Abstract
Genetic association analyses suggest that certain common single nucleotide polymorphisms (SNPs) may adversely impact recovery from traumatic brain injury (TBI). Delineating their causal relationship may aid in development of novel interventions and in identifying patients likely to respond to targeted therapies. We examined the influence of the (C/T) SNP rs1800497 of ANKK1 on post-TBI outcome using data from two prospective multicenter studies: the Citicoline Brain Injury Treatment (COBRIT) trial and Transforming Research and Clinical Knowledge in Traumatic Brain Injury Pilot (TRACK-TBI Pilot). We included patients with ANKK1 genotyping results and cognitive outcomes at six months post-TBI (n = 492: COBRIT n = 272, TRACK-TBI Pilot n = 220). Using the California Verbal Learning Test Second Edition (CVLT-II) Trial 1-5 Standard Score, we found a dose-dependent effect for the T allele, with T/T homozygotes scoring lowest on the CVLT-II Trial 1-5 Standard Score (T/T 45.1, C/T 51.1, C/C 52.1, ANOVA, p = 0.008). Post hoc testing with multiple comparison-correction indicated that T/T patients performed significantly worse than C/T and C/C patients. Similar effects were observed in a test of non-verbal processing (Wechsler Adult Intelligence Scale, Processing Speed Index). Our findings extend those of previous studies reporting a negative relationship of the ANKK1 T allele with cognitive performance after TBI. In this study, we demonstrate the value of pooling shared clinical, biomarker, and outcome variables from two large datasets applying the NIH TBI Common Data Elements. The results have implications for future multicenter investigations to further elucidate the role of ANKK1 in post-TBI outcome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Traumatic brain injury (TBI) is a complicated injury in a complex organ. Each year in the USA, at least 2.5 million people suffer TBIs. This includes 52,000 deaths, 275,000 hospitalizations, and 1.365 million treated and released from an emergency department (ED) [1]. TBI is a contributing factor to 30 % of all injury-related deaths in the USA [1]. An estimated 3.2 to 5.3 million persons currently live with long-term physical, cognitive, and neuropsychiatric disabilities attributable to TBI [2]. Heterogeneity of the primary injury is complicated by a host of patient-specific factors that together determine clinical outcome [3]. Understanding how physiological factors influence patient outcome provides an avenue for identifying methods of clinical intervention, as well as the patients most likely to benefit. The advent of the Human Genome Project and genetic association analyses has allowed the identification of several polymorphic alleles of candidate genes that may signal disparate outcomes following TBI. However, examination of large numbers of genes results in high chance of type 1 error, underscoring the need for repeat studies of larger samples and high statistical power [4].
Cognitive deficits are among the leading sources of morbidity in TBI patients, and the underlying mechanisms are poorly understood. Patients presenting with similar injuries exhibit disparate patterns of cognitive impairment. The source of this variability is presently unknown but may involve genetic modulation as well as subtle morphometric differences in injury characteristics, highlighting the importance of investigating genetic differences that modulate cognitive function [5]. Previous studies have examined genes that modulate the dopaminergic pathway, which is critical to attention, memory, and executive function. As a result, the dopaminergic system is frequently targeted, through pharmacologic manipulation, to ameliorate chronic deficits in these areas following TBI [6].
Ankyrin repeat and kinase domain-containing 1 (ANKK1) is a candidate gene involved in dopamine transmission [7, 8]. In human adults, ANKK1 mRNA and protein is expressed in the central nervous system (CNS), exclusively in astrocytes [9]. The ANKK1 protein, also known as SgK288, shares structural homology with a family of serine/threonine receptor-interacting protein kinases (RIPKs) potentially responsible for signal transduction and cellular response modulation of dopaminergic reward processes [10].
A common single nucleotide polymorphism (SNP) in the ANKK1 gene may impact outcome after TBI [11, 12]. The C/T SNP rs1800497, also known as Taq1A, is located on chromosome band 11q23.1 in exon 8 of ANKK1 and causes a p. Glu713Lys amino acid change in the C-terminal ankyrin repeat domain, which is involved in protein-protein interaction [10]. Rs1800497 is located 10 kB downstream of the DRD2 gene. While unlikely to directly control DRD2 expression, it may be located within a regulatory region for a functional SNP in the DRD2 gene [10]. Positron emission tomography (PET) studies have shown that rs1800497 affects dopamine binding in the striatum in healthy volunteers [13]. Presence of a single T allele is associated with a 30–40 % reduction of dopamine D2 receptor (DRD2) density in the ventral striatum compared to homozygotes with C alleles, suggesting that T allele carriers may require increased dopaminergic tone to achieve similar levels of reinforcement and reward as C/C individuals. Studies have shown that one or two copies of the T allele of rs1800497 associates with disorders of reward deficiency such as alcohol dependence, smoking, and addictive behavior [14–17]. McAllister et al. found that rs1800497 allele status was associated with cognitive function following mild to moderate TBI (N = 141: 93 TBI patients, 48 healthy controls) as defined by initial Glasgow Coma Scale (GCS) score of 9–15 and/or loss of consciousness (LOC) ≤24 h [11, 12]. The TBI group included 65 T-allele negative and 28 T-allele positive patients. T-allele positive patients showed worse episodic memory at 1 month post-TBI on the California Verbal Learning Test (CVLT) recognition trial, a result not observed in controls with the T allele. T-allele positive patients in the TBI group also exhibited slower performance on measures of response latency than those without the T allele [11, 12].
The present study extends this work in evaluating whether variation at rs1800497 within ANKK1 associates with verbal learning and non-verbal learning after acute TBI in a large multicenter cohort. We combined clinical and outcome data from two large prospective multicenter studies, The Citicoline Brain Injury Treatment Trial (COBRIT) [18, 19] and the Transforming Research and Clinical Knowledge in Traumatic Brain Injury Pilot (TRACK-TBI Pilot) [20] to create the largest sample size to date of adult TBI patients with rs1800497 genotyping and six-month outcome testing after acute TBI (N = 492). The merging of these two large datasets was made possible by their shared common standards—the National Institute of Neurological Disorders and Stroke (NINDS) TBI Common Data Elements (CDEs) [21]. We tested the primary hypothesis that the rs1800497 associates with reduced performance on the CVLT as previously described by McAllister et al. [11, 12] and assessed secondary endpoints and tertiary endpoints including a measure of non-verbal processing (Wechsler Adult Intelligence Scale Processing Speed Index (WAIS-PSI)).
Materials and methods
Study design
COBRIT is a multicenter, two-group, phase three, double-blind randomized placebo-controlled clinical trial conducted at eight U.S. Level I Trauma Centers [18, 19]. Inclusion criteria were patients with blunt force trauma to the head requiring inpatient hospitalization for TBI, with either: (1) Glasgow Coma Scale (GCS) score 3–12 and GCS motor score <6, or (2) GCS 3–12 with motor score 6 or GCS 13–15 or paralyzed after administration of paralytics as part of the clinical course with ≥1 of the following CT parameters: ≥10-mm diameter intraparenchymal hemorrhage, ≥5-mm extra-axial hematoma, subarachnoid hemorrhage visible on two or more 5-mm slices, or midline shift ≥5 mm. TRACK-TBI Pilot is a multicenter prospective observational study with patients recruited through convenience sampling at three U.S. Level I Trauma Centers [20]. Inclusion criteria were external force trauma to the head and clinically indicated head CT scan within 24 h of injury.
Exclusion criteria for both studies included positive pregnancy test result or known pregnancy, imminent death or current life-threatening disease, incarceration, or evidence of serious psychiatric and neurologic disorders that interfere with outcome assessment. Non-English speakers were not enrolled due to inability to participate in outcome assessments, which are normed and administered in English. The COBRIT study also excluded patients with bilaterally fixed and dilated pupils, those with prior TBI requiring hospitalization, concurrent enrollment in another study, and/or acetylcholinesterase inhibitor use within two weeks prior to injury. One trauma center (University of Pittsburgh) participated in both COBRIT and TRACK-TBI Pilot, but patients at this site were not co-enrolled into both studies.
The institutional review boards of all participating sites approved the protocols for each study. Patients were approached for informed consent before enrollment. For patients unable to give consent, due to their injury, consent was obtained from their legally authorized representative (LAR). Patients consented by LAR were approached for informed consent to continue participation while in the hospital or during follow-up assessment time-points.
These two studies enrolled a large number of TBI patients through acute and intermediate care to provide an ethnically and demographically diverse patient population. In TRACK-TBI Pilot, a comprehensive acute clinical profile was obtained from each patient in accordance with the National Institutes of Health (NIH) and NINDS CDEs across demographics, medical history, injury characteristics, acute hospital clinical care, and neuroimaging [22–26]. Enrollment in COBRIT began prior to the release of the NIH NINDS CDEs, but variables were collected in a standardized fashion with a high degree of concordance with the CDE effort [18], which enabled data pooling between the two studies. The pharmacological intervention in COBRIT consisted of daily enteral/oral citicoline (2000 mg) or placebo for 90 days. As the primary report by Zafonte et al. in 2012 found no association between citicoline use and improvement in functional and cognitive outcome [19], we did not pursue outcome analysis between treatment and control arms for this study.
Patient selection
All adult patients with complete 6-month outcomes and an acute blood biospecimen drawn for DNA were selected for this analysis from the COBRIT and TRACK-TBI Pilot studies. In both studies, patients without genotyping results and/or complete 6-month outcomes were excluded. Of 1213 total adult patients in COBRIT, 739 patients did not have blood genotyping results available and 202 of the remaining 474 patients had no or incomplete outcomes, leaving a final N of 272 patients for analysis. Of 650 total patients in TRACK-TBI Pilot, 51 patients were excluded from the non-acute TBI site and 27 patients were under the age of 18. Of the remaining 572 adult patients, 166 did not have blood genotyping results and 186 had genotyping but no or incomplete outcomes, leaving a final N of 220 for analysis. A comparison between included and excluded adult patients for this analysis, by study, is discussed in the Results section and in Online Resource 1 and 2. The distributions of demographic and clinical descriptors for COBRIT patients by treatment group are summarized in Online Resource 3.
Blood collection and genotyping
Specimen acquisition was performed as previously described [20]. In brief, blood samples for DNA genotyping analysis were collected via peripheral venipuncture or existing peripheral venous indwelling catheters within 24 h of injury. Samples were collected in BD Vacutainer K2-EDTA vacutainer tubes, and subsequently aliquoted and frozen in cryotubes at −80 °C within 1 h of collection in accordance with recommendations from the NIH-CDE Biomarkers Working Group [25]. DNA was extracted from isolated leukocytes using the Wizard® Genomic DNA Purification Kit as described by the manufacturer (Promega, Madison, WI). The ANKK1 C/T SNP (rs1800497) was genotyped utilizing TaqMan®SNP Genotyping Assay as described by the manufacturer (Applied Biosystems, Carlsbad, CA). Patients were categorized by genotype: T/T, C/T, or C/C.
Outcome measures
The primary outcome measure was the California Verbal Learning Test, Second Edition (CVLT-II) Trials 1-5 Standard Score [27], which is one of the “core” TBI CDE outcome measures and was collected in both COBRIT and TRACK-TBI Pilot [28, 29]. The CVLT-II is a verbal learning and memory task in which there are five learning trials, an interference trial, an immediate recall trial, and a post-20 min recall trial. The CVLT-II Trials 1–5 Standard Score (CVLT-TSS) is normed for age and sex, and provides a global index of verbal learning ability [27]. The Wechsler Adult Intelligence Scale processing speed index (WAIS-PSI) was used as a secondary outcome measure [30]. Tertiary outcome measures collected across both studies include the Glasgow Outcome Scale-Extended (GOSE) [31], the Satisfaction with Life Scale (SWLS) [32], the Trail Making Test (TMT) Trail B minus Trail A Score (TMT B-A) [33], and the Brief Symptom Inventory 18 (BSI18) Global Severity Index Score (BSI18 GSI) [34].
Statistical analysis
Primary analysis assessed the impact of the T allele (T/T, C/T, C/C) on the chosen cognitive outcome measures. Group differences in demographic and clinical descriptors across ANKK1 genotypes (T/T, C/T, C/C) were assessed by Pearson’s chi-squared test (X 2) for categorical variables and analysis of variance (ANOVA) for continuous variables. Row categories with average cell counts of less than 5 by ANKK1 were combined into a single row category during analysis. Fisher’s exact test was used for comparisons with more than 20 % of individual cell counts less than 5. A two-way ANOVA was performed to assess the main effects of ANKK1 dose and study cohort (COBRIT vs. TRACK-TBI) as well as their interaction on 6-month CVLT-TSS. If the interaction was not significant, significant main effects were confirmed with a two-way ANOVA omitting the interaction term, using Tukey’s post hoc test with multiple-comparison correction. Fisher’s permutation test [35] was performed as a sensitivity analysis to address the unequal distribution of ANKK1 across races. Fifty thousand permutations, within study and race, were used to evaluate the effect of ANKK1. Significance was assessed at α = 0.05 for all analyses. Fisher’s permutation test was performed using Statistical Analysis System (SAS), Version 9.4 (SAS Institute, Cary, NC). All other analyses were performed using Statistical Package for the Social Sciences (SPSS), Version 21 (IBM Corporation, Chicago, IL).
Results
Demographic and clinical descriptors
A total of 492 patients were included in the analysis (COBRIT N = 272 (55 %), TRACK-TBI Pilot N = 220 (45 %)). The overall mean age was 40 years old (standard deviation (SD) 16), and subjects were 75 % male (Table 1). The overall race distribution was 78 % Caucasian, 15 % African American/African, and 2 % or less of each of the other races. Mechanisms of injury were 35 % fall, 24 % motor vehicle accident, 16 % motorcycle/bicycle accident, 13 % assault, 7 % pedestrian struck by vehicle, 3 % struck by/against object, and 2 % other. TBI classification by emergency department (ED) arrival GCS was as follows: 21 % severe (GCS 3–8), 8 % moderate (GCS 9–12), and 71 % mild (GCS 13–15).
Comparison by study demonstrated that there was a lower proportion of African/American-African patients and higher proportions of non-Caucasian, non-African-American/African patients in TRACK-TBI Pilot (Caucasian 75 %, African-American/African 11 %, other 14 %) than in COBRIT (80, 19, and 1 %, respectively, p < 0.001). Mechanism of injury differed by study (p < 0.001) with more falls (43 vs. 28 %), fewer motor vehicle accidents (19 vs. 28 %), and fewer motorcycle/bicycle accidents (10 vs. 21 %) observed in TRACK-TBI Pilot than in COBRIT, respectively. COBRIT patients presented with lower GCS (28 % severe, 10 % moderate, 62 % mild) than TRACK-TBI Pilot patients (12 % severe, 5 % moderate, 83 % mild, p < 0.001). No differences by study were observed in age, gender, or ANKK1 genotype (Table 2).
ANKK1 genotype distribution
ANKK1 genotype distribution was 8 % T/T (N = 40), 36 % C/T (N = 175), and 56 % C/C (N = 277) consistent with the HapMap Phase III average across all races [36]. ANKK1 allelic frequencies (T = 0.26, C = 0.74) were found to be at or near Hardy–Weinberg equilibrium (p = 0.263, Pearson X 2). T allele distribution differed across races (p < 0.001) but conformed to known HapMap Phase III frequencies [36]. Distributions across the two primary race groups in this study were assessed: Caucasians (5 % T/T, 34 % C/T, 61 % C/C) did not differ from the expected CEU HapMap (5 % T/T, 28 % C/T, 66 % C/C (p = 0.291)), and African American/Africans (21 % T/T, 42 % C/T, 37 % C/C) did not differ from the expected YRI HapMap (16 % T/T, 50 % C/T, 34 % C/C (p = 0.606)). HapMap comparisons for ANKK1 were not performed for the other races due to small sample sizes of n ≤ 10. No differences in ANKK1 genotype distribution were observed by age, gender, mechanism of injury, or GCS.
Comparison of descriptors between included and excluded patients by study
In both studies, there was a higher proportion of African-American/African patients included in this analysis (COBRIT N = 270, 80 % Caucasian, 19 % African-American/African, 1 % other; TRACK-TBI Pilot N = 220, 75 % Caucasian, 11 % African-American/African, 14 % other) compared to patients not included (COBRIT N = 938, 83 % Caucasian, 13 % African-American/African, 4 % other, p = 0.033; TRACK-TBI N = 348, 85 % Caucasian, 6 % African-American, 9 % other, p = 0.043). The included COBRIT patients had less severe injuries by GCS (N = 271, 28 % severe, 11 % moderate, 62 % mild) compared to those not included (N = 936, 39 % severe, 10 % moderate, 51 % mild, p = 0.004). The included TRACK-TBI Pilot patients were younger (N = 220, mean 41, SD 16) compared to those not included (N = 352, mean 46, SD 19). No differences in other baseline descriptors or ANKK1 genotype distribution were observed between included and excluded adult patients within each study (Online Resource 1 and 2).
Comparison of descriptors between COBRIT treatment and control arms
The COBRIT patients included in this analysis (N = 272) distributed evenly across citicoline (N = 137 (50 %)) and placebo arms (N = 135 (50 %)). No differences in any demographic and clinical descriptors were observed by treatment arm (Online Resource 3).
Relationship of ANKK1 to CVLT-TSS
Our analyses were designed to address potential confounding created by pooling COBRIT and TRACK-TBI Pilot data for the effects of the following: (1) the particular study and (2) interaction between ANKK1 and the particular study on CVLT-TSS. First, we performed a two-way ANOVA with CVLT-TSS as the dependent variable to assess the main effects of ANKK1 and study, plus the interaction term ANKK1 X study. Table 3 shows that ANKK1 had a statistically significant association at α = 0.05 with CVLT-TSS (F(2,486) = 4.964, p = 0.007), while particular study and ANKK1 X study did not. We then re-ran the model, omitting the interaction term, to confirm the significant association between ANKK1 and CVLT-TSS (F(2,486) = 4.893, p = 0.008), and not between particular study and CVLT-TSS (F(1,486) = 0.117, p = 0.732). We performed Tukey’s post-hoc test for ANKK1 in the same model to assess for differences in CVLT-TSS across the three ANKK1 genotypes. Figure 1 shows the CVLT-TSS means by ANKK1, and that mean CVLT-TSS of T/T patients differed significantly from that of C/T and C/C patients, with a mean decrease of 6.0 points against C/T and 7.0 points against C/C.
Comparison of 6-month CVLT-TSS means across ANKK1 genotypes. Graph shows 6-month CVLT-TSS mean ± SE by ANKK1 genotype. Tukey’s post hoc test was used to assess mean differences (MD) in CVLT-TSS between genotypes. Only significant MDs at α = 0.05 are shown in the table. Mean difference is calculated by the mean CVLT-TSS of the first genotype (I) minus that of the second genotype (J). CVLT-TSS California Verbal Learning Test, Second Edition Trials 1–5 Standard Score, SE standard error, CI confidence interval
Based on our initial descriptive statistics (Table 1), there were subpopulation differences in the distribution of ANKK1 genotypes across races. As a sensitivity analysis, we ran Fisher’s permutation test as a distribution-free alternative to the parametric model [35]. The association between ANKK1 and six-month CVLT-TSS remained significant (p = 0.026) when controlling for race and particular study.
Exploratory analysis of ANKK1 on other outcome measures
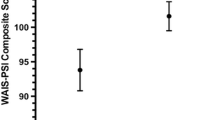
To explore the common six-month outcome measures in our pooled multicenter dataset, we assessed the association between ANKK1 genotype on a non-verbal cognitive test, the WAIS-PSI, as well as with four other measures: GOSE, SWLS, TMT B-A, BSI18 GSI. We performed identical analyses as above to assess the main effect of ANKK1 genotype and particular study, plus the interaction factor ANKK1 X study, using two-way ANOVA with each outcome measure as the dependent variable. There was a significant association at α = 0.05 between ANKK1 and WAIS-PSI (F(2,486) = 3.225, p = 0.041), and particular study and WAIS-PSI (F(1,486) = 7.01, p = 0.008), with no effect of ANKK1 X study. No significant pairwise differences at α = 0.05 were observed in WAIS-PSI means across ANKK1 (T/T: 94.1, SE 2.5; C/T: 95.9, SE 1.3; C/C: 98.8; SE 0.9) on Tukey’s post-hoc test. Mean WAIS-PSI scores in COBRIT were lower than in TRACK-TBI Pilot (COBRIT: 95.9, SE 1.0; TRACK-TBI Pilot 99.3, SE 1.1, p = 0.02). There was no significant association between ANKK1, study or ANKK1 X study with GOSE, SWLS, or TMT B-A. There was a marginal association at α = 0.05 between ANKK1 and BSI18 GSI (F(2,486) = 3.0, p = 0.052), with no effect of particular study or ANKK1 X study. On Tukey’s post hoc test, BSI18 GSI means (T/T 60.1, SE 2.1; C/T 54.7, SE 0.9; C/C 56.3, SE 0.7) differed significantly at α = 0.05 between T/T and C/T only (95 % CI 0.3 to 10.4, p = 0.036).
Discussion
Over the past decade, genetic association studies have contributed to our understanding of the molecular mechanisms of multiple common human diseases, including Alzheimer disease, heart disease, and diabetes, among others [37–42]. In each case, molecular mechanisms suspected to be involved in disease pathogenesis based on preclinical or pathologic studies were confirmed by human genetics. In addition, human genetic association studies have uncovered new molecular pathways previously unsuspected to play a role in disease pathogenesis [43–47]. The overwhelming majority of these genetic discoveries, however, have applied to disease risk [48–52]. TBI presents special challenges for genetic association studies [53]. First, there is a prominent and stochastic environmental factor: the traumatic injury. Second, premorbid personality and developmental factors play a clear role in recovery from injury. Thus, in order to identify molecular pathways in resilience to or recovery from TBI, large sample sizes and collection of comprehensive data, which allow for consideration of premorbid factors and assessment of injury severity, are essential [54, 55]. The use of CDEs is fundamental to the success of these efforts and the NIH-NINDS TBI CDEs were designed to address this need [21]. Investigators of COBRIT and TRACK-TBI Pilot were among the leaders in this effort, and the present study was feasible because of the high degree of overlap between the assessment tools and outcome measures utilized in the two studies.
Our robust sample permitted confirmation of the hypothesis concerning the effects of the T allele on cognitive outcome. Indeed, we found an association between ANKK1 and poorer performance on 6-month CVLT-TSS specifically tied to the T/T genotype. The C/T group alone did not show any differences from the C/C group on CVLT-TSS. Although this does not align perfectly with previous findings in TBI, where T-allele carriers showed worse performance on an episodic memory task of the CVLT, our overall result remains more confirmatory than divergent. McAllister et al. reported only one T/T individual in a sample size of 141, which could not enable a T-dose-dependent analysis. The distribution ANKK1 genotypes in our analysis approaches that of the general population according to HapMap Phase III and therefore allows us more statistical power to investigate the differential relationships between genotype and cognition. Secondly, it may be that differential genotypic associations with specific symptoms are more easily identified on specialized verbal memory trials such as the CVLT recognition task while the deleterious effect of a double dose of T allele manifest on the CVLT-TSS, a more highly generalizable and normative global index of verbal learning ability.
Our study reinforces the benefits of pooling multicenter trials into a unified data commons. There were no differential study effects by COBRIT and TRACK-TBI Pilot, nor were there ANKK1 X study interactions, on six-month verbal learning. This validates data sharing as a mechanism to raise statistical power for hypothesis testing and increases our confidence in the associations of ANKK1 T/T with verbal learning across a large, heterogeneous TBI population.
As well, merging COBRIT and TRACK-TBI Pilot data effectively captures patients across the entire TBI spectrum. As COBRIT excluded patients with GCS 13–15 presenting with negative head CTs, it targeted patients with more moderate and severe TBI whereas TRACK-TBI Pilot enrolled patients with similar TBI incidence as reported in literature and the population, which is predominantly mild [56, 57]. Indeed, COBRIT patients in the current analysis presented with more severe TBI compared to TRACK-TBI Pilot, and this difference may account for the observed differences by study in some of our analyses of secondary outcomes. For example, the study effect on WAIS-PSI scores reached significance. It is also interesting to note the marginal signal of ANKK1 T/T with the BSI18 GSI, which corroborates the range of studies interrogating ANKK1 in the context of neuropsychiatric disorders.
Our study has clarified several key areas identified by McAllister et al. as areas of further investigation concerning the relationship of ANKK1 with TBI outcome [11, 12]. The authors questioned whether their results would hold in a larger, more diverse racial and ethnic population, with varying injury severity and in outcomes at a longer post-injury interval. By utilizing two multicenter studies (COBRIT: eight centers, TRACK-Pilot: three centers, one center participated in both studies), the sample size was expanded to encompass a total of 10 Level I trauma centers across the USA. This heterogeneous population covers the full severity spectrum from concussion to coma, which previous studies did not have an opportunity to evaluate in the context of ANKK1. Regarding outcomes, McAllister et al. were only able to access CVLT at 1-month post-injury and expressed concern about generalizability at later timepoints. With a larger multicenter sample and long-term follow-up (the 6-month clinical standard), the present study is more resilient to local demographic and practice effects providing a strong replication test of McAllister et al.’s results.
Limitations
Although we have improved upon the breadth and generalizability of previous studies, we recognize several limitations in the current analysis. First, we could not fully account for the impact of TBI pathology and lesion types on recovery, the lack of pre-injury psychometric tests, other genetic predispositions, and non-TBI control groups. As our primary analysis was confirmatory in nature, we pursued similar inclusion criteria as McAllister et al. for general TBI and did not explore the structure-function implications of ANKK1 with intracranial lesion types or baseline mental health variables. Given the heterogeneity of TBI, subjects may never be perfectly matched by type, location, and extent of injury. Despite this fact, convincing evidence of genetic association can be clarified by sufficiently large sample sizes. The ability to comment on causative or confounding relationships between ANKK1 and pre- or post-injury risk factors is beyond the scope of the current analysis. As T/T has been associated with propensity for addiction and poor coping strategies [8, 14–17, 58, 59], the acquisition and analysis of detailed pre-injury addictive behavior, post-acute treatment, and recovery variables are relevant next steps in delineating the contribution of ANKK1 to both TBI risk and outcome variability. We are also constrained by the lack of genome-wide data, which makes it difficult to fully control for population stratification, as evidenced by the observed differences for patients who met the inclusion criteria for this analysis compared their excluded counterparts in COBRIT and TRACK-TBI Pilot. The proportion of T/T within our sample is still rather small, limiting our ability to assess whether there is a differential influence of ANKK1 genotypes on other domains of outcome, or in different races. The robustness of the association between ANKK1 and a given outcome domain such as working memory or processing speed, which encompasses multiple individual outcome measures, can be interrogated using multivariate integration and correlated with specific injuries in the dorsolateral prefrontal cortex—where working memory processes are known to be confined [60–62]. Work of this type is ongoing in the TRACK-TBI consortium.
In analyzing patients with full outcomes, there is an inherent risk of selecting for patients able to return for follow-up. For example, in our study, the COBRIT patients with genotyping and complete six-month outcomes presented with less severe injuries than those who had incomplete outcomes. This may be attributable in part to better cognition and functional ability to return for follow-up. As observed in TRACK-TBI Pilot, patients of younger age may be more mobile and/or available to return for full outcomes assessment. In some ways, the selection bias relates to the primary goal of this analysis, which was to assess the association of ANKK1 with outcome measures common to both studies and hence contingent on patients with valid scores. It is difficult to capture reasons for incomplete outcomes in patients who are lost to follow-up, as in many cases contact is never made.
The molecular mechanism and active location of ANKK1 remains a topic of ongoing study, with further experiments needed in cellular and animal models, as well as human trials. There is a need to examine gene-gene interaction with other loci of susceptibility for prognostic phenotyping within the dopaminergic system to elucidate an ANKK1 molecular pathway in local CNS physiology, contingent on detailed structure-function analysis from the comprehensive mapping of the human connectome [63]. Alternatives to the limitations of conventional imaging modalities such as CT are being explored with TRACK-TBI Pilot data. Early results indicate that prediction models including contusion on 3T MRI and axonal injury by diffusion tensor imaging (DTI) surpass other predictors for global outcome prediction in a subset of patients after mild TBI [64]. Advanced diffusion imaging modalities targeting the dorsal prefrontal cortex have been reported for healthy and diseased states [65–69]. Increased precision in characterizing regional pathophysiology will enable more objective control of injury type and severity in order to distill the specific mechanism by which ANKK1 modulates working memory, as a subset of the disparate patterns of cognitive impairment observed in the current TBI classification system of mild, moderate, and severe. In a broader sense, further development of classification approaches based on quantitative morphometry [70], in conjunction with appropriate computational methods [71] and data integration processes [72], will aid in deconstructing the contribution of genetic modulation to multidimensional domains of outcome after TBI.
Greater sample size and more extensive genotyping will overcome our current limitations to allow for stratification across known genetic profiles and TBI severities, as well as raise statistical power to levels appropriate for phase III clinical trials. We successfully pooled COBRIT and TRACK-TBI Pilot data through outcome measures common to both studies, but we were still constrained in our scope of data pooling. Clearer evaluations of the effects of risk factors and predictors of TBI outcome, including ANKK1 and other SNPs, await the expanded initiatives of current multicenter studies such as the Transforming Research and Clinical Knowledge in TBI study (TRACK-TBI) [73] and the Collaborative European NeuroTrauma Effectiveness Research in TBI study (CENTER-TBI) [74], which will enroll 3000 and 5000 patients with controls, respectively, over the next five years, using the expanded Version 2 of the NIH-NINDS TBI CDEs [21, 75]. Adopting an international approach [76] to this standardized set of variables with wide scope, utility, and applicability will allow us to converge and leverage research efforts to achieve the sample sizes we truly need for delineating the effects of the ANKK1 polymorphism in TBI.
Conclusions
In the largest prospective multicenter study to date examining the incidence of the rs1800497 SNP in TBI, enabled by data pooling of shared common variables, we report that the ANKK1 T/T genotype associates with poorer verbal learning performance on CVLT-TSS at six months post-injury across the spectrum of TBI severity. With the augmented statistical power of this analysis, successful replication of the association between ANKK1 and cognition reinforces the potential implication of a DRD2-dependent biological mechanism underlying cognitive performance after TBI.
References
Faul M, Xu L, Wald MM, Coronado VG (2010) Traumatic brain injury in the United States: emergency department visits, hospitalizations and deaths 2002-2006. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control, Atlanta, GA
Thurman DJ, Alverson C, Dunn KA, Guerrero J, Sniezek JE (1999) Traumatic brain injury in the United States: a public health perspective. J Head Trauma Rehabil 14:602–615
Saatman KE, Duhaime A-C, Bullock R, Maas AI, Valadka A, Manley GT (2008) Classification of traumatic brain injury for targeted therapies. J Neurotrauma 25:719–738
Diaz-Arrastia R, Baxter VK (2006) Genetic factors in outcome after traumatic brain injury: what the human genome project can teach us about brain trauma. J Head Trauma Rehabil 21:361–374
McAllister TW, Flashman LA, Sparling MB, Saykin AJ (2004) Working memory deficits after traumatic brain injury: catecholaminergic mechanisms and prospects for treatment—a review. Brain Inj 18:331–350
Bales JW, Wagner AK, Kline AE, Dixon CE (2009) Persistent cognitive dysfunction after traumatic brain injury: a dopamine hypothesis. Neurosci Biobehav Rev 33:981–1003
Jonsson EG, Nothen MM, Grunhage F, Farde L, Nakashima Y, Propping P, Sedvall GC (1999) Polymorphisms in the dopamine D2 receptor gene and their relationships to striatal dopamine receptor density of healthy volunteers. Mol Psychiatry 4:290–296
Noble EP (2003) D2 dopamine receptor gene in psychiatric and neurologic disorders and its phenotypes. Am J Med Genet B Neuropsychiatr Genet 116:103–125
Hoenicka J, Quinones-Lombrana A, Espana-Serrano L, Alvira-Botero X, Kremer L, Perez-Gonzalez R, Rodriguez-Jimenez R, Jimenez-Arriero MA, Ponce G, Palomo T (2010) The ANKK1 gene associated with addictions is expressed in astroglial cells and upregulated by apomorphine. Biol Psychiatry 67:3–11
Neville MJ, Johnstone EC, Walton RT (2004) Identification and characterization of ANKK1: a novel kinase gene closely linked to DRD2 on chromosome band 11q23.1. Hum Mutat 23:540–545
McAllister TW, Rhodes CH, Flashman LA, McDonald BC, Belloni D, Saykin AJ (2005) Effect of the dopamine D2 receptor T allele on response latency after mild traumatic brain injury. Am J Psychiatry 162:1749–1751
McAllister TW, Flashman LA, Harker Rhodes C, Tyler AL, Moore JH, Saykin AJ, McDonald BC, Tosteson TD, Tsongalis GJ (2008) Single nucleotide polymorphisms in ANKK1 and the dopamine D2 receptor gene affect cognitive outcome shortly after traumatic brain injury: a replication and extension study. Brain Inj 22:705–714
Hirvonen M, Laakso A, Nagre K, Rinne JO, Pohjalainen T, Hietala J (2004) C957T polymorphism of the dopamine D2 receptor (DRD2) gene affects striatal DRD2 availability in vivo. Mol Psychiatry 9:1060–1061
Comings DE, Comings BG, Muhleman D, Dietz G, Shabahrami B, Tast D, Knell E, Kocsis P, Baumgarten R, Kovacs BW, Levy DL, Smith M, Borison RL, Durrell Evans D, Klein DN, MacMurray J, Tosk JM, Sverd J, Gysin R, Flanagan SD (1991) The dopamine D2 receptor locus as a modifying gene in neuropsychiatric disorders. J Am Med Assoc 266:1793–1800
Munafo MR, Matheson IJ, Flint J (2007) Association of the DRD2 gene Taq1A polymorphism and alcoholism: a meta-analysis of case-control studies and evidence of publication bias. Mol Psychiatry 12:454–461
Smith L, Watson M, Gates S, Ball D, Foxcroft D (2008) Meta-analysis of the association of the Taq1A polymorphism with the risk of alcohol dependency: a HuGE gene-disease association review. Am J Epidemiol 167:125–138
David SP, Strong DR, Munafo MR, Brown RA, Lloyd-Richardson EE, Wileyto PE, Evins EA, Shields PG, Lerman C, Niaura R (2007) Bupropion efficacy for smoking cessation is influenced by the DRD2 Taq1A polymorphism: analysis of pooled data from two clinical trials. Nicotine Tob Res 9:1251–1257
Zafonte R, Friedewald WT, Lee SM, Levin B, Diaz-Arrastia R, Ansel B, Eisenberg H, Timmons SD, Temkin N, Novack T, Ricker J, Merchant R, Jallo J (2009) The citicoline brain injury treatment (COBRIT) trial: design and methods. J Neurotrauma 26:2207–2216
Zafonte RD, Bagiella E, Ansel BM, Novack TA, Friedewald WT, Hesdorffer DC, Timmons SD, Jallo J, Hl E, Hart T, Ricker JH, Diaz-Arrastia R, Merchant RE, Temkin NR, Melton S, Dikmen SS (2012) Effect of citicoline on functional and cognitive status among patients with traumatic brain injury: Citicoline Brain Injury Treatment Trial (COBRIT). JAMA 308:1993–2000
Yue JK, Vassar MJ, Lingsma H, Cooper SR, Yuh EL, Mukherjee P, Puccio AM, Gordon W, Okonkwo DO, Valadka A, Schnyer DM, Maas A, Manley GT; TRACK-TBI Investigators. Transforming research and clinical knowledge in traumatic brain injury pilot: multicenter implementation of the common data elements for traumatic brain injury. J Neurotrauma 30:1831–1844
“Traumatic Brain Injury Standards” (2014) National Institute of Neurological Disorders and Stroke Common Data Elements. http://www.commondataelements.ninds.nih.gov/tbi.aspx. Accessed 15 Jul 2014
Menon DK, Schwab K, Wright DW, Maas AI et al (2010) Position statement: definition of traumatic brain injury. Arch Phys Med Rehabil 91:1637–1640
Maas AI, Steyerberg EW, Marmarou A, McHugh GS, Lingsma HF, Butcher I, Lu J, Weir J, Roozenbeek B, Murray GD (2010) IMPACT recommendation for improving the design and analysis of clinical trials in moderate to severe traumatic brain injury. Neurotherapeutics 7:127–134
Duhaime AC, Gean AD, Haacke EM, Hicks R, Wintermark M, Mukherjee P, Brody D, Latour L, Riedy G, Common Data Elements Neuroimaging Working Group Members, Pediatric Working Group Members (2010) Common data elements in radiologic imaging of traumatic brain injury. Arch Phys Med Rehabil 91:1661–1666
Manley GT, Diaz-Arrastia R, Brophy M, Engel D, Goodman C, Gwinn K, Veenstra TD, Ling G, Ottens AK, Tortella F, Hayes RL (2010) Common data elements for traumatic brain injury: recommendations from the biospecimens and biomarkers working group. Arch Phys Med Rehabil 91:1667–1672
Maas AI, Harrison-Felix CL, Menon D, Adelson PD, Balkin T, Bullock R, Engel DC, Gordon W, Langlois-Orman J, Lew HL, Robertson C, Temkin N, Valadka A, Verfaellie M, Wainwright M, Wright DW, Schwab K (2011) Standardizing data collection in traumatic brain injury. J Neurotrauma 28:177–187
Delis DC, Kramer JH, Kaplan E, Ober BA (2000) California Verbal Learning Test, Second Edition. Psychological Corporation, San Antonio, TX
Whyte J, Vasterling J, Manley GT (2010) Common data elements for research on traumatic brain injury and psychological health: current status and future development. Arch Phys Med Rehabil 91:1692–1696
Wilde EA, Whiteneck GG, Bogner J, Bushnik T, Cifu DX, Dikmen S, French L, Giacino JT, Hart T, Malec JF, Millis SR, Novack TA, Sherer M, Tulsky DS, Vanderploeg RD, von Steinbuechel N (2010) Recommendations for the use of common outcome measures in traumatic brain injury research. Arch Phys Med Rehabil 91:1650–1660
Wechsler D (2008) Wechsler Adult Intelligence Scale, Fourth Edition. Psychological Corporation, San Antonio, TX
Wilson JT, Pettigrew LE, Teasdale GM (1998) Structured interviews for the Glasgow outcome scale and the extended Glasgow outcome scale: guidelines for their use. J Neurotrauma 15:573–585
Diener E, Emmons RA, Larsen RJ, Griffin S (1985) The satisfaction with life scale. J Pers Assess 49:71–75
Reitan RM (1958) Validity of the Trail Making Test as an indicator of organic brain damage. Percept Mot Skills 8:271–276
Derogatis LR (2000) Brief symptom inventory 18 administration, scoring, and procedures manual. Pearson Inc, Minneapolis, MN
Fisher RA (1936) Coefficient of racial likeness and the future of craniometry. J Roy Anthrop Soc 66:57–63
“Reference SNP (refSNP) Cluster Report: rs1800497”. (2014) National Center for Biotechnology Information. [http://www.ncbi.nlm.nih.gov/projects/SNP/snp_ref.cgi?rs=1800497]. Accessed 15 Jul 2014
Ku CS, Loy EY, Pawitan Y, Chia KS (2010) The pursuit of genome-wide association studies: where are we now? J Hum Genet 55:195–206
Wang Q (2005) Molecular genetics of coronary artery disease. Curr Opin Cardiol 20:182–188
Ross OA, Worrall BB, Meschia JF (2007) Advancing stroke therapeutics through genetic understanding. Curr Drug Targets 8:850–859
Tsai CT, Lai LP, Hwang JJ, Lin JL, Chiang FT (2008) Molecular genetics of atrial fibrillation. J Am Coll Cardiol 52:241–250
Wheeler E, Barrosso I (2011) Genome-wide association studies and type 2 diabetes. Brief Funct Genomics 10:52–60
Tosto G, Reitz C (2013) Genome-wide association studies in Alzheimer’s disease: a review. Curr Neurol Neurosci Rep 13:381
Wang Q (2005) Advances in the genetic basis of coronary artery disease. Curr Atheroscler Rep 7:235–241
Cresci S (2008) From SNPs to functional studies in cardiovascular pharmacogenomics. Methods Mol Biol 448:379–393
Van de Bunt M, Gloyn AL (2010) From genetic association to molecular mechanism. Curr Diab Rep 10:452–466
Moraes CF, Lins TC, Carmargos EF, Naves JO, Pereira RW, Nobrega OT (2012) Lessons from genome-wide association studies findings in Alzheimer’s disease. Psychogeriatrics 12:62–73
Brunetti A, Chiefari E, Foti D (2014) Recent advances in the molecular genetics of type 2 diabetes mellitus. World J Diabetes 5:128–140
Gulcher JR, Gretarsdottir S, Helgadottir A, Stefansson K (2005) Genes contributing to risk for common forms of stroke. Trends Mol Med 11:217–224
Bettens K, Sleegers K, Van Broeckhoven C (2013) Genetic insights in Alzheimer’s disease. Lancet Neurol 12:92–104
Gershon ES, Alliey-Rodriguez N, Liu C (2011) After GWAS: searching for genetic risk for schizophrenia and bipolar disorder. Am J Psychiatry 168:253–256
Doris PA (2012) Genetic susceptibility to hypertensive renal disease. Cell Mol Life Sci 69:3751–3763
Van der sijde MR, Ng A, Fu J (2014) Systems genetics: From GWAS to disease pathways. Biochem Biophys Acta May 2. Epub ahead of print
Diaz-Arrastia R, Baxter VK (2006) Genetic factors in outcome after traumatic brain injury: what the human genome project can teach us about brain trauma. J Head Trauma Rehabil 21:361–374
Jordan BD (2007) Genetic influences on outcome following traumatic brain injury. Neurochem Res 32:905–915
Dardiotis E, Fountas KN, Dardioti M, Xiromerisiou G, Kapsalaki E, Tasiou A, Hadjigeorgiou GM (2010) Genetic association studies in patients with traumatic brain injury. Neurosurg Focus 28:E9
Cassidy JD, Carroll LJ, Peloso PM, Borg J, von Holst H, Holm L, Kraus J, Coronado VG, WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury (2004) Incidence, risk factors and prevention of mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehab Med 43(Suppl):28–60
Borg J, Holm L, Cassidy JD, Peloso PM, Carroll LJ, von Holst H, Ericson K; WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury (2004) Diagnostic procedures in mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med (43 Suppl):61–75
Ponce G, Jimenez-Arriero MA, Rubio G, Hoenicka J, Ampuero I, Ramos JA, Palomo T (2003) The A1 allele of the DRD2 gene (TaqI A polymorphisms) is associated with antisocial personality in a sample of alcohol-dependent patients. Eur Psychiatry 18:356–360
Klein TA, Neumann J, Reuter M, Hennig J, von Cramon DY, Ullsperger M (2007) Genetically determined differences in learning from errors. Science 318:1642–1645
NcNab F, Klingberg T (2008) Prefrontal cortex and basal ganglia control access to working memory. Nat Neurosci 11(1):103–107
Baier B, Karnath HO, Dieterich M, Birklein F, Heinze C, Muller NG (2010) Keeping memory clear and stable—the contribution of human basal ganglia and prefrontal cortex to working memory. J Neurosci 30(29):9788–9792
Van Hecke J, Gladwin TE, Coremans J, Destoop M, Hulstijn W, Sabbe B (2010) Prefrontal, parietal and basal activation associated with the reordering of a two-element list held in working memory. Biol Psychol 85(1):143–148
Toga AW, Clark KA, Thompson PM, Shattuck DW, Van Horn JD (2012) Mapping the human connectome. Neurosurgery 71(1):1–5
Yuh EL, Cooper SR, Mukherjee P, Yue JK, Lingsma HF, Gordon WA, Valadka AB, Okonkwo DO, Schnyer DM, Vassar MJ, Maaas AI, Manley GT, Investigators TRACK-TBI (2014) Diffusion tensor imaging for outcome prediction in mild traumatic brain injury: a TRACK-TBI study. J Neurotrauma 31(17):1457–1477
Malykhin N, Concha L, Seres P, Beaulieu C, Coupland NJ (2008) Diffusion tensor imaging tractography and reliability analysis for limbic and paralimbic white matter tracts. Psychiatry Res 164(2):132–142
Takahashi E, Ohki K, Kim DS (2013) Dissociation and convergence of the dorsal and ventral visual working memory streams in the human prefrontal cortex. Neuroimage 65:488–498
Zhang K, Johnson B, Pennell D, Ray W, Sebastianelli W, Slobounov S (2010) Are functional deficits in concussed individuals consistent with white matter structural alterations: a combined FMRI and DTI study. Exp Brain Res 204(1):57–70
Zarei M, Patenaude B, Damoiseaux J, Morgese C, Smith S, Matthews PM, Barkhof F, Rombouts SA, Sanz-Argita E, Jenkinson M (2010) Combining shape and connectivity analysis: an MRI study of thalamic degeneration in Alzheimer’s disease. Neuroimage 49(1):1–8
Rose SE, Chalk JB, Janke AL, Strudwick MW, Windus LC, Hannah DE, McGrath JJ, Pantelis C, Wood SJ, Mory BJ (2006) Evidence of altered prefrontal-thalamic circuitry in schizophrenia: an optimized diffusion MRI study. Neuroimage 32(1):16–22
Yuh EL, Cooper SR, Ferguson AR, Manley GT (2012) Quantitative CT improves outcome prediction in acute traumatic brain injury. J Neurotrauma 29(5):735–746
Lum PY, Singh G, Lehman A, Ishkanov T, Vejdemo-Johansson M, Alagappan M, Carlsson J, Carlsson G (2013) Extracting insights from the shape of complex data using topology. Sci Rep 3:1236
Sorani MD, Ortmann WA, Bierwagen EP, Behrens TW (2010) Clinical and biological data integration for biomarker discovery. Drug Discov Today 15(17–18):741–748
Norris J (2013) “Traumatic Brain Injury Research Advances with $18.8 M NIH Award.” University of California, San Francisco. http://www.ucsf.edu/news/2013/10/109851/traumatic-brain-injury-research-advances-188-million-nih-award-administered-ucsf. Accessed 15 Jul 2014
“CENTER-TBI” (2014) https://www.center-tbi.eu. Accessed 15 Jul 2014
Hicks R, Giacino J, Harrison-Felix C, Manley G, Valadka A, Wilde EA (2013) Progress in developing common data elements for traumatic brain injury research: version two—the end to the beginning. J Neurotrauma 30:1852–1860
Manley GT, Maas AI (2013) Traumatic brain injury: an international knowledge-based approach. JAMA 310:473–474
Acknowledgments
The authors would like to thank the COBRIT and TRACK-TBI Investigators, without whom this work would not have been possible. The authors would like to thank the following contributors to the development of the TRACK-TBI database and repositories by organization and alphabetical order by last name -- QuesGen Systems, Inc.: Vibeke Brinck, MS, and Michael Jarrett, MBA; One Mind: General Peter Chiarelli, U.S. Army (Ret.), and Garen Staglin; and Thomson Reuters: Sirimon O’Charoen, PhD. This work was supported by the following Grant Numbers: NIH U01 HD42652, NIH R01 HD48179 (to R.D.-A.), and NIH RC2 NS0694909, DOD USAMRAA W81XWH-13-1-0441 (to G.T.M.).
Compliance with Ethical Standards
ᅟ
Disclosure of Potential Conflicts of Interest
The authors declare that they have no conflicts of interest.
Research Involving Human Participants
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
John K. Yue and Angela M. Pronger contributed equally to the manuscript
The COBRIT Investigators and the TRACK-TBI Investigators are listed in the Appendix in alphabetical order by last name
Registry: ClinicalTrials.gov Identifier NCT01565551
Electronic supplementary material
Below is the link to the electronic supplementary material.
Online Resource 1
(PDF 69 kb)
Online Resource 2
(PDF 70 kb)
Online Resource 3
(PDF 67 kb)
Appendix
Appendix
COBRIT investigators
The following are COBRIT investigators: Howard M. Eisenberg, MD (Department of Neurosurgery, University of Maryland School of Medicine, Baltimore, MD), Jack Jallo, MD, PhD (Temple University Hospital and Moss Rehabilitation Research Institute, Philadelphia, PA; Division of Neurotrauma and Critical Care, Thomas Jefferson University, Philadelphia, PA), Randall E. Merchant, PhD (Department of Neurosurgery, Virginia Commonwealth University, Richmond, VA), Thomas A. Novack, PhD (Department of Physical Medicine and Rehabilitation, University of Alabama at Birmingham, Birmingham, AL), Joseph H. Ricker, PhD (University of Pittsburgh School of Medicine, Pittsburgh, PA), Shelly D. Timmons, MD, PhD (Department of Neurosurgery, Geisinger Medical Center, Danville, PA; University of Tennessee Health Science Center, Memphis, TN), and Ross D. Zafonte, DO (Department of Physical Medicine and Rehabilitation, Harvard Medical School and Massachusetts General Hospital, Boston, MA).
TRACK-TBI investigators
The following are TRACK-TBI investigators: Kristen Dams-O’Connor, PhD (Department of Rehabilitation Medicine, Mount Sinai School of Medicine, New York, NY), Wayne A. Gordon, PhD (Department of Rehabilitation Medicine, Mount Sinai School of Medicine, New York, NY), Allison J. Hricik, MS (Department of Neurological Surgery, University of Pittsburgh Medical Center, Pittsburgh, PA), Andrew I. R. Maas, MD, PhD (Department of Neurosurgery, Antwerp University Hospital, Edegem, Belgium), David K. Menon, MD, PhD (Division of Anaesthesia, University of Cambridge, Addenbrooke’s Hospital, Cambridge, United Kingdom), Diane J. Morabito, RN, MPH (Department of Neurological Surgery, University of California, San Francisco, San Francisco, CA), Pratik Mukherjee, MD, PhD (Department of Radiology, University of California, San Francisco, San Francisco, CA), David M. Schnyer, PhD (Department of Psychology, University of Texas at Austin, Austin, TX), Alex B. Valadka, MD (Seton Brain and Spine Institute, Austin, TX), Mary J. Vassar, RN, MS (Department of Neurosurgery, University of California, San Francisco, San Francisco, CA), and Esther L. Yuh, MD, PhD (Department of Radiology, University of California, San Francisco, San Francisco, CA).
Rights and permissions
About this article
Cite this article
Yue, J.K., Pronger, A.M., Ferguson, A.R. et al. Association of a common genetic variant within ANKK1 with six-month cognitive performance after traumatic brain injury. Neurogenetics 16, 169–180 (2015). https://doi.org/10.1007/s10048-015-0437-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10048-015-0437-1