Abstract
Hyperplastic fibro-epithelial lesions are the most common tumor-like swellings in the mouth. The neodymium yttrium aluminium garnet (Nd:YAG) laser appears to be useful for the surgical treatment of these lesions. Some controversies of laser surgery concern the accuracy of pathological diagnosis as well as the control of thermal damage on the target tissue. The aim of this study was to establish if the thermal changes induced by the Nd:YAG laser may affect the histopathological diagnosis and the evaluation of the resection margins. Furthermore, we compared the histological features of oral benign fibro-epithelial lesions excised through Nd:YAG laser and traditional scalpel. Twenty-six benign fibro-epithelial oral lesions from 26 patients, localized in the same oral subsites (cheek and buccal mucosa), were collected at the Unit of Oral Pathology and Oral Laser-assisted Surgery of the Academic Hospital of the University of Parma, Italy. Specimens were subclassified into three groups according to the tool used for the surgical excision. Group 1 included six specimens excised through Nd:YAG laser with an output power of 3.5 W and a frequency of 60 Hz (power density 488,281 W/cm2); Group 2 included nine specimens excised through Nd:YAG laser with an output power of 5 W and a frequency of 30 Hz; Group 3 included 11 specimens excised through a Bard-Parker scalpel blade no. 15c. Epithelial changes, connective tissue modifications, presence of vascular modifications, incision morphology and the overall width of tissue modification were evaluated. Differences between specimens removed with two different parameters of Nd:YAG laser were not significant with regard to stromal changes (p = 0.4828) and vascular stasis (p = 0.2104). Analysis of regularity of incision revealed a difference which was not statistically significant (p = 1.000) between group 1 and group 2. Epithelial and stromal changes were significantly more frequent in specimens with a mean size less than 7 mm (p < 0.0001). Nd:YAG laser induced serious thermal effects in small specimens (mean size less than 7 mm) independently from the frequency and power employed. The quality of incision was better and the width of overall tissue injuries was less in the specimens obtained with higher frequency and lower power (group 1: Nd:YAG laser at 3.5 W and 60 Hz).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Numerous options for interventions on oral tissues and collection of mucosal biopsies are available to dental and/or oral maxillofacial surgeons: electrosurgery, techniques employing high frequency, radiosurgery, piezosurgery, and lasers with different wavelengths.
The use of laser technology in surgical treatment of oral lesions aims to provide benefits for both operators and patients. The rapidity and precision of the surgical technique may improve the healing processes and decrease the postoperative discomfort [1].
The most frequent tumors in the mouth arise from the stratified squamous oral epithelium. These are closely similar to tumors of stratified epithelium occurring at other sites and usually present no diagnostic problems. Hyperplastic fibro-epithelial lesions are the most common tumor-like swelling in the mouth and are often related to some source of chronic traumatism (e.g., cheek or tongue biting). The mucosa of these lesions is regular, smooth, pink, and usually not ulcerated, even though chronic traumatisms may cause injuries. Clinically, the lesions are painless masses of fibrous tissue usually unchanged for years [2].
The histologic pattern of benign fibro-epithelial lesions usually consists of hyperplastic fibrous connective tissue covered by hyperplastic and acanthotic epithelium. Fibroblasts are mature and widely scattered in a dense collagen matrix. Sparse chronic inflammatory cells may be seen usually in a perivascular distribution. The area of neoplasm in contact with gingival plaque or prosthetic resin is usually inflamed and superficially infected by Candida albicans [3].
The neodymium yttrium aluminium garnet (Nd:YAG) laser has been reported to be useful for treatment of benign, vascular, and premalignant lesions of the oral mucosa [4]. Some controversies of laser surgery concern the accuracy of pathological diagnosis as well as the control of thermal damage on the target tissue. However, if precise operative procedures and the appropriate power and frequency parameters are respected it seems possible to reduce collateral thermal injuries and support the wound-healing processes.
The aim of this study was to establish if the thermal changes induced by the Nd:YAG laser may affect the histopathological diagnosis and the accuracy of the resection margins evaluation. Furthermore, we compared the histological appearance of oral benign fibro-epithelial lesions excised with Nd:YAG laser and traditional scalpel.
Material and methods
Twenty-six surgical samples of benign fibro-epithelial oral lesions were collected at the Unit of Oral Pathology and Laser-assisted Surgery of the Academic Hospital of the University of Parma, Italy, between May 2007 to May 2008. Samples were obtained from 26 patients (nine males, 17 females, ranging in age from 35 to 65 years, mean age 53 years). All the lesions were localized in the same oral subsites (cheek and buccal mucosa). All the surgical excisions were performed by the same operator. Surgical specimens were subclassified according to the instrument used for excision: group 1 (six specimens)-Nd:YAG laser: output power: 3.5 W; frequency: 60 Hz; fiber diameter: 320 µm (power density 488,281 W/cm2); group 2 (nine specimens)-Nd:YAG laser: output power 5 W; frequency 30 Hz; fiber diameter 320 µm (power density 300,000 W/cm2); group 3 (11 specimens): Bard-Parker scalpel blade no. 15c. Nd:YAG laser had a pulse width of 100 µs (very short pulse–VSP). The device was based on a flashlamp pump.
Histopathological diagnosis included epithelial and fibrous overgrowth such as denture-induced fibrous hyperplasias, fibromas, and fibropapillomas.
The specimens were fixed in a 10% buffered formalin solution, cut into slices, and embedded in paraffin blocks, according to conventional methods. Sections 5 µm thick were obtained for hematoxylin and eosin staining. The histopathological sections were evaluated under low- and high-power light microscopy (Nikon Labophot) by a pathologist unaware of the excision method. Specimens were observed at two different magnifications (40× and 100×) for measurement of tissue injuries widths. The extension of tissue injuries was measured with an ocular micrometer (Olympus BX 51). The main size of histological sections ranged from 3 to 14 mm (mean 8.58 mm; mean size group 1 = 10.5 mm, mean size group 2 = 8.12 mm mean size group 3 = 8 mm).
Tissue modifications were evaluated on the basis of the following histological features:
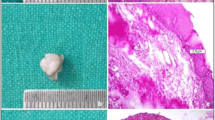
(1) epithelial changes in proximity of the resection margin, evaluated from the edge of the margin to a depth of 1,000 µm. Variables evaluated include nuclear changes, (presence of picnotic, spindle-shaped and hyperchromic nuclei), cytoplasmatic and cell membrane changes (hyperchromic cytoplasm, cell fusion and/or loss of normal cell adhesion), possible intraepithelial or subepithelial loss of attachment on the basis of a cut-off value of 10% of altered tissue in the whole resection margin (Fig. 1); (2) connective tissue modifications, evaluated from the edge of the margins to a depth of 1,000 µm. Variables evaluated included carbonization (thermal necrosis), desiccation (presence of dense eosinophilic layer underlying the possible carbonization area and mainly consisting of collagen denaturation and tissue hyalinization) (Figs. 2, 3, 4); (3) presence or absence of vascular modifications, analyzed by considering a cut-off value of 10% of the above-mentioned changes in the observed area (from the edge of the margin to a depth of 1,000 µm), presence of thrombosed or collapsed blood and lymphatic vessels (including presence of intraluminal clotted erythrocytes, presence of vascular stasis (not-collapsed vessels associated to the presence of gathered erythrocytes); (4) incision morphology, subclassified into regular (presence of a linear, smooth edge for more than 90% of the whole resection margin) and irregular (presence of a rough, uneven edge for more than 90% of the whole resection margin) (Fig. 5). An overall quality score (ranging 0–4) was assigned to each incision, score “4” representing the highest quality.; (5) overall width of tissue modifications, defined as the width of tissue injuries (expressed in micrometers and separately evaluated for the epithelium, fibrous tissue and vascular structures), taking into account the minimum damage width from the resection margin. The area with the most evident damage, perpendicular to the cut margin, was chosen for the evaluation. Changes grossly exceeding the average width of the damage and presumptively associated to manipulation artifacts, were excluded from the evaluation.
Epithelial modifications extending to a depth of ca. 350 μm. (Nd:YAG laser output power 5 W; frequency 30 Hz; fiber diameter 320 µm): nuclear changes, (presence of picnotic, spindle-shaped, and hyperchromic nuclei), cytoplasmatic and cell membrane changes (original magnification: 20×; H&E stained section)
Changes in the connective tissue extending to a depth of ca. 500 μm. (Nd:YAG laser-output power 5 W; frequency 30 Hz; fiber diameter 320 µm): carbonization, presence of dense eosinophilic layer underlying the carbonization area (collagen denaturation) and presence of vascular modifications. (original magnification: 4X; H&E stained section)
Higher magnification of Fig. 2 (magnification: 20×). Presence of collapsed blood and lymphatic vessels and presence of intraluminal clotted erythrocytes
Specimens were subclassified taking into account the main size, the cut-off being 7 mm.
Statistical analysis was performed using non-parametric statistical tests (Chi-square test and Fisher’s exact test) elaborated by the GraphPad Instat software. Variables where compared using contingency tables filled with the “two rows, two columns” or with the “larger contingency table” entry formats. A p value < 0.05 was considered significant (very significant p < 0.001, and extremely significant p < 0.0001) .
Results
Traditional scalpel produces less changes in the epithelium when compared to Nd:YAG laser, independently from the emission parameters employed (Tables 1 and 2).
However, the results where not statistically significant when separately analyzed with regard to nuclear, cytoplasmatic or overall epithelial changes (p = 0.2241; p = 0.2148; p = 0.4828).
Differences between specimens removed with different parameters of emission of Nd:YAG laser were not significant with regard to stromal changes (p = 0.4828) and vascular stasis (p = 0.2104) (Table 3).
Analysis of regularity of incision revealed a difference which was not statistically significant (p = 1.000) regarding the two parameters of emission of the Nd:YAG laser. The specimens in group 1 had a better quality of incision (score 1.5) in comparison to group 2 (score 1.4) (Table 4).
The width of overall tissue injuries in the specimens of group 2 was higher than those of group 1 (Table 5). Statistical analysis of the differences between two parameters of Nd:YAG laser emission was not significant with regard to epithelial changes (p = 0.2370 with Fisher’s test and p = 0.2371 with Chi-square test) but the differences in the stromal layer were “quite significant” (p = 0.0644 with Fisher’s test and p = 0.0646.with Chi-square test).
When the specimens were separately analyzed according to the main size (smaller or larger than 7 mm) (Table 6), epithelial and stromal changes in histological sections less than 7 mm were more evident, independently from parameters of the laser emission used (Fig. 6). The result was extremely significant with both Chi-square and Fisher’s exact test (p < 0.0001). The differences regarding vascular changes were “quite” significant (Fisher’s exact test: p = 0.0827 and Chi-square test: p = 0.0832) (Fig. 5).
Discussion
The interaction between the laser beam and the tissue consists of four processes: reflection, scattering, transmission, and absorption, the latter being employed in clinical practice. The energy transferred results in tissue heating, which is followed by ablation and/or coagulation on the target tissue.
The Nd:YAG laser beam is poorly absorbed by water and selectively absorbed by hemoglobin [4–6]. Due to the poor absorption by water, this wavelength penetrates deeply into the tissue, to a depth of about 4–5 mm. The laser beam transfers heat to the tissues producing a selective coagulation within blood vessels to a depth of about 7–10 mm. This process is called photocoagulation. These properties are used specifically for treating head and neck vascular lesions [7].
The thermal effects of lasers on biological tissue result from three phenomena: conversion of light to heat, transfer of heat and the tissue reaction, which is related to the temperature, and the time of exposure. This interaction leads to denaturation or to tissue destruction. The laser effects depend on the parameters used (wavelength, power, time and mode of emission, beam profile, and spot size) altogether with the chemical and physical characteristics of the target tissue.
Hyperthermia consists of a moderate rise in temperature, ranging from 41 to 44°, for some tens of minutes and resulting in cell death due to changes of enzymatic processes.
Coagulation is an irreversible necrosis without immediate tissue destruction. The temperature reached (50–100°C) for around 1 s, produces desiccation, blanching, and a shrinking of tissues by denaturation of proteins and collagen.
Volatilization induces tissue transformation into smoke at above 100°C in a relatively short time of around one-tenth of a second. At the edges of the volatilization zone there is a region of coagulative necrosis. There is a gradual transition between the volatilization and healthy zones [6].
Treatment performed with the Nd:YAG laser at 1,064 nm also comprises mechanical effects which can result from either the creation of a plasma, an explosive vaporization, or the phenomenon of cavitations, all of these effects being associated with the production of a shock wave. With nano- or pico-second pulsed Nd:YAG lasers, a very high intensity of luminous flux over a small area (between 1,010 and 1,012 W/cm2) ionizes atoms and creates a plasma. At the limit of the ionized region, there is a very high pressure gradient that causes the propagation of a shock wave. It is the expansion of such a shock wave that causes the destructive effect.
It seems reasonable to think that differences between specimens obtained with different surgical techniques could be influenced by their sizes and by distortion of tissues due to physical trauma at the moment of surgery. However, the presence of those artifacts is supposed to be comparable in each specimen independently form the technique employed. Artifacts are caused by the traction with forceps on the edge of the tissue and they are not linked to thermal energy that causes contraction of collagen in the dermal tissue and subsequent possible irregular shrinking [8]. This distortion, together with changes associated with fixation and cutting artifacts, may have contributed to some of the irregular margins observed [9].
However, tissue fragmentation and thermal injury with cytologic artifacts did not affect histologic diagnosis and accuracy of resection margins evaluation.
The use of an Nd:YAG laser requires a period of training. The choice of appropriate parameters is connected to thermal injuries and healing of oral tissues [10]. Average output power, frequency, energy density, pulse duration, and energy per pulse influence the laser beam interaction with the target tissue, particularly with regard to coagulation of the target tissue.
Insufficient laser energy levels do not initiate tissue ablation, while excessive energy can lead to carbonization and possibly to thermal damages [11, 12].
If small fragments of carbonized tissue are present on the fiber tip or on surgical anatomical surfaces, they can absorb all light wavelengths and quickly provoke overheating. It is therefore indispensable to remove the charred layer with a damp gauze [13].
For most intra-oral minor surgical procedures, the average Nd:YAG laser power (J/s) should be between 2 and 5 W [14].
In the present study, the laser incision was considered regular only in half of the specimens and the overall quality score was 1.46 in comparison to the sharp surgical cut obtained with traditional scalpel, which scored a 4. The parameter employed in group 1 (higher frequency and lower energy) revealed a better quality of incision and a lower width of thermal damages in the epithelial and stromal layers.
With regard to “small” (mean size of specimen less than 7 mm) and “large” biopsies (size of specimen greater than 7 mm) the Nd:YAG laser induces serious thermal effects in small specimens independently from the frequency and energy employed. The vascular modifications were one-third higher, the epithelial changes were double, and the stromal damages were four times higher in small biopsies.
Conclusions
Three parameters are important for the evaluation of new technologies in oral surgery: (1) Patient compliance during pre- and post-operative phases, (2) comfort of the surgeon, and (3) possible histological damages [15].
The analysis of the literature shows that laser treatment represents a procedure with minimal short- and long-term morbidity [16, 17]. The laser is also well accepted in pediatric patients [18].
The possible problems of such a tool are the inflammation in the incisional area, the control of thermal modifications, and the quality of excised tissues [19–22]. In our experience, thermal artifacts did not limit the histopathological diagnosis in any case.
We conclude that the Nd:YAG laser induces serious thermal effects in small specimens (less than 7 mm) independently form the frequency and power employed. The quality of incision was better and the width of overall tissue injuries was minor in the specimens obtained with higher frequency and lower power (group 1: Nd:YAG laser at 3.5 W and 60 Hz). Differences between two groups of specimens obtained by different parameters of laser were not statistically significant. This preliminary information supports further investigations.
References
Rocca J-P: Les laser en odontologie. Editions CdP-Wolters Kluver France-Avril 2008
Wenig BM (2008) Atlas of head and neck pathology, 2nd edn. Saundeish Elsevier, China
Fletcher Christopher DM (2007) Diagnostic histopathology of tumors, 3rd edn. Churchill Livingston–Elsevier, China
Clayman L, Kuo P (1997) Lasers in Maxillofacial Surgery and Dentistry. Thieme Medical Publishers, USA
Svelto O (1998) Principles of Lasers, 4th edn. Springer Sciences–Business Media Inc, USA
Niemz MH (2004) Laser-tissue interactions. Fundamental applications, 3rd edn. Springer-Verlag, Berlin Heidelberg Germany
Vesnaver A, Dovŝak DA (2006) Treatment of vascular lesions in the head and neck using Nd:YAG laser. J Cranio Maxillofac Surg 34:17–24
Reimer SB, Sequin B, Hilde HD et al (2005) Evaluation of the effect of routine histologic processing on the size of skin samples obtained from dogs. Am J Vet Res 66:500–505
Silveberg SG (2006) Surgical Pathology and cytopathology, 4th edn. Churchill Livingston–Elsevier, China
Yukna RA, Carr RL, Evans GH (2007) Histologic evaluation of an Nd:YAG laser-assisted new attachment procedure in humans. Int J Periodontics Restorative Dent 27(6):577–587
Howell R, Hammond R, Pryse-Davies J (1991) The histologic reliability of laser come biopsy of the cervix. Obstet Gynecol 77:905–911
Lewis PL, Lasghari M (1994) A comparison of the cold knife, CO2 laser and electrosurgical loop conization in the treatment of cervical intraepithelial neoplasia. J Gynecol Surg 10:905–911
Spencer P, Coob CM, Weliczka DM, Glaros A, Morris P (1998) Change in temperature of subjacent bone during soft tissue laser ablation. J Periodontol 69:287–291
Parker S (2007) Laser and soft tissue: “loose” soft tissue surgery. Br Dent J (4):185–191. Feb202
Fornaini C, Rocca JP, Bertrand MF, Merigo E, Nammour S, Vescovi P (2007) Nd:YAG and Diode laser in the surgical management of soft tissues related to orthodontic treatment. Photomed and Laser Surg 25(5):381–392
Nammour S, Dourov N (1992) Removal of benign tumors using CO2 laser. J Clin Laser Med Surg 4:109–113
Gutknecht N (2007) Proceedings of the 1st international workshop of Evidence-Based Dentistry on laser in dentistry. Quintessence Publishing Co Ltd, Chicago
Genovese MD, Olivi G (2008) Laser in paediatric dentistry: patient acceptance of hard and soft tissue therapy. Eur J Paediatr Dent 9(1):13–17
Taylor DL, Schafer S, Nordquist R, Payton ME, Dickley DT, Bartelas KE (1997) Comparison of a high power diode laser with the Nd:YAG laser using in situ wound strength analysis of healing cutaneous incisions. Laser Med Surg 21:248–254
Liboon J, Funkhouser W, Terris DJ (1997) A comparison of mucosal incisions made by scalpel, CO2 laser, electrocautery and constant-voltage electrocautery. Otolaryngol Head Neck Surg 116:379–385
Sinha UK, Gallagher LA (2003) Effects of steel scalpel, ultrasonic scalpel, CO2 laser and monopolar and bipolar electrosurgery on wound healing in Guinea pig oral mucosa. Laryngoscope 113(2):228–236
D’Arcangelo C, Di Nardo Di Maio F, Prosperi GD, Conte E, Baldi M, Caputi S (2007) A preliminary study of healing of diode laser versus scalpel incisions in rat oral tissue: a comparison of clinical, histological, and immunohistochemical results. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 103(6):764–773
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vescovi, P., Corcione, L., Meleti, M. et al. Nd:YAG laser versus traditional scalpel. A preliminary histological analysis of specimens from the human oral mucosa. Lasers Med Sci 25, 685–691 (2010). https://doi.org/10.1007/s10103-010-0770-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-010-0770-4