991) Photoablation with the Er:YAG laser in ocular tissues. Fortschr Ophthalmol 88(1):12–16
Walsh JT Jr, Flotte TJ, Deutsch TF (1989) Er:YAG laser ablation of tissue: effect of pulse duration and tissue type on thermal damage. Lasers Surg Med 9(4):314–326
Araki AT, Ibraki Y, Kawakami T, Lage-Marques JL (2006) Er:Yag laser irradiation of the microbiological apical biofilm. Braz Dent J 17(4):296–299
Lopes BM, Theodoro LH, Melo RF, Thompson GM, Marcantonio RA (2010) Clinical and microbiologic follow-up evaluations after non-surgical periodontal treatment with erbium:YAG laser and scaling and root planing. J Periodontol 81(5):682–691
Gentil De Moor RJ, Delme KI (2010) Laser-assisted cavity preparation and adhesion to erbium-lased tooth structure: part 2. present-day adhesion to erbium-lased tooth structure in permanent teeth. J Adhes Dent 12(2):91–102
Hamamci N, Akkurt A, Başaran G (2010) In vitro evaluation of microleakage under orthodontic brackets using two different laser etching, self etching and acid etching methods. Lasers Med Sci 25(6):811–816
Catone GA, Alling CC (1997) Laser application in oral and maxillofacial surgery, 1st edn. W.B. Saunders, Philadelphia
Pick RM, Colvard MD (1993) Current status of lasers in soft tissue dental surgery. J Periodontol 64:589–602
Goharkhay K, Moritz A, Wilder-Smith P, Schoop U, Kluger Wakolitsch S et al (1999) Effects on oral soft tissue produced by a diode laser in vitro. Lasers Surg Med 25:401–406
Eversole LR (1997) Laser artifacts and diagnostic biopsy. Oral Surg Med Oral Pathol Oral Radiol Endod 83:639–640
Convissar RA (1997) Laser biopsy artefact. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 84:458
Rizoiu IM, DeShazer LG, Eversole LR (1995) Soft tissue cutting with a pulsed 20 Hz Er,Cr:YSGG laser. Proc SPIE 2396:273–283
Gendelman H, Actis AB, Ouri HO (1993) Neodymium-YAG and CO2 lasers in treatment of pre-cancerous lesions of the oral cavity. Acta Stomatol Belg 90(2):95–101
Romeo U, Del Vecchio A, Ripari F, Palaia G, Chiappafreddo C, Tenore G, Visca P (2007) Effects of different laser devices on oral soft tissue: in vitro experience. J Oral Laser Appl 7:155–159
Romeo U, Palaia G, Del Vecchio A, Tenore G, Gambarini G, Gutknecht N, De Luca M (2010) Effects of KTP laser on oral soft tissues. An in vitro study. Lasers Med Sci 25(4):539–543
Van As G (2004) Erbium laser in dentistry. Dent Clin North Am 48:1017–1059
Fried NM, Tesfaye Z, Ong AM, Rha KH, Hejazi P (2003) Optimization of the erbium:YAG laser for precise incision of ureteral and urethral tissues: in vitro and in vivo results. Lasers Surg Med 33(2):108–114
Stübinger S, Ghanaati S, Saldamli B, Kirkpatrick CJ, Sader R (2009) Er:YAG laser osteotomy: preliminary clinical and histological results of a new technique for contact-free bone surgery. Eur Surg Res 42(3):150–156
Romeo U, Del Vecchio A, Palaia G, Tenore G, Visca P, Maggiore C (2009) Bone damage induced by different cutting instruments – an in vitro study. Braz Dent J 20(2):162–168
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Romeo, U., Libotte, F., Palaia, G. et al. Histological in vitro evaluation of the effects of Er:YAG laser on oral soft tissues. Lasers Med Sci 27, 749–753 (2012). https://doi.org/10.1007/s10103-011-0969-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-011-0969-z