Abstract
The emergence of antimicrobial resistance (AMR) in lactic acid bacteria (LAB) raises questions on qualified presumptive safety status and poses challenge of AMR transmission in food milieu. This study focuses on isolation, identification and characterization of AMR in LAB prevalent in traditional fermented Indian food products. The analysis of 16SrRNA based phylogenetic tree showed placements of isolates among four different genera Lactobacillus, Enterococcus, Weissella and Leuconostoc. In E-strip gradient test of susceptibility to 14 different antibiotics, over 50% of isolates showed resistance to ampicillin, chloramphenicol, ciprofloxacin, erythromycin, kanamycin, linezolid, streptomycin, trimethoprim and vancomycin. A multivariate principal component analysis, an antibiogram and multiple antibiotic resistance index-values (> 0.2) indicated presence of multidrug-resistance among the isolates. This study reports prevalence of an alarmingly high rate of AMR LAB strains in traditional fermented foods and is important to regulators and public health authorities for developing strategies to control transmission in food systems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With the advent of antibiotics in the medical system, pathologists anticipated and managed to control several serious outbreaks of infections. This led to the use of antibiotics globally at a broad level. However, over the years, rampant and non-judicious use of antibiotics led to the evolution of antibiotic resistance throughout the planet (Blair et al., 2015; György et al., 2021; Ojha et al., 2021). According to the UN, 700,000 people die every year as a result of diseases caused by AMR pathogens. Thus, AMR has posed a serious challenge to the investigators working in this domain of research. The food ecosystem has become a hotspot for the transmission of AMR in the bacterial community across the globe (Founou et al., 2016; Zarzecka et al., 2020a). Bacterial strains harboring resistance genes may enter the food supply chain system from the environment and get transmitted to feed animals and humans (Dutta and Ramamurthy, 2020; György et al., 2021; Thapa et al., 2020).
Lactic acid bacteria (LAB) are Gram-positive bacteria comprising a large group of genera with potential probiotic properties in several fermented foods (Bhushan et al., 2021; Erginkaya et al., 2018; Mohammed and Çon, 2021). Species of this group are known to produce lactic acid, a principal metabolic end product, through carbohydrate fermentation. The common genera include Pediococcus, Leuconostoc, Enterococcus, Streptococcus, Lactococcus and Lactobacillus. Lactobacillus is the largest group of the genus used commercially in fermented foods and pharmaceuticals (Dutta and Ramamurthy, 2020). Recently, the genus Lactobacillus has been re-classified into 25 genera by Zheng and his team (Zheng et al., 2020). They are known as Generally recognized as safe (GRAS) organisms due to their extensive use in fermented foods. However, LAB are known to act as a reservoir of antibiotic resistance in the food ecosystem (Ojha et al., 2021; Zarzecka et al., 2020a). They harbour genes that encode antibiotic resistance against various antibiotics of clinical importance such as tetracycline [tet(M), tet(K) and tet(W)], macrolide [(erm(B), erm(C) and erm(G)], glycopeptide like vancomycin [van(A), van(B), van(C), van(D), van(E) and van(G)], and ampicillin (bla, blaZ and mecA) (Álvarez-cisneros and Ponce-alquicira, 2021; Guo et al., 2017; Nunziata et al., 2022; Ojha et al., 2021; Zarzecka et al., 2020b). LAB can transfer the resistance genes to commensal bacteria of humans and animals. Horizontal gene transfer (HGT) has been a major mechanism of the transfer of resistance among these groups of bacteria (Dec et al., 2018; Ojha et al., 2021). Here, bacteria resort three prime approaches to transfer their resistance to sensitive groups. These are mainly: transformation (uptake of extracellular DNA by competent bacterial cell), transduction (incorporation of a bacterial DNA segment to the other bacterial cell with the help of a bacteriophage), conjugation (DNA translocation from one bacterial cell to another through physical mating with a surface pilli protein) (Ojha et al., 2021).Therefore, the usage of strains carrying antibiotic resistance must be ceased in food and feed additives (Casado Muñoz et al., 2014; Dec et al., 2018).
LAB have been reported to exhibit resistance to a plethora of antibiotics. Many genera like Lactobacillus, Lactococcus, Pediococcus, Enterococcus and Leuconostoc were reported to display resistance against some common classes of antibiotics such as aminoglycosides (streptomycin, gentamicin and kanamycin), β-lactams (ampicillin), macrolides (erythromycin) and tetracycline (Ivanova et al., 2016). Over a decade, LAB have been isolated from fermented foods, dairy products, vegetables, cheese, wine, and many animal feeds. A high rate of prevalence of antibiotic resistance was observed among dairy associated LAB. Where dairy based fermented food (milk) has been a major source of LAB harbouring antibiotic resistance genes (ARGs) (Blanco-Picazo et al., 2022; Wang and Lu, 2019) and may be a potential source of transfer of these gene determinants. Additionally, the residue of antibiotics have also been ascertained in milk (Blanco-Picazo et al., 2022; Landers et al., 2012; Sachi et al., 2019). Due to a global public health concern about antibiotic resistance in foods, determination of AMR in LAB in traditional fermented foods is a prerequisite to determining their food safety. Thus, a thorough investigation is needed to study the resistance profile of these bacteria. Therefore, the present study focused on the determination of phenotypic antibiotic resistance profile of LAB isolates of fermented Indian foods and beverages and map the status of AMR populations using principal component analysis (PCA) and MAR assessments. This is a key to the determination of food safety and public health of the nation and device strategies to control the spread of AMR via food systems.
Materials and methods
Food sampling and microbial cultures
To obtain LAB strains, food samples were collected from nearby markets (including street vendors), local households, milkmen and commercial food products. A total of 53 food samples were collected from different sources (curd, lassi, commercial fermented milk products, fermented batters) in a sterile container (100 ml). The collected samples were transported to the lab immediately from the site of collection for the isolation of LAB cultures. Twelve different strains of Lactobacillus species were also procured from the National Collection of Dairy Cultures (NCDC), NDRI, Karnal, in the state of Haryana, India.
Isolation and phenotypic identification of LAB
Isolation was accomplished from different food sources by using standard plating technique and preliminary identification was carried out using morphological and biochemical methods. Briefly, an appropriate amount of sample was diluted in sterile peptone water followed by serial dilution and plating on MRS (de Man, Rogosa and Sharpe, Hi-Media Ltd., Mumbai, India) agar. All plates with respective samples were incubated at 37 °C aerobically for 24–48 h. The colonies with typical LAB morphology (creamy-white small to medium sized round colonies with entire margins) were selected for further characterization studies.
All the putative isolates (n = 18) were tested for their morphology by microscopic examination and biochemical confirmation by catalase and oxidase test. For growth under anaerobic conditions, an Anaero Gas Pack jar (Hi-media, Mumbai, India) consisting Anaero gas pack sachet (LE002A, Hi-media, Mumbai, India) was used. Gram-positive rods and catalase/oxidase-negative isolates were taken for further identification based on carbohydrate fermentation (KB009 HiCarbo Kit, Hi-Media, Mumbai, India).
All the isolates were designated with a unique identification code and maintained in MRS broth at 4 °C and sub-cultured after every 15 days. The stocks of all the isolates were preserved in 25% (v/v) glycerol stock medium at − 80 °C. The cultures were activated before use by sub-culturing them twice in MRS broth. The details of all the isolates used and their sources are given in Table S1.
Genotypic identification
In order to identify and characterize the LAB isolates (up to the species level), partial 16 S rRNA gene was selected for PCR amplification and sequencing. For this complete genomic DNA was extracted according to the methods of Pospiech and Neumann (1995) with some modifications (Pospiech and Neumann, 1995). Briefly, an active bacterial culture (1.5 ml) grown overnight in MRS broth was centrifuged at 6010×g for 10 min. The supernatant was discarded and the pellet was washed with 1X PBS (phosphate buffer saline, pH 7.0) followed by centrifugation at 6010×g for 10 min. To the resultant pellet, 0.5 ml of SET buffer (75 mM NaCl, 25 mM EDTA, 20 mM Tris, pH 7.5) and 100 µl of lysozyme (1 mg/ml) were added followed by an hour of incubation at 37 °C. A 50 µl solution of 10% (w/v) SDS was added to the above mixture, followed by the addition of 10 µl of proteinase K. The tube was incubated for 2 h at 56 °C with intermittent inversion. After this, 550 µl chloroform and 180 µl of 5 M NaCl were added and the contents were mixed by inverting the tube, followed by incubation at room temperature for 30 min. The tube was centrifuged at 9391×g for 15 min. DNA was precipitated by adding chilled isopropanol to the uppermost aqueous phase from the above step. The tube was centrifuged for 7 min at 9391×g. The pellet was washed with 70% (v/v) ethanol and air-dried subsequently to remove any residual ethanol. TE buffer (100 µL) was added to dissolve DNA and the tube was incubated at 37 °C overnight. This extracted DNA was quantified by using a nanodrop spectrophotometer (DeNovix, USA) and used for amplifying the 16S rRNA gene by PCR using universal primers: 63F (5′CAGGCCTAACACATGCAAGTC3′) and 1387R (5′GGGCGGWGTGTACAAGGC3′). The reactions for PCR were set up as per the instructions provided by the manufacturer on the PCR reagent kit (ThermoFisher Scientific, USA).
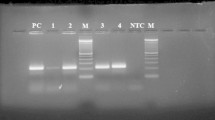
The PCR was programmed with the conditions of initial denaturation at 95 °C for 5 min followed by 35 cycles of denaturation at 95 °C for 1 min, annealing at 55 °C for 1 min, amplification at 72 °C for 1 min and a final extension at 72 °C for 8 min (Eppendorf, Germany). The amplified PCR product was resolved using 1.2% (w/v) agarose gel stained with ethidium bromide (0.5 µg/ mL) through gel electrophoresis at 80 V for 30 min using 1X TAE buffer (Mini/ Maxi submarine, Hoeffer, USA). Gels images were taken using a UV Transilluminator (Gel capture, DNR Bio-Imaging System) (Fig. 1B). The PCR products were sequenced commercially by Chromous Biotech (Bengaluru, India) using an automated DNA sequencer (ABI Prism 3100 Genetic Analyzer, Applied Biosystems, Foster, CA, USA) with specific primers. Sequences were analyzed by using the NCBI GenBank database BLAST programme (Altschul et al., 1990). All sequences were submitted to NCBI GenBank database to retrieve their accession numbers. The accession numbers were from OP932065–OP932078 for fourteen isolates while for other four isolates (AKO 94.6, NIFTEM 95.8, DVM 95.7 and SKL1), these were from MW647658–MW647661 in the database. The 16S rRNA sequences of all the 18 isolates were aligned by using an algorithm of clustalW programme in MEGA 7.0 software analysis. Phylogenetic dendrogram reconstruction was done by using MEGA version 7.0 to check the placements of the isolates in a phylogenetic tree (Altschul et al., 1990; Kumar et al., 2016).
Determination of susceptibility pattern
To evaluate the minimum inhibitory concentration (MIC) of selected isolates, commercial antibiotic E-strips (Hi-Media Ltd., Mumbai, India) were used. The concentrations ranged from 0.016 to 256 µg/ml in all the antibiotic strips except in rifampicin and trimethoprim where 0.002 to 32 µg/ml of concentration was used. The test was performed against fourteen clinically important antibiotics i.e. ampicillin, vancomycin, ciprofloxacin, tigecycline, rifampicin, clindamycin, trimethoprim, streptomycin, chloramphenicol, linezolid, kanamycin, erythromycin, gentamycin, and tetracycline.
Actively grown cultures (100 µL) of all the isolates and reference strains containing 108 cells (0.5 Mcfarland turbidity) were taken onto MRS agar media plates and uniformly spread over MRS agar plates using a sterile swab. To ensure effective absorption, the plates were kept at 40 °C for 1 h. The commercial strips were put on the agar surface and it was ensured that the strip was uniformly in contact with the agar surface. The plates were kept at 37 °C for 24 h and MIC values were recorded in triplicates. The organisms were classified as resistant/ susceptible based on the European Food Safety Authority (EFSA) guidelines (Danielsen and Wind, 2003; European Food Safety Authority (EFSA), 2012).
Genotypic detection of genetic determinants of antibiotic resistance
The resistance gene loci were detected using PCR amplification reaction as per the protocol mentioned in the above section of ‘genotypic identification’ method. The PCR reactions were set up as mentioned briefly in the above protocol of genotypic identification by 16S rRNA gene amplification except for the primers and annealing temperatures used for different reactions which are given in the Table S2. The PCR products of different resistance gene loci [(erm(B), tet(M) and xis] of the isolates were also sequenced by dideoxy chain termination protocol commercially at Eurofins Genomics Ltd. (Bengaluru, India). The sequences were analyzed through BLAST search programme of NCBI and submitted to the GenBank database of NCBI to retrieve the accession numbers. The identified sequences of tet(M) gene loci in the isolates NIFTEM 51, SKL1, NIFTEM 63 and NIFTEM 67, were deposited with the accession numbers as OQ448895, OQ450369, OQ448894 and OQ421194 respectively. Sequences for transposable genetic element (xis, excisionase) from Tn916 family were submitted with the accession numbers OQ504985–OQ504987, respectively for the isolates NIFTEM 95.8, DVM 95.7 and NIFTEM 63.
MAR (multiple antibiotic resistance) index bar plot determination
The multiple antibiotic resistance (MAR) index of the isolated LAB strains was computed by using the following formula: MAR index = x/y, where (x) is representing the number of antibiotics to which the isolates were resistant and (y) is the total number of antibiotics used in susceptibility test (Costa et al., 2020).
Statistical analysis
To evaluate the pattern of resistance among the LAB isolates, a multivariate PCA analysis was performed using Past 4.10 software (Hammer et al., 2001). A numerical code 1 (for resistance) and 2 (for susceptible) was used to represent the variability among resistant phenotypes (Alves et al., 2014; Kappell et al., 2015). An antibiogram through the heatmap was constructed to depict the incidence of resistance among the isolates using GraphPad Prism 8.0 software.
Results and discussion
Isolation and phenotypic characterization of LAB
The maximum number of isolates was obtained from curd (n = 45). The microscopic characterization of isolates showed variations in cell sizes, shapes, and arrangements. All the isolates were Gram-positive rods/cocci, arranged either singly/pairs/chain or in a cluster-like arrangement on microscopic observation. All strains were negative to catalase and oxidase and able to grow at 37 °C optimally under anaerobic conditions (Table S3). Curd and lassi are fermented milk products consumed very commonly in the Indian sub-continent. In the current study, 30 LAB strains were taken, of which, 18 organisms were isolated from the fermented dairy food sources and the remaining, 12 organisms were procured from a national culture collection centre (NCDC).
Identification using 16S rRNA gene
The presumptive LAB isolates were confirmed based on 16S rRNA gene sequencing followed by alignment using BLAST search tool. All 18 isolates were from the four different genera of LAB when analyzed through the BLAST search programme. There were 4 strains (AKO 95.4, DVM 95.7, NIFTEM 95.8 and SKL1) that belonged to the genus Lactobacillus. All these four strains showed > 98% similarity to the species of Limosilactobacillus fermentum whereas the other genera were Enterococcus (n = 10), Weissella (n = 3) and Leuconostoc (n = 1). Analysis of the reconstructed phylogenetic tree of 16S rRNA gene sequences further confirmed the placement of respective isolates of LAB within the different species of four genera of LAB (Lactobacillus, Enterococcus, Weissella and Leuconostoc). This was also validated through a high bootstrap similarity percentage (> 70%) value (Fig. 1A). The major occurrence of the species in our isolation study was Enterococcus indicating the abundance of these bacteria in fermented foods or beverages. The isolated strains were identified to belong to the different genera of LAB group namely Lactobacillus, Enterococcus, Weissella and Leuconostoc. The highest frequency of abundant species of Lactobacillus and Enterococcus in these fermented samples indicates that these bacteria are present dominantly in fermented food matrices of dairy origin in North India. Importantly, few recent studies have reported the presence of pathogenic species in Enterococcus (Stefańska et al., 2021). However, many species of this genus are widely used in fermented foods without any QPS or GRAS status (Hanchi et al., 2018; Krawczyk et al., 2021; Saeed et al., 2014). Lactobacillus as discussed by several groups of investigators is the most commonly and extensively used bacteria in fermented foods and feeds. Due to its large benefits, these bacteria are given GRAS and QPS status by USFDA (United States Food and Drug Administration) and EFSA (European Food Safety Authority) (EFSA, 2012). The genus Weissella and Leuconostoc are a group of bacteria that look similar to each other and share their phenotypic and biochemical characteristics to a large extent. Both these bacteria are abundantly found in fermented milk as reported previously (Fusco et al., 2015; Holland and Liu, 2011). Several previous studies have illustrated the dominance of LAB in fermented foods like fruits, vegetables, meat, fish, plants and dairy based products (Stefańska et al., 2021; Saeed et al., 2014). Hence, in consonance with the previous studies, we have also obtained the commonly distributed LAB in fermented milk products.
Antibiotic susceptibility
All strains showed variable responses against different antibiotics. The antibiotic susceptibility results of all 30 strains are shown in Table 1. Determination of resistance and sensitivity was based upon the MIC breakpoints cut-off values of the respective species of the LAB microbes given by EFSA (2012) and Danielsen & Wind, (2003). These cut-off values varied depending on the different genera of this group of LAB. Here if the MIC values were beyond the maximum range of concentration (> 256 µg/ml) it was categorized as completely resistant to the specific antibiotic. Whereas if the MIC values fell within the test concentration range (0.016–256 µg/ml), the strain was classified as resistant as per the MIC breakpoints given earlier (Danielsen and Wind, 2003; European Food Safety Authority (EFSA), 2012). It was found that most of the strains were strictly sensitive to clindamycin, gentamycin, rifampicin, tetracycline and tigecycline except for a few strains (Enterococcus thailandicus NIFTEM 30, Enterococcus durans NIFTEM 50, Enterococcus durans NIFTEM 58 and Enterococcus faecium NIFTEM 75) which showed resistance against clindamycin, gentamycin and rifampicin. With the 14 antibiotics used for testing the susceptibility of 30 isolates, all thirty isolates were susceptible to only tigecycline. Most of the isolates showed resistance to ampicillin (63%), chloramphenicol (73%), ciprofloxacin (80%), erythromycin (86%), kanamycin (86%), linezolid (56%), streptomycin (70%), trimethoprim (83%) and vancomycin (63%) (Fig. 2A). A noteworthy result is that 86% of the strains showed resistance to erythromycin and kanamycin. The pattern of susceptibility of each strain against 14 antibiotics was interpreted through a bar chart as shown in Fig. 2B. There were three strains (Enterococcus durans NIFTEM 50, Enterococcus faecium NIFTEM 75, and Lactobacillus delbreukii NCDC 405) that were resistant to a maximum of 11 antibiotics out of 14 used in the study. Similarly, strain NCDC 400 was sensitive to 10 antibiotics out of 14 included in the investigation. Resistance to multiple classes of antibiotics was discernible in our study. It is evident from our study that more than 56% of the strains were resistant to antibiotics used in the investigation.
(A) Number of isolates showing resistance and sensitivity towards different antibiotics; Maximum % of resistance was observed in kanamycin (86%), erythromycin (86%) and trimethoprim (83%). LR LAB resistant; LS LAB sensitive. (B) Prevalence of antibiotic susceptibility of LAB isolates; NIFTEM 50, NIFTEM 75 and NCDC 405 showed resistance to a maximum 11 antibiotics out of 14 used. R Resistant; S Sensitive
The prevalence of antibiotic resistance in bacteria associated with food and feeds may be one of the challenges to overcome with the already existing menace of antibiotic drug resistance globally. The potential threat of transfer through horizontal gene transfer mechanism is even more worrisome (Das et al., 2020; Lin et al., 2015; Nawaz et al., 2011; Ojha et al., 2021; Tan, 2003; Thumu and Halami, 2019; Von Wintersdorff et al., 2016; Zarzecka et al., 2022). Regardless of their wide application with profound benefits, health risks associated with them cannot be overlooked. This study was carried out to assess the antibiotic resistance in LAB isolates to find out the probable risk of transfer of resistance.
The fourteen antibiotics used in study, represented mainly three prominent classes of drugs based on their site of action in bacterial cells. These are (1) cell wall synthesis inhibitors (ampicillin and vancomycin); (2) nucleic acid inhibitors (ciprofloxacin, rifampicin and trimethoprim) and (3) protein synthesis inhibitors (chloramphenicol, clindamycin, erythromycin, gentamycin, kanamycin, linezolid, streptomycin, tetracycline and tigecycline) (Álvarez-cisneros and Ponce-alquicira, 2021; Nunziata et al., 2022). The results of the study showed that most of the strains were phenotypically resistant to a majority of the antibiotics. Therefore, a pattern of multidrug resistance was demonstrated by all the strains. This is analogous to other previous studies where similar observations were reported (Erginkaya et al., 2018; Ivanova et al., 2016; Saeed et al., 2014). A high level of resistance to chloramphenicol, erythromycin, kanamycin, streptomycin and trimethoprim was observed in species of Lactobacillus, Enterococcus, and Leuconostoc. This result is similar to the earlier published reports by other research groups (Danielsen and Wind, 2003; Erginkaya et al., 2018; Flórez et al., 2005). Multi drug resistance in Enterococcus spp. from fermented dairy based foods to such a high degree could be due to the source of contaminated milk either through equipment or environmental source such as contaminated water (Abriouel et al., 2015). Enterococci have shown resistance to aminoglycosides (gentamycin and kanamycin) due to the presence an enzyme called 2′-phosphotransferase-6′- acetyltransferase, which promotes the ATP-dependent phosphorylation of aminoglycosides (Miller et al., 2014). Therefore, in certain cases, enterococci of fermented food origin have been reported to behave like nosocomial emergent pathogenic enterococci with MDR mechanism of resistance (Alvarez-Cisneros et al., 2017; Miller et al., 2014). AMR in Leuconostoc spp. have been reported by Flórez and group in 2008, where resistance to antibiotics class like glycopeptides (vancomycin), aminoglycosides (kanamycin and gentamycin) and sulfonamides (trimethoprim) was observed (Flórez et al., 2008). The possible role of Leuconostoc species was acceded in AMR gene dissemination when Florez and his co-workers in 2016, demonstrated the transfer of erm(B) resistance from Enterococcus faecalis to Leuconostoc spp. both under wet lab as well as in cheese environment. This restates the possibility of AMR transfer in food matrix (Flórez et al., 2016).
The presence of erythromycin resistance in most of the strains is alarming as it is an acquired resistance. This could lead to the transfer of resistance to other bacteria through plasmid gene transfer using conjugation (Anisimova and Yarullina, 2019, 2018; Nawaz et al., 2011; Thumu and Halami, 2019). Likewise, tetracycline resistance is also an acquired resistance that occurs through mobile genetic elements (Tn916 family) present in bacterial strains (Chandra et al., 2012; Flórez et al., 2008; Gazzola et al., 2012; Gevers et al., 2003; Jacobsen et al., 2007; Ojha et al., 2021; Thumu and Halami, 2019; Zarzecka et al., 2020a; Zonenschain et al., 2009). However, very few strains (30%) showed resistance to tetracycline in our study. The report on susceptibility of Weissella spp. was published recently by Jang and his team (Jang et al., 2021). They showed that Weissella spp. were resistant to vancomycin and kanamycin with MIC cut off more than 256 mg/L. Whereas, in our analysis resistance was also recorded for ampicillin and erythromycin besides vancomycin and kanamycin. This variation between the two studies indicates the complexity of food matrices which may facilitate the resistance gene transfer through the HGT mechanism.
PCR amplification of resistance gene loci of LAB isolates
The isolates were tested for the presence of antibiotic resistance gene determinants by using PCR amplification method. The tested isolates are given in the Table S4, where 18 isolates were subjected to check the presence of different gene loci of resistance through PCR amplification reaction protocol. Isolates DVM 95.7, NIFTEM 95.8, NIFTEM 51, NIFTEM 63, SKL1 and NIFTEM 67 showed tet(M) resistance gene in PCR amplification reaction while isolates DVM 95.7 and NIFTEM 95.8 showed the presence of erm(B) gene (Fig. S1 a–c).
The isolate AKO 94.6 did not show any of the genetic determinants in PCR reactions but it was discerned to show the phenotypic resistance for both erythromycin and tetracycline. This might be either due to the presence of specific resistant gene loci [tet(W) and erm(C)] other than that were studied here in the isolates or due to some unknown underlying genetic determinants. It could also be explained with the fact that the resistance might have occurred due to mutations in the strain during study (Wang and Lu, 2019). However, there was no resistance offered phenotypically by the isolates NIFTEM 63 (Enterococcus thailandicus) and NIFTEM 67 (Enterococcus faecium) against the tetracycline antibiotic. Similarly, in the isolate DVM 95.7, no phenotypic resistance was observed for erythromycin but resistance gene loci erm(B) was detected in PCR amplification results. This could be explained with a reasoning that the genes are expressed either at a very low level or they are down regulated or could be due to some inactive gene product (Eaton and Gasson, 2001; Wang and Lu, 2019). The result can also be explained by the method dependent susceptibility tests where some strains showed higher MICs when inoculum size was increased with longer incubation time period (Anisimova and Yarullina, 2018; Egervärn et al., 2007). The determinant tet(M) was found linked with chromosomal genes in the tested isolates while we detected that the loci of erm(B) gene was associated with plasmid DNA of Lactobacillus isolates. We also detected the presence of xis (excisionase) gene from Tn916 family of transposons in our study in the isolates DVM 95.7, NIFTEM 95.8 NIFTEM 63 and NIFTEM 67 only (Fig. S2 a & b). The result could be interpreted with the fact that xis gene belonged to conjugative transposon family (Tn916) linked with tet and erm gene loci. Therefore, presence of xis gene of transposon may lead to the transfer of the resistance gene loci to the other sensitive LAB species or pathogenic bacteria (Ammor et al., 2007; Anisimova and Yarullina, 2018; Bellanger et al., 2014; Devirgiliis et al., 2009; Gazzola et al., 2012; Gueimonde et al., 2013; Ojha et al., 2021; Preethi et al., 2017; Thumu and Halami, 2019; Zarzecka et al., 2020).
Lactobacillus spp. are the dominant group of LAB found in fermented food and harbor erm(B) gene frequently in their several strains (Ammor et al., 2007; Anisimova and Yarullina, 2019, Anisimova and Yarullina, 2018; Gueimonde et al., 2013; Guo et al., 2017; Huys et al., 2007; Nawaz et al., 2011; Zarzecka et al., 2020). Whereas tet(M) gene was reported by many group of researchers earlier in strains Lactobacillus species (Anisimova and Yarullina, 2019, Anisimova and Yarullina, 2018; Campedelli et al., 2019; Huys et al., 2007; Nawaz et al., 2011; Zarzecka et al., 2022). The genetic determinant erm(B) is a macrolide resistance gene which codes for the enzyme rRNA methylase acting on the 23S ribosomal subunit whereas, gene tet(M) codes for the ribosomal protection proteins. Previously many strains of Lactobacillus have been reported to contain these genes coding for macrolide resistance and ribosomal protection proteins (Anisimova and Yarullina, 2019, Anisimova and Yarullina,2018; Ashraf and Shah, 2011; Chandra et al., 2012; Gueimonde et al., 2013; Microbiol et al., 2011; Preethi et al., 2017; Thumu and Halami, 2019; Zarzecka et al., 2020). Since, genes encoding these resistance are found frequently on mobile genetic elements like conjugative plasmid and transposons. Therefore, these genes must be investigated in detail along with their ability of transfer through horizontal gene mechanisms. The other genetic determinants such as tet(S), tet(O), erm(A) and int for resistance were not detected in PCR gene amplification study.
MAR index bar plot/heatmap antibiogram
Based on the resistance to n number of antibiotics (Table 2), a MAR index bar plot was constructed. The index plot indicates the occurrence of multi drug resistance (MDR) among a majority of the strains included in our study. Except for the strain Limosilactobacillus fermentum NCDC 400, the MAR index is higher in all the strains (> 0.3) (Fig. 3a). Bacteria with a value of MAR index less than 0.2 are considered less risky from the food safety aspects. But a higher MAR index value (> 0.2) makes a strain riskier and more hazardous for consumption through food. Three strains (Enterococcus durans NIFTEM 50, Enterococcus faecium NIFTEM 75, and Lactobacillus delbreukii NCDC 405) were showing the MAR value much higher (0.79) making these strains highly risky for usage in food. High MAR index (> 0.3) in all the isolates (except NCDC 400) suggests the overexposure of isolates to the antibiotics at a sub-therapeutic level. Several factors like the presence of resistance genes, previous source/niche of isolates and indiscriminate use of antibiotics in humans and food/feed animals, could lead to the emergence of AMR (Nataraj et al., 2021). An antibiogram of resistant phenotypes against 14 different antibiotics showed the prevalence of phenotypic AMR in fermented food isolates (Fig. 3b). The heatmap indicates the existence of MDR among the LAB cultures (> 50%) and verifies the MAR index data. Antibiogram heatmap analysis is a two-dimensional data visualization technique that depicts the values in color codes. Nonetheless, mostly the heatmaps are used in the field related to omics studies to visualize the large data sets. However, to envisage the clustering pattern, limited attempts have been made to segregate and thereafter select the target bacteria with desired phenotypes (Nataraj et al., 2021). In this study, the generated heatmap showed a high pattern of resistance among the LAB.
(A) MAR (Multiple antibiotic resistance) index plot of LAB isolates isolated from fermented food stuffs. Values > 0.2 indicates multiple drug resistance incidence. A maximum value (0.79) was recorded in NCDC 405, NIFTEM 75 and NIFTEM 50. NCDC 400 showed the least MAR score (0.29). B Antibiogram represented through heatmap. Rows represent the LAB isolates while columns are the 14 antibiotics used in the study. Blocks in red represent the isolates showing resistance to antibiotics while green blocks represent the susceptible strains. Abbreviations of antibiotics are mentioned in “materials and methods” section
Principal component analysis (PCA)
A multivariate analysis through PCA biplot was carried out on phenotypic resistance in the isolates against different classes of antibiotics. Biplot represents here both the score (dots) and loading (vectors) plots. The vectors (variables) representing the different classes of antibiotics are positively correlated except for the antibiotics trimethoprim and gentamycin which are negatively correlated. Variability among the resistant phenotypes was discernible with a pattern of antibiotic resistance (Fig. 4a). A total of 44.8% variance was observed by the first two components (PC1 and PC2) with 30.2% and 14.6% variance by each of them respectively. The majority of the isolates belonging to different Lactobacillus species were clustered in the upper right part of the quadrant. Very few isolates were placed in the slightly lower left of the quadrant with negative PC2. A scree plot confirms the extent of variation of each component represented by the components PC1 and PC2 (Fig. 4b). PCA is a widely used statistical approach for reducing the complexity of multivariate data and explaining the inter-relationship between multiple variables by reducing the dimensions of the original variables (György et al., 2021; Nataraj et al., 2021). PCA has been used to classify the antibiotic resistance bacteria in the environment (Alves et al., 2014; György et al., 2021; Kappell et al., 2015). In the present study, multi drug resistance of 30 LAB isolates against 14 different antibiotics was graphically interpreted through a three-dimensional plot.
(A) Principal Component Analysis (PCA) biplot of LAB isolates on the basis of phenotypic antibiotic resistance against different classes of antibiotics. The red points represent all the isolates of Lactobacillus species, black points for Enterococcus spp. isolates, blue for Weissella spp. isolates whereas aqua point represents the isolate from Leuconostoc species. Green arrows denote the antibiotics used to check the susceptibility of the isolates. A cumulative variability of 44.8% was observed from the two components PC1 (30.2%) and PC2 (14.6%) representing the total variability of all the variables. (B) Scree plot showing the different components with their respective eigen values
Antibiotic resistant LAB may be advantageous for patients with antibiotic-induced diarrhoea because these strains can survive under the selective pressure of antibiotic resistance and contribute to the restoration of gastrointestinal stasis (Charteris et al., 1998; Stefańska et al., 2021; Wang et al., 2019). However, LAB employed in food fermentation should not be antibiotic resistant from a food safety standpoint. A large number of LAB cells enter the human gut through various food sources and starter cultures and interact with the resident intestinal microbiota. Since antibiotic resistance genes have previously been shown to have the ability to be transferred horizontally to commensal bacteria or enteric pathogenic bacteria posing a major hazard to food safety and public health, hence they are essential to be mapped (Mathur and Singh, 2005; Ojha et al., 2021; Preethi et al., 2017). LAB used in the food industry must not carry the transferable antibiotic resistance determinants. Therefore, a critical inspection of antibiotic resistance in LAB is required to cease the undesirable resistant gene transfer. Most importantly, a high percentage of strains showing resistance is of grave concern to the food and healthcare sectors and must be taken sternly with proper management of antibiotic usage.
A variety of traditional Indian dairy products studied to determine/estimate the incidence of antibiotic resistance in LAB isolates showed a high rate of prevalence of antibiotic resistance among the strains. All the tested isolates showed resistance to multiple antibiotics (≥ 1.0 or ≤ 256 µg/ml) used in the study. A pattern of multidrug resistance was observed among the strains. Although the strains belong to a group (LAB) that is known to provide benefits rather than being detrimental to human health. However, the presence of acquired resistance to antibiotics such as erythromycin, tetracycline, and vancomycin cannot be undermined. Since these antibiotics are amongst the majorly used drugs in the clinical and animal feed system. Hence, a successful screening of antibiotic resistant LAB cultures is required in fermented food products. The genes responsible for the resistance should be detected to check the probability of their transfer through HGT. The judicious use of antibiotics must be implemented through general awareness. Further, the safety evaluation of LAB consumption must be guided through standard and regulatory methods established by competent authorities.
References
Abriouel H, Casado Muñoz M del C, Lavilla Lerma L, Pérez Montoro B, Bockelmann W, Pichner R, Kabisch J, Cho GS, Franz CMAP, Gálvez A, Benomar N. New insights in antibiotic resistance of Lactobacillus species from fermented foods. Food Research International. 78: 465-481 (2015)
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. Journal of Molecular Biology. 215: 403-410 (1990)
Alvarez-Cisneros YM, Fernández FJ, Sainz-Espuñez T, Ponce-Alquicira E. Assessment of virulence factors, antibiotic resistance and amino-decarboxylase activity in Enterococcus faecium MXVK29 isolated from Mexican chorizo. Letters in Applied Microbiology. 64: 171-176 (2017)
Álvarez-cisneros YM, Ponce-alquicira E. (2021) Antibiotic resistance in lactic acid bacteria: a review. International Journal of Biology, Pharmacy and Allied Sciences. Doi: 10.5772/intechopen.80624
Alves MS, Pereira A, Araújo SM, Castro BB, Correia ACM, Henriques I. Seawater is a reservoir of multi-resistant Escherichia coli, including strains hosting plasmid-mediated quinolones resistance and extended-spectrum beta-lactamases genes. Frontiers in Microbiology. 5: 1-10 (2014)
Ammor MS, Belén Flórez A, Mayo B. Antibiotic resistance in non-enterococcal lactic acid bacteria and bifidobacteria. Food Microbiology. 24: 559-570 (2007)
Anisimova E, Yarullina D. Characterization of erythromycin and tetracycline resistance in Lactobacillus fermentum strains. International Journal of Microbiology. 11: 2018: (2018)
Anisimova E, Yarullina D. Antibiotic resistance of Lactobacillus strains. Current Microbiology. 76: 1407-1416 (2019)
Ashraf R, Shah NP. Antibiotic resistance of probiotic organisms and safety of probiotic dairy products. International Food Research Journal. 18: 837-853 (2011)
Bellanger X, Payot S, Leblond-Bourget N, Guédon G. Conjugative and mobilizable genomic islands in bacteria: evolution and diversity. FEMS Microbiology Reviews. 38(4): 720-760 (2014)
Bhushan B, Sakhare SM, Narayan KS, Kumari M, Mishra V, Dicks LMT. Characterization of riboflavin producing strains of Lactobacillus plantarum as potential probiotic candidate through in vitro assessment and principal component analysis. Probiotics and Antibiotic Proteins. 13: 453-467 (2021)
Blair JMA, Webber MA, Baylay AJ, Ogbolu DO, Piddock LJV. Molecular mechanisms of antibiotic resistance. Nature Reviews Microbiology. 13: 42-51 (2015)
Blanco-Picazo P, Gómez-Gómez C, Morales-Cortes S, Muniesa M, Rodríguez-Rubio L. Antibiotic resistance in the viral fraction of dairy products and a nut-based milk. International Journal of Food Microbiology. 367: 109-590 (2022)
Campedelli I, Mathur H, Salvetti E, Clarke S, Rea MC, Torriani S, Ross RP, Hill C, O’Toole PW. Genus-wide assessment of antibiotic resistance in Lactobacillus spp. Applied and Environmental Microbiology. 85 (2019)
Casado Muñoz M del C, Benomar N, Lerma LL, Gálvez A, Abriouel H. Antibiotic resistance of Lactobacillus pentosus and Leuconostoc pseudomesenteroides isolated from naturally-fermented Aloreña table olives throughout fermentation process. International Journal of Food Microbiology. 172: 110-118 (2014)
Chandra S, Thumu R, Halami PM. Presence of erythromycin and tetracycline resistance genes in lactic acid bacteria from fermented foods of Indian origin. Antonie Van Leewenhoek. 102: 541-551 (2012)
Charteris WP, Kelly HPM, Morelli L, Collins JK. Antibiotic susceptibility of potentially probiotic Lactobacillus species. Journal of Food Protection. 61: 1636-1643 (1998)
Costa da MC, Cruz AIC, Bispo da ASR, Ferreira MA, Costa JA, Evangelista-Barreto NS. Occurrence and antimicrobial resistance of bacteria in retail market spices. Ciência Rural. 17: 50 (2020)
Danielsen M, Wind A. Susceptibility of Lactobacillus spp. to antimicrobial agents. International Journal of Food Microbiology. 82: 1-11 (2003)
Das DJ, Shankar A, Johnson JB, Thomas S. Critical insights into antibiotic resistance transferability in probiotic Lactobacillus. Nutrition. 69: 110567 (2020)
Dec M, Nowaczek A, Stȩpień-Pyśniak D, Wawrzykowski J, Urban-Chmiel R. Identification and antibiotic susceptibility of lactobacilli isolated from turkeys. BMC Microbiology. 18: 1-14 (2018)
Devirgiliis C, Coppola D, Barile S, Colonna B, Perozzi G. Characterization of the Tn916 conjugative transposon in a food-borne strain of Lactobacillus paracasei. Applied and Environmental Microbiology. 75: 3866-3871 (2009)
Dutta S, Ramamurthy T. Influence of abiotic factors in the emergence of antibiotic resistance. Antimicrobial Resistance. (2020) Doi: 10.1007/978-981-15-3658-8_4
Eaton TJ, Gasson MJ. Molecular screening of Enterococcus virulence determinants and potential for genetic exchange between food and medical isolates. Applied and Environmental Microbiology. 67: 1628–1635 (2001)
Egervärn M, Lindmark H, Roos S, Huys G, Lindgren S. Effects of inoculum size and incubation time on broth microdilution susceptibility testing of lactic acid bacteria. Antimicrobial Agents and Chemotherapy. 51: 394–396 (2007)
Erginkaya Z, Turhan EU, Tatli D. Determination of antibiotic resistance of lactic acid bacteria isolated from traditional Turkish fermented dairy products. Iranian Journal of Veterinary Research. 19: 53-56 (2018)
European Food Safety Authority (EFSA). European Food Safety Authority, EFSA panel on additives and products or substances used in animal feed (FEEDAP). Scientific opinion guidance on the assessment of bacterial susceptibility to antimicrobials of human and veterinary importance. EFSA Journal. 10: 2740 (2012)
Flórez AB, Ammor MS, Mayo B. Identification of tet(M) in two Lactococcus lactis strains isolated from a Spanish traditional starter-free cheese made of raw milk and conjugative transfer of tetracycline resistance to lactococci and enterococci. International Journal of Food Microbiology. 121: 189-194 (2008)
Flórez AB, Campedelli I, Delgado S, Alegría Á, Salvetti E, Felis GE, Mayo B, Torriani S. Antibiotic susceptibility profiles of dairy Leuconostoc, analysis of the genetic basis of atypical resistances and transfer of genes in vitro and in a food matrix. PLoS ONE. 11: 1-20 (2016)
Flórez AB, Delgado S, Mayo B. Antimicrobial susceptibility of lactic acid bacteria isolated from a cheese environment. Canadian Journal of Microbiology. 51: 51-58 (2005)
Founou LL, Founou RC, Essack SY. Antibiotic resistance in the food chain: A developing country-perspective. Frontiers in Microbiology. 7: 1-19 (2016)
Fusco V, Quero GM, Cho G, Kabisch J, Meske D, Neve H, Bockelmann W, Franz CMAP. The genus Weissella : taxonomy, ecology and biotechnological potential. Frontiers in Microbiology. 6: 155 (2015)
Gazzola S, Fontana C, Bassi D Cocconcelli PS. Assessment of tetracycline and erythromycin resistance transfer during sausage fermentation by culture-dependent and -independent methods. Food Microbiology. 30: 348–354 (2012)
Generally Recognized as Safe (GRAS) | FDA. Available from: https://www.fda.gov/food/food-ingredients-packaging/generally-recognized-safe-gras. Accessed December 16, 2021
Gevers D, Huys G, Swings J. In vitro conjugal transfer of tetracycline resistance from Lactobacillus isolates to other Gram-positive bacteria. FEMS Microbiology Letters. 225: 125-130 (2003)
Gueimonde M, Sánchez B, Reyes-gavilán CGDL, Margolles A. Antibiotic resistance in probiotic bacteria. Frontiers in Microbiology. 4 (2013)
Guo H, Pan L, Li L, Lu J, Kwok L, Menghe B, Zhang H, Zhang W. Characterization of antibiotic resistance genes from Lactobacillus isolated from traditional dairy products. Journal of Food Science 82: 724-730 (2017)
György É, Laslo É, Antal M, András CD. Antibiotic resistance pattern of the allochthonous bacteria isolated from commercially available spices. Food Science and Nutrition. 9: 4550-4560 (2021)
Hammer, Øyvind, David AT Harper, Paul D. Ryan. PAST: Paleontological statistics software package for education and data analysis. Palaeontologia electronica 4:9 (2001)
Hana J, Chena D, Lic S, Lia X, Zhoud WW, Zhangb B, Jiaa Y. Antibiotic susceptibility of potentially probiotic Lactobacillus strains. Italian Journal of Food Science. 27: 282-289 (2015)
Hanchi H, Mottawea W, Sebei K, Hammami R. The genus Enterococcus: between probiotic potential and safety concerns—An Update. Frontiers in Microbiology. 9: 1791 (2018)
Jacobsen L, Wilcks A, Hammer K, Huys G, Gevers D, Andersen SR. (2007) Horizontal transfer of tet(M) and erm(B) resistance plasmids from food strains of Lactobacillus plantarum to Enterococcus faecalis JH2-2 in the gastrointestinal tract of gnotobiotic rats. FEMS Microbiology Ecology. 59: 158-166
Jang Y, Gwon H, Jeong W, Yeo S, Kim S. Safety Evaluation of Weissella cibaria JW15 by phenotypic and genotypic property analysis. Microorganisms. 9: 2450 (2021)
Kappell AD, De Nies MS, Ahuja NH, Ledeboer NA, Newton RJ, Hristova KR. Detection of multi-drug resistant Escherichia coli in the urban waterways of Milwaukee, WI. Frontiers in Microbiology. 6: 336 (2015)
Krawczyk B, Wityk P, Gałecka M, Michalik M. The many faces of Enterococcus spp.—commensal, probiotic and opportunistic pathogen. Microorganisms. 9:1900 (2021)
Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular Biology and Evolution. 33: 1870-1874 (2016)
Landers TF, Cohen B, Wittum TE, Larson EL. A review of antibiotic use in food animals: perspective, policy, and potential. Public Health Reports. 127: 4–22 (2012)
Lin J, Nishino K, Roberts MC, Tolmasky M, Aminov RI, Zhang L. Mechanisms of antibiotic resistance. Frontiers in Microbiology. 6: 2013-2015 (2015)
Mathur S, Singh R. Antibiotic resistance in food lactic acid bacteria - a review. International Journal of Food Microbiology. 105: 281-295 (2005)
Miller WR, Munita JM, Arias CA. Mechanisms of antibiotic resistance in enterococci. Expert Review of Anti-Infective Therapy. 12: 1221-1236 (2014)
Mohammed S, Çon AH. Isolation and characterization of potential probiotic lactic acid bacteria from traditional cheese. Lwt. 152: 112319 (2021)
Nataraj BH, Ramesh C, Mallappa RH. Characterization of antibiotic resistance and virulence traits present in clinical methicillin-resistant Staphylococcus aureus isolates. Current Microbiology. 78: 2001-2014 (2021)
Nawaz M, Wang J, Zhou A, Ma C, Wu X, Moore JE, Millar BC, Xu J. Characterization and transfer of antibiotic resistance in lactic acid bacteria from fermented food products. Current Microbiology. 62: 1081-1089 (2011)
Holland R, Liu SQ. Lactic acid bacteria - Leuconostoc spp. Encyclopedia of Dairy Sciences 138-142 (2011)
Nunziata L, Brasca M, Morandi S, Silvetti T. Antibiotic resistance in wild and commercial non-enterococcal lactic acid bacteria and bifidobacteria strains of dairy origin: an update. Food Microbiology. 104: 103999 (2022)
Ojha AK, Shah NP, Mishra V. Conjugal transfer of antibiotic resistances in Lactobacillus spp. Current Microbiology. 78: 2839-2849 (2021)
Pospiech A, Neumann B. A versatile quick-prep of genomic DNA from Gram-positive bacteria. Trends in Genetics. 11: 217-218 (1995)
Preethi C, Thumu SCR, Halami PM. Occurrence and distribution of multiple antibiotic-resistant Enterococcus and Lactobacillus spp. from Indian poultry: in vivo transferability of their erythromycin, tetracycline and vancomycin resistance. Annals of Microbiology. 67: 395-404 (2017)
Sachi S, Ferdous J, Sikder MH, Hussani SMAK. Antibiotic residues in milk: past, present, and future. Journal of Advanced Veterinary and Animal Research. 6: 315-332 (2019)
Saeed RM, Elyas YYA, Yousif NME, Eltayeb MM, Ahmed IAM. Incidence of antibiotic resistance of lactic acid bacteria (LAB) isolated from various Sudanese fermented foods. Journal of Food & Nutritional Disorders. 3: 6 (2014)
Stefańska I, Kwiecień E, Jóźwiak-Piasecka K, Garbowska M, Binek M, Rzewuska M. Antimicrobial susceptibility of lactic acid bacteria strains of potential use as feed additives - the basic safety and usefulness criterion. Frontiers in Veterinary Science. 8: 1-11 (2021)
Tan TY. Use of molecular techniques for the detection of antibiotic resistance in bacteria. Expert Review of Molecular Diagnostics. 3: 93-103 (2003)
Thapa SP, Shrestha S, Anal AK. Addressing the antibiotic resistance and improving the food safety in food supply chain (farm-to-fork) in Southeast Asia. Food Control. 108: 106809 (2020)
Thumu SCR, Halami PM. Conjugal transfer of erm(B) and multiple tet genes from Lactobacillus spp. to bacterial pathogens in animal gut, in vitro and during food fermentation. Food Research International. 116: 1066-1075 (2019)
Wang K, Zhang H, Feng J, Ma L, Fuente-Núñez C de la, Wang S, Lu X. Antibiotic resistance of lactic acid bacteria isolated from dairy products in Tianjin, China. Journal of Agriculture and Food Research. 1: 100006 (2019)
Von Wintersdorff CJH, Penders J, Van Niekerk JM, Mills ND, Majumder S, Van Alphen LB, Savelkoul PHM, Wolffs PFG. Dissemination of antimicrobial resistance in microbial ecosystems through horizontal gene transfer. Frontiers in Microbiology. 7: 1-10 (2016)
Zarzecka U, Chajęcka-Wierzchowska W, Zadernowska A. Microorganisms from starter and protective cultures - occurrence of antibiotic resistance and conjugal transfer of tet genes in vitro and during food fermentation. Lwt. 153: 112-490 (2022)
Zarzecka U, Zadernowska A, Wierzchowska WC. Starter cultures as a reservoir of antibiotic resistant microorganisms. Lwt. 127: 109424 (2020)
Zheng J, Wittouck S, Salvetti E, Franz CMAP, Harris HMB, Mattarelli P, O’toole PW, Pot B, Vandamme P, Walter J, Watanabe K, Wuyts S, Felis GE, Gänzle MG, Lebeer S. A taxonomic note on the genus Lactobacillus: description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. International Journal of Systematic and Evolutionary Microbiology. 70: 2782-2858 (2020)
Zonenschain D, Rebecchi A, Morelli L. Erythromycin and tetracycline resistant lactobacilli in Italian fermented dry sausages. Journal of Applied Microbiology. 107: 1559-1568 (2009)
Acknowledgements
We are thankful to Dr. Barjinder Pal Kaur, Department of Food Engineering, NIFTEM for support in PCA analysis. The technical support of Mr. Rishi Bhatia and Dr. Kuljinder Kaur, Microbiology lab is duly acknowledged.
Funding
The work is funded by the National Institute of Food Technology Entrepreneurship and Management (NIFTEM) Kundli, Sonepat (India).
Author information
Authors and Affiliations
Contributions
AKO contributed to conceiving, data acquisition and writing the manuscript. NPS carried out analysis, data interpretation and critical revision. NE contributed to refining the manuscript. VM conceived the research work. NKT analyzed, interpreted, refined and critically revised the manuscript. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
There is no declared conflict of interest.
Ethics approval
The paper does not contain any study on human participants or animals.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ojha, A.K., Shah, N.P., Mishra, V. et al. Prevalence of antibiotic resistance in lactic acid bacteria isolated from traditional fermented Indian food products. Food Sci Biotechnol 32, 2131–2143 (2023). https://doi.org/10.1007/s10068-023-01305-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-023-01305-1