Abstract
Since the introduction of laser in clinical practice, different wavelengths have been used for oral surgery on the basis of the different characteristics and affinities of each one. The aim of this study was a comparison of different laser wavelengths in relation to both thermal increase and “histological quality” in a model of soft tissue surgery procedures. Thermal evaluation was realized, during laser-assisted surgery excision performed on a bovine tongue, by a thermal camera device to evaluate thermal increase on the surface of the sample and with four thermocouples to evaluate thermal increase on the depth of the specimen; temperature was recorded before starting surgical procedure and at the peak of every excision. The quality of excision, in terms of tissue damage and regularity, was realized by two blind examiners on the basis of established criteria. The highest superficial thermal increase was recorded for Superpulse 5-W CO2 laser, the lowest one for Er:YAG laser. The highest in depth thermal increase was recorded for 5 W Diode laser, the lowest one for Er:YAG laser. The best quality of incision was obtained with a 3-W CO2 laser and 3-W diode laser; epithelial, stromal, and vascular damages were evaluated with different degrees for all the used wavelengths with the best result, in terms of “tissue respect,” for Er:YAG laser. In all the surgical procedures performed, thermal increase was evaluated until the end of the procedure; at remaining tissue level, thermal decrease was evaluable in the few seconds after surgery. The Er:YAG laser was the device with a lower influence on thermal increase; CO2 and diode lasers revealed a good histological quality. Further studies may be necessary to test the reliability of laser devices for the excision of all the types of specimens needing histological evaluation and diagnosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Laser technology was employed for the first time in dentistry in 1988 [1] for soft tissue surgery, and since then, it has greatly spreading due the evolution of devices and techniques. Lasers used in dentistry emit in pulse durations of microseconds and, for this reason, are effective in the tissues thanks to the so-called “photo thermal effect” consisting of the transformation of light into heat with the consequence of ablative capacity [2]. Unfortunately, thermal elevation caused by a laser beam spreads also from the impact point to the surrounding areas and this may cause inconvenience in the healing wound with oedema, pain after the intervention and other problems related to difficulty to make a real histological diagnosis in the samples.
As referred by several studies, thermal increase during laser-assisted surgical procedures is influenced particularly on the basis of peak absorption and working mode of laser devices and on the characteristics of tissues. Thermal increase may be different at different levels, superficial and deep, and for both removed tissue and remaining tissue. In order to avoid these problems, many systems have been carried out, such as, the pulsed and super pulsed mode [3], the association of an air/water spray [4] and the energy reduction [5].
With this preliminary study, we would like to evaluate, in an ex vivo model, the thermal elevation at both superficial and deep level with five wavelengths used with surgical common laser parameters. In order to evaluate every laser device, we performed a histological evaluation of excised samples for epithelial, stromal and vascular damage, but also an evaluation of the quality of incision by a scoring system realized by two blind examiners.
One of the basic concept to understand the interaction laser–tissues is the affinity between the wavelength and specific biological molecules: every wavelength has one or more peaks of affinity for variable tissue components such as water, hemoglobin and melanin [2]; these are the photoacceptors we used to reach the thermal effect linked to the different properties and capacities of laser light, also in laser-assisted surgical procedures.
Material and methods
Samples collection and thermal evaluation
Freshly extracted calf tongues were used to carry out this study. Specimens were kept at room temperature and were used within 6 h of the animal sacrifice. As reported in literature [6], specimens were stored during transit at 2–4°C and 100% of humidity to prevent tissue degradation. All measurements were made at room temperature between 22°C and 24°C. In the tongue margins, areas measuring about 2 cm2 (2 × 1 cm) were identified and circumscribed with a pen in order to help the operator in the excision procedure and to position thermocouples.
Before starting with experimental protocol, every laser device was checked with power meter devices (Nova II, Ophir, Jerusalem, Israel) in order to check the real emitted power. For all the used laser devices, the loss of power was between 18% and 25%.
In order to evaluate the in-depth variations of temperature, we placed four naked-bead chrome–alumel (K-type) thermocouples (TP-01, Lutron, Taiwan) with a 0.5-mm diameter probe sensitive to temperature variations between −40°C and 250°C; the thermocouples were connected to a four-channel thermometer (TM-946, Lutron, Taiwan), sensitive to temperature variations (for k-type thermocouples) between −100°C and 1,300°C, with an accuracy of 0.1°C.
A thermoconductor paste (Warme Leftpast WPN 10, Austerlitz Electronic, Germany) was spread on thermocouple probes to ensure good thermal contact; the thermal conductivity of the paste was 0.4 cal s−1 m−1 K−1, in a way comparable with thermal conductivity of human tissues. The four thermocouples were placed at a distance of 2–5 mm from the margins of incision and at a depth of 2–5 mm in the four margins of specimens in order to monitor the temperature in every direction and depth (Fig. 1).
Due to the growing distance during excision between the specimen and thermocouples probes, in order to calculate the deep temperature, we recorded the peak temperature for every thermocouple. In order to evaluate the surface variations of the temperature, we used an infrared camera, a tool able to measure the emitted infrared radiation from an object.
Surface temperature was checked during all procedures with a thermal camera (Thermovision A 800, Flyr Systems, Stockolm, Sweden) connected to a PC and working with the Software Thermacam Researcher; this thermal camera device has a 320 × 240 pixel detector with a resolution of 0.08°C.
The infrared camera is a reliable and reproducible technique that allows non-contact high-resolution monitoring surface temperature in different medical application, such as, monitoring temperature during surgical procedure or use the thermal evaluation as diagnostic tool [7, 8].
Image camera was performed with the video camera on a fixed pedestal at a preset distance from the sample of about 100 cm. Images were analyzed by calculating the mean temperature within a standard region of interest of a 1-cm diameter. Temperature was recorded before starting surgical procedure for the initial temperature and at the peak temperature, this one identified by means the analysis of the temperature graphs.
Surgical procedures
Laser-assisted surgical procedures were performed with different wavelengths and different parameters, choose in order to couple the most used parameters in clinical practice and the parameters compatible with the surgical excision in the ex vivo conditions, for example, the decreased presence of blood and the consequent loss of pigmentation of the samples made possible the Nd:YAG surgical procedure with a minimal power of 4 Watts and a frequency of 80 Hz. Every excision was performed on two different samples.
-
1.
CO2 laser (wavelength, 10,600 nm) (Miran 25, Mediclase, Israel) used with the non-contact handpiece with working distance of 20 mm (spot size, 0.4 mm) with three different types of application
-
a.
3 W in continuous wave (power density, 2,388 W/cm2)
-
b.
5 W in continuous wave (power density, 3,980 W/cm2)
-
c.
5 W in superpulsed mode at 500 Hz of frequency and 300 μs pulse duration (power density, 3,980 W/cm2).
-
a.
-
2.
KTP laser (532 nm) (Lasermar 800, Eufoton, Trieste, Italy) used with 320 μm diameter in contact mode with twoz different types of application:
-
a.
2 W in continuous wave (power density, 2,500 W/cm2)
-
b.
4 W in continuous wave (power density, 5,000 W/cm2)
-
a.
-
3.
Er:YAG Laser (2,940 nm) (Fidelis Plus®; Fotona, Slovenia) in VSP (100 μs) using the non-contact handpiece with working distance of 8 mm (R02-spot size, 0.9 mm) with 250 mJ of energy, 20 Hz frequency and with air and water spray.
-
4.
Nd:YAG Laser (1,064 nm) (Fidelis Plus®; Fotona, Slovenia) in VSP (100 μs) using a 320-μm diameter fibre in contact mode with a power of 4 W and a frequency of 80 Hz (power density, 5,000 W/cm2).
-
5.
GaAlAs laser (wavelength 808 nm) (Fotona XD-2; Fotona, Lubiana, Slovenia) using a 320-μm diameter fibre in contact mode with two different types of application
-
a.
3 W in continuous wave (power density, 3,750 W/cm2)
-
b.
5 W in continuous wave (power density, 6,250 W/cm2)
-
a.
For each surgical procedure the operative time was considered from the starting of laser application on tissue until the complete excision of sample. Each test was repeated four times and for the final result we considered mean values and standard deviations.
Histological samples collection and analysis
The specimens were fixed in a 10% buffered formalin solution, cut into slices and embedded in paraffin blocks according to conventional methods. Sections of 5-μm thick were obtained for hematoxylin and eosin staining. The histopathological sections were evaluated under low-power and high-power light microscopy (Eclipse 80i, Nikon) by two pathologists unaware of the excision method and in a blind manner. Specimens were observed at two different magnifications (40× and 100×) for measurements of tissue injuries widths. The main size of histological sections ranged from 13 to 25 mm.
On the basis of criteria established by Vescovi and coworkers in 2010 [9], tissue modifications were evaluated in every part of the tissue (bottom, middle and margins) on the basis of the following histological features:
-
1.
Epithelial changes in proximity of the resection margin, evaluated from the edge of the margin to a depth of 1,000 μm. Variables evaluated include nuclear changes (presence of picnotic, spindle-shaped and hyperchromic nuclei), cytoplasmatic and cell membrane changes (hyperchromic cytoplasm, cell fusion and/or loss of normal cell adhesion), possible intraepithelial or subepithelial loss of attachment on the basis of a cutoff value of 10% of altered tissue in the whole resection margin;
-
2.
Connective tissue modifications, evaluated from the edge of the margins to a depth of 1,000 μm. Variables evaluated included thermal necrosis, presence of dense basophilic layer underlying the possible carbonization area and mainly consisting of collagen denaturation and homogenization;
-
3.
Presence or absence of vascular modifications (from the edge of the margin to a depth of 1,000 μm), presence of thrombosed or collapsed blood and lymphatic vessels (including presence of intraluminal clotted erythrocytes), presence of vascular stasis (not-collapsed vessels associated to the presence of gathered erythrocytes);
-
4.
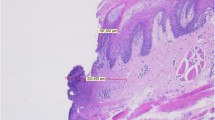
Incision morphology was subclassified into “regular” (presence of a linear, smooth edge for the most part of resection margin) and “irregular” (presence of a rough, uneven edge for the most part of resection margin). An overall quality score (ranging 0–4) was assigned to each incision, score “4” representing the highest quality (Fig. 2); a score was assigned by two blind examiners. To determine the overall score, we added every score, obtaining values ranging from 0 to 16.
-
5.
Overall width of tissue modifications, defined as the width of tissue injuries (expressed in micrometre and separately evaluated for the epithelium, fibrous tissue and vascular structures), taking into account the minimum damage width of the damage presumptively associated to manipulation artefact, were excluded from the evaluation.
We used as controls two samples excised with 15c Bard-Parker scalpel.
Statistical analysis
Statistical analysis of the results was performed using one-way ANOVA test. A p value <0.05 was considered significant (very significant, <0.01; extremely significant, <0.001).
Results
Excision time
The mean time necessary to perform the excision was variable between 227 s (KTP laser with 4 W of power) and 764 s (diode laser with 3 W of power); in fact, the fastest performance was for KTP laser at 4 W of power and then, with increasing times, for CO2 in superpulsed mode (342 s), CO2 at 5 W of power (351 s), KTP laser at 2 W of power (375 s), Er:YAG laser (400 s), Nd:YAG laser (431 s), CO2 laser at 3 W of power (615 s) and diode laser at 3 W of power (764 s).
Temperature elevations
The increase of temperature at superficial level was more consistent in CO2 and diode laser, at different parameters, with variations between 20°C and 30°C and was less important, with values between 5°C and 15°C for KTP and Nd:YAG lasers. Conversely, with all the other wavelengths, Er:YAG laser, used with air-water spray (as normally used in surgery), was positive for a decrease of superficial temperature (mean temperature variation, 0.8°C) (Table 1). Statistical analysis retrieved a very significant result (p < 0.0001).
The increase of temperature in depth was more consistent for diode and Nd:YAG laser, with an increase between 8°C and 16°C and less important for CO2, Er:YAG and KTP laser, with an increase between 2°C and 8°C (Table 2). Statistical analysis retrieved a significant result (p < 0.05), particularly for the comparison between the 3-W CO2 laser and the 5-W diode laser (p = 0.0027).
In order to evaluate, in a global mode, the temperature variations induced by laser treatments, we added variations for superficial and deep variations: the most consistent temperature increase was linked to the 5-W diode laser (about 40°C); temperature elevation for CO2 laser (with every used parameter) and 3-W diode laser was between 25°C and 35°C, as for Nd:YAG laser. KTP laser (at 2 and 4 W) was related to an increase of about 15°C. Temperature increase for Er:YAG laser was less than 5°C.
Histological evaluations
From a histological point of view (Fig. 3), regularity of incision was mainly observed for CO2 and diode lasers—considering that in the chosen scoring system “good quality” for score 12 or greater, “mean quality” for score between 8 and 12 and “poor quality” for score less than 8, “good quality” was reached with CO2 and 3-W diode laser; the mean quality of incision was observed for the 5-W diode laser and 2-W KTP laser, poor quality for Er:YAG laser, Nd:YAG and 4-W KTP laser (Table 3).
With all the used wavelengths and parameters, epithelial changes were observed at both nuclear and cytoplasmic level, even if in a slight mode, particularly with the Er:YAG laser. Loss of attachment was observed only in the sample excised with the Nd:YAG laser.
In terms of epithelial width changes, the most important result was found with the Nd:YAG laser (width about 750 μm), lesser with Er:YAG laser (width about 130 μm) with intermediate values for CO2 laser at different parameters (Fig. 4).
Stromal changes, considered as collagen fusion and homogenization, were observed in all the samples with width variables between about 50 and 500 μm and were greatest for the 5 W diode laser (Fig. 5) and Nd:YAG laser (between 500 and 600 μm) (Fig. 5) and slightest for Er:YAG laser (less than 100 μm). For all the other wavelengths, the mean changes in width were between 100 and 300 μm.
Vascular changes were observed in all the samples with the only exception of Er:YAG excised samples. Mean changes in width were between 200 and 400 μm with the greatest values for Nd:YAG laser (mean width about 1,000 μm) and 4-W KTP laser (mean width about 600 μm) (Fig. 6).
Discussion
Similar to previous published data [10], our evaluation retrieved that the higher the power settings, the quicker and easier it is to create the incision. Wavelengths we used with different parameters (CO2–KTP–diode) highlighted this result. These results may be explainable by the fact that making more performing laser parameters, laser light has a more ablating action thus reducing excision time.
One of the aims of this study was the evaluation of the laser effect on the global temperature of surgically treated tissue. The coupling of thermal camera, a device able to record surface temperature and thermocouples, tools used to record deep temperature [5], allows us to obtain a global image for thermal effect of different laser wavelengths on laser surgical treated tissue.
The evaluation of superficial temperature identified a relevant difference for CO2 and diode laser and Er:YAG laser in comparison to Nd:YAG laser and KTP laser. In fact, the first laser group reported a greater increase of temperature, maybe for the greater absorption, particularly for CO2 laser, in superficial layer. The superficial action is also a characteristic of the Er:YAG laser, but the presence of the air–water spray, determining the ablative effect, permits to control the temperature of the surgical site. The Nd:YAG and the KTP laser revealed an increase of temperature of about 10°C, probably because of the deeper absorption linked with a deeper increase of temperature. In fact, the increase of temperature in depth was more consistent for the diode and Nd:YAG lasers, with a differential value of about 10–15°C, but it was relatively limited for the KTP laser (increase of 3–6°C) and for the CO2 laser. This result could be clearly related with width of tissue alterations. In fact, higher deep temperatures were recorded for the laser with a higher width of alterations (diode and Nd:YAG laser).
In order to evaluate, with a certain degree of approximation, the total temperature variation caused at the tissue level, we added superficial and deep temperature. This analysis highlighted the best performance in terms of reduced thermal injury for Er:YAG and KTP laser.
Literature reports an increase of temperature for dental pulp vitality of 5.5°C during laser treatments in conservative, prosthetic or aesthetic dentistry as critical [11–13]; moreover, it has been reported that cellular death is immediately valuable with temperature above 70°C [14, 15] and that bone necrosis is caused by a temperature of 47°C for 5 min, 50°C for 1 min or 56°C for less than 1 min. Considering that the starting temperature for the evaluated samples was less than body temperature (room temperature between 19°C and 24°C) and that this difference do not change the properties of tissue [16] also during laser treatments, we can evaluate our observations in order to apply this study to the laser-assisted surgical practice. In fact, the deep temperature increase was between 13°C and 16°C for both 5-W diode and Nd:YAG laser, and this maybe pass the limit of 50°C considered dangerous for bone safety, even if we have to consider that in the ex vivo model, the absence of vascularization may decrease the natural cooling capacity of tissue.
Considering these results, we could agree with Romanos and colleagues [17] that affirm that Nd:YAG and diode lasers must be used with special care because of the higher penetration depth and the possible damage to the bone. Moreover, as reported for other surgical procedures [18] superficial treatment with surface temperatures exceeding 47°C typically caused epidermal and dermal injury that may influence the healing process of non-excised tissue.
The use of a cooling system by an air and/or water spray as reported in literature [19] and observed in our experience could significantly reduce the heat transfer to the tissues.
These considerations could influence the surgical concept of treatment, suggesting the use of pulsed modes or a faster surgical movement in order to give to the tissue the relaxation time; the accurate operative procedures can reduce collateral thermal damage.
In the histological evaluation, firstly, we have to consider, as limit for this analysis (particularly on the quantification of tissue changes), the presence of processing artefacts caused by routine procedures [20, 21]; we tried to reduce this “distortion”, anyway comparable in each specimen, by removing samples of at least 12–15 mm as main size.
The quality of incision was good and regular for the CO2 and diode laser but was poor for the Nd:YAG laser, with an intermediate condition for KTP laser. This may be due to the fact that CO2 (but not in superpulsed mode that may be represent as a quasi-continuous mode) and diode lasers were used in continuous mode, and this could determine a more precise cut than for the Er:YAG laser, this last working in a pulsed mode and in a non-contact way that may be more difficult to the precision of cut; this may be correlated with the different factors not temperature-related such as presence of water, overlapping of the spots and bigger spot size. However, even if with an irregular aspect for the incision, the Er:YAG laser gained the best performance in terms of histological anatomy, with limited changes at the different levels.
Epithelial and stromal changes were limited for the CO2, diode (particularly for 3-W power) and KTP laser, but very important for the Nd:YAG laser, maybe for the heating effects on tissues and for the deeper absorption than the other wavelength; as already reported [10], the primarily absorbed light by melanin and hemoglobin permits the energy to penetrate deeper into the tissue.
Vascular changes, in a predictable manner, depending on the very important affinity for the hemoglobin, reported the most important vascular changes, with a great number of vessels with signs of coagulation. This result had to be considered in light of the fact that it may be connected with anatomical differences of mucosal vascularizations.
Conclusion
Transferring the present ex vivo study to in vivo surgery, the clinicians should take into account the heating of collateral tissue up to 53°C (37°C of body temperature plus 16°C of deep increase in temperature by the diode laser) and maybe the adaptation, particularly for continuous mode, of a surgical technique and systems allowing the cooling of tissues.
In this study, we reported positive results for the evaluation of laser-excised specimens in relation to their readability and diagnostic reliability. To better assess whether the laser-induced histological artefacts and changes determine some limit for histological analysis, a study comparing larger groups of samples and several blinded pathologists should be made.
Clinicians should be aware of small biopsies requiring histological analysis that a portion of a biopsy specimen could not be used for histological evaluation; however, if adequate margins around the tissue of interest are respected, lasers could be useful tools.
Results of this study have to be considered in light of the limits for an ex vivo study. Loss of perfusion, even if reduced by the fresh sample used, may influence performance of lasers due to the loss of cromophores; this may be balanced by the fact that all the lasers found this as an influencing factor.
References
Frame JW, Morgan D, Rhys Evans PH (1988) Tongue resection with the CO2 laser: the effects of past radiotherapy on postoperative complications. Br J Oral Maxillofac Surg 26(6):464–471
Wang X, Ishizaki NT, Matsumoto K (2005) Healing process of skin after CO2 laser ablation at low irradiance: a comparison of continuous-wave and pulsed mode. Photomed Laser Surg 23(1):20–26
Niemz MH (2007) Laser-tissue interactions. Fundamentals and applications. 3rd edn, Springer
Sperandio FF, Meneguzzo DT, Ferreira LS, da Ana PA, Azevedo LH, de Sousa SC (2011) Different air–water spray regulations affect the healing of Er, Cr:YSGG laser incisions. Lasers Med Sci 26(2):257–265
Fornaini C, Rocca JP, Merigo E, Meleti M, Manfredi M, Nammour S, Vescovi P (2011) Low energy KTP laser in oral soft tissue surgery: a 52 patients clinical study. Med Oral Med Buc Cir Buc 17(2):e287–e291
Gómez-Santos L, Arnabat-Domínguez J, Sierra-Rebolledo A, Gay-Escoda C (2010) Thermal increment due to ErCr:YSGG and CO2 laser irradiation of different implant surfaces. A pilot study. Med Oral Patol Oral Cir Bucal 15(5):e782–e787
Sun H, Mikula E, Kurtz RM, Juhasz T (2010) Temperature increase in human cadaver retina during direct illumination by femtosecond laser pulses. J Refract Surg 26(4):272–277
George J, Bensafi A, Schmitt AM, Black D, Dahan S, Loche F, Lagarde JM (2008) Validation of a non-contact technique for local skin temperature measurements. Skin Res Technol 14(4):381–384
Vescovi P, Corcione L, Meleti M, Merigo E, Fornaini C, Manfredi M, Bonanini M, Govoni P, Rocca JP, Nammour S (2010) Nd:YAG laser versus traditional scalpel. A preliminary histological analysis of specimens from the human oral mucosa. Lasers Med Sci 25(5):685–691
Rizzo LB, Ritchey JW, Higbee RG, Bartels KE, Lucroy MD (2004) Histologic comparison of skin biopsy specimens collected by use of carbon dioxide or 810-nm diode lasers from dogs. J Am Vet Med Assoc 225(10):1562–1566
Oelgiesser D, Blasbalg J, Ben-Amar A (2003) Cavity preparation by Er-YAG laser on pulpal temperature rise. Am J Dent 16(2):96–98
Martins GR, Cavalcanti BN, Rode SM (2006) Increases in intrapulpal temperature during polymerization of composite resin. J Prosthet Dent 96(5):328–331
Sulieman M, Rees JS, Addy M (2006) Surface and pulp chamber temperature rises during tooth bleaching using a diode laser: a study in vitro. Br Dent J 200(11):631–634
Augustin G, Davila S, Mihoci K, Udiljak T, Vedrina DS, Antabak A (2008) Thermal osteonecrosis and bone drilling parameters revisited. Arch Orthop Trauma Surg 128(1):71–77
Augustin G, Davila S, Udiljak T, Vedrina DS, Bagatin D (2009) Determination of spatial distribution of increase in bone temperature during drilling by infrared thermography: preliminary report. Arch Orthop Trauma Surg 129(5):703–709
Sedlin ED, Hirsch C (1966) Factors affecting the determination of the physical properties of femoral cortical bone. Acta Orthop Scand 37(1):29–48
Geminiani A, Caton JG, Romanos GE (2011) Temperature change during non-contact diode laser irradiation of implant surfaces. Lasers Med Sci 27(2):339–342
DiBernardo BE, Reyes J, Chen B (2009) Evaluation of tissue thermal effects from 1064/1320-nm laser-assisted lipolysis and its clinical implications. J Cosmet Laser Ther 11(2):62–69
Miserendino LJ, Abt E, Wigdor H, Miserendino CA (1993) Evaluation of thermal cooling mechanisms for laser application to teeth. Lasers Surg Med 13(1):83–88
Silverman EB, Read RW, Boyle CR, Cooper R, Miller WW, McLaughlin RM (2007) Histologic comparison of canine skin biopsies collected using monopolar electrosurgery, CO2 laser, radiowave radiosurgery, skin biopsy punch, and scalpel. Vet Surg 36(1):50–56
Reimer SB, Séguin B, DeCock HE, Walsh PJ, Kass PH (2005) Evaluation of the effect of routine histologic processing on the size of skin samples obtained from dogs. Am J Vet Res 66(3):500–505
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Merigo, E., Clini, F., Fornaini, C. et al. Laser-assisted surgery with different wavelengths: a preliminary ex vivo study on thermal increase and histological evaluation. Lasers Med Sci 28, 497–504 (2013). https://doi.org/10.1007/s10103-012-1081-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-012-1081-8