Abstract
Objective
To systematically compare the tacker mesh fixation (TMF) with the suture mesh fixation (SMF) in laparoscopic incisional and ventral hernia (LIVH) repair.
Methods
Trials evaluating the TMF with the SMF in LIVH repair were analysed using the statistical tool RevMan®. Combined dichotomous and continuous data were expressed as odds ratio (OR) and mean difference (MD), respectively.
Results
Four trials (2 randomised and 2 non-randomised) encompassing 207 patients undergoing LIVH repair with TMF versus SMF were retrieved from the standard electronic databases and analysed systematically. Ninety-nine patients underwent TMF and 108 patients underwent SMF in LIVH repair. There was no statistically significant heterogeneity (p = 0.27)] among trials. In the fixed-effects model, LIVH repair with TMF was associated with shorter operation time (MD, −23.65; 95 % CI, −31.06, −16.25; z = 6.26; p < 0.00001). Four- to six-week postoperative pain score was significantly lower (MD, −0.69; 95 % CI, −1.16, −0.23; z = 2.92; p < 0.004) following TMF. Peri-operative complications (p = 0.65), length of hospital stay (p = 1) and risk of hernia recurrence (OR, 1.54; 95 % CI, 0.38, 6.27; z = 0.61; p = 0.54) following TMF and SMF were statistically not different.
Conclusion
TMF in LIVH repair is associated with shorter operative time and lesser postoperative pain. TMF is comparable with SMF in terms of peri-operative complications, length of hospital stay and hernia recurrence. Therefore, TMF may be used in LIVH repair. However, further randomised trials recruiting higher number of patients are required to validate these findings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Primary ventral hernia (umbilical hernia, para-umbilical hernia, epigastric hernia and spigelian hernia) is a common surgical disorder which may or may not require surgical repair depending on the size and consequent symptoms. Secondary ventral hernia commonly known as an incisional hernia has a reported incidence of 3–15 % of patients undergoing laparotomy and laparoscopy [1–6]. In the USA, approximately 4–5 million laparotomies are performed annually [4], leading to at least 400,000–500,000 incisional hernias, of which approximately 200,000 repairs are performed [4, 7]. Estimated rate of incisional/ventral hernia repair is about 300,000 per annum in Europe. In terms of success rate, mortality, morbidity and recurrence rate, the superiority of laparoscopic incisional/ventral hernia (LIVH) repair as opposed to traditional open repair has been widely accepted in surgical fraternity after the publications of at least seven systematic reviews including one Cochrane review [8–14] over a span of last 9 years. Although LIVH repair has become almost a universal standard, but significant controversy still exists regarding the management of large hernial sac, postoperative seroma, mesh selection and technique of intra-abdominal mesh fixation. In addition, patients undergoing LIVH repair tend to have higher pain score in early postoperative pain than after any other laparoscopic abdominal procedure [15–18]. Etiology and pathogenesis of postoperative complications is being investigated extensively leading to the introduction of new generation of meshes (lightweight) and new approaches for mesh fixation.
In LIVH repair, various mesh fixation techniques have been reported including tacker mesh fixation (TMF), suture mesh fixation (SMF), combined TMF and SMF using either absorbable or non-absorbable sutures, resorbable fixation devices and fibrin glue [19–24]. TMF and SMF are two most commonly used techniques but both are associated with variable risk of postoperative complications. TMF is associated with relatively weaker fixation because tacker clips do not fix mesh with muscle and fascia and it merely penetrates through few millimeters of inner abdominal wall and peritoneum which potentially may lead to partial or complete mesh dislodgment leading to hernia recurrence [25]. Since SMF penetrates through full thickness of muscle and fascial sheath, therefore, the tensile strength of SMF has shown to be up to 2.5 times more than TMF [25]. TMF may also contribute into the hernia recurrence due to tacker-induced mesh shrinkage [20]. Other reported implications of spiral tacks include intestinal erosion, intestinal fistula formation and intra-abdominal adhesions leading to small bowel obstruction [26, 27]. SMF is also an effective mesh fixation technique but has been reported with prolonged postoperative pain, longer operative time, prolonged hospital stay and hernia recurrence through fixation holes [28–30].The objective of this article is to systematically analyse the role of TMF with SMF in LIVH repair in terms of operation time, postoperative pain, surgical site infection, peri-operative complications, length of hospital stay and recurrence of incisional and ventral hernia.
Methods
Relevant randomised and non-randomised controlled trials (irrespective of type, language, blinding, sample size or publication status) evaluating the role of TMF with SMF during LIVH repair until July 2012 were included in this review. The Cochrane Colorectal Cancer Group (CCCG) Controlled Trial Register, the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library, Medline, Embase and Science Citation Index Expanded were searched until July 2012 using the medical subject headings (MeSH) terms “incisional hernia” and “ventral hernia.” These headings were used in combination with “laparoscopic repair,” “minimal invasive surgery,” “surgical mesh,” “mesh,” “suture fixation” and “tacker fixation.” The “related article” function was used to widen the search criteria. All abstracts, comparative studies, non-randomised trials and citations scanned were reviewed in order to get the maximum results by comprehensive literature search. A filter for identifying relevant studies recommended by the Cochrane collaboration [31] was used to filter out irrelevant studies in Medline and Embase. The references of the included studies were searched to identify further trials. We included all types of comparative trials evaluating the role of TMF with SMF in LIVH repair. Two authors independently identified the relevant studies for inclusion: extracted data related to the outcomes and secured data on a Microsoft Excel spread sheet. Conflict about data was resolved by mutual agreement among authors. The software package RevMan 5.0.1 [32] provided by the Cochrane collaboration was used for analysis. The odds ratio (OR) with a 95 % confidence interval (CI) was calculated for binary data variables. If the standard deviation was not available in case of continuous variables, it was calculated according to the guidelines of the Cochrane collaboration [31]. This involved assumptions that both groups have the same variance, which may not be true. The random-effects model [33] and the fixed-effects model [34] were used to calculate the combined outcome in both binary and continuous variables. In cases of heterogeneity, only the results of the random-effects model were reported. Heterogeneity was explored using the χ2 test, with significance set at p < 0.05, and quantified using I 2 [35] test [31]. The Mantel–Haenszel method was used for the calculation of OR under the fixed- and random-effect models [36]. In a sensitivity analysis, 0.5 was added to each cell frequency for trials in which no event occurred in either the treatment or control group, according to the method recommended by Deeks et al. [37]. The estimate of the difference between both techniques was pooled depending upon the effect weights in results determined by each trial estimate variance. A forest plot was used for the graphical display of results from the meta-analysis. The square around the estimate stood for the accuracy of the estimation (sample size) and the horizontal line represented the 95 % CI.
Results
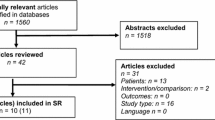
Figure 1 depicts the literature search strategy, methodology and trial selection based on the published trials. Four trials (two randomised and 2 non-randomised) [19–22] on 207 patients undergoing LIVH repair were retrieved from the electronic databases. Ninety-nine patients of LIVH repair underwent TMF and 108 patients underwent SMF. The important features of included trials are given in Table 1. Extracted data of the reported variables used to achieve a combined outcome are given in Table 2. In all four included studies, non-absorbable tacks were used to fix mesh. The data pertaining to different types of tacks were not reported and therefore it was not possible to generate a combined outcome depending upon type of the tacks.
Methodological quality of included studies
The methodological quality of included randomised, controlled trials was assessed by the published guidelines of Jadad et al. [38] and Chalmers et al. [39]. The methodological quality of included non-randomised trials was assessed by the published guidelines of Scottish Intercollegiate Guidelines Network (SIGN) and Rangel et al. [40, 41]. Based on the quality of included trials [19–22], the strength and the summary of evidence was further evaluated by GradePro® [42], a statistical tool provided by Cochrane collaboration (Fig. 2). The Mantel–Haenszel fixed-effects model was used to compute robustness and susceptibility to an outlier among these trials. The allocation concealment and blinding of investigator or assessor were not clearly reported.
Operative time
Statistically, there was no significant heterogeneity [χ2 = 7.41, df = 3, (p = 0.06); I 2 = 59 %] among four trials. Therefore, in the fixed-effects model (MD, −10; 95 % CI, −19.77, −0.23; z = 6.20; p < 0.00001; Fig. 3), TMF took shorter operative time as opposed to SMF.
Postoperative pain
Variable of postoperative pain was not thoroughly investigated in all included studies. Timing of postoperative pain data recording was also variable among studies. Combined outcome of 4–6 weeks postoperative pain was achieved by meta-analysis. Statistically, there was no significant heterogeneity [χ2 = 0.20, df = 1, (p = 0.65); I 2 = 0 %] among three [19, 20, 22] included trials. Therefore, in the fixed-effects model (MD, −0.69; 95 % CI, −1.16, −0.23; z = 2.92; p < 0.004; Fig. 4), TMF was associated with lesser postoperative pain.
Peri-operative complications
The reported peri-operative complications in included trials were bleeding, wound infection, seroma formation, urinary retention, urinary tract infection, lower respiratory tract infection and the development of any condition requiring medical, surgical and pharmacological treatment or delaying the discharge from the hospital. Statistically, there was no significant heterogeneity [χ2 = 7.79, df = 3, (p = 0.05); I 2 = 62 %] among four trials. Therefore, in the fixed-effects model (OR, 1.22; 95 % CI, 0.51, 2.92; z = 0.45; p = 0.65; Fig. 5), there was no statistical difference in the incidence of peri-operative complications in the use of both types of mesh fixation techniques.
Recurrence of hernia
Statistically, there was no significant heterogeneity [χ2 = 0.12, df = 1, (p = 0.73); I 2 = 0 %] among four trials. Therefore, in the fixed-effects model (OR, 1.54; 95 % CI, 0.38, 6.27; z = 0.61; p = 0.54; Fig. 6), the risk of developing recurrent incisional or ventral hernia was similar following TMF and SMF during LIVH repair.
Length of hospital stay
Statistically, there was no significant heterogeneity [χ2 = 5.93, df = 3, (p = 0.12); I 2 = 49 %] among four trials. Therefore, in the fixed-effects model (MD, 0.07; 95 % CI, −0.14, 1.37; z = 0.63; p = 0.53; Fig. 7), there was no statistical difference in the total length of hospital stay following the use of TMF and SMF.
Subgroup analysis
Subgroup analysis was performed on randomised trials and non-randomised trials separately and combined outcomes were compared. Statistically, TMF was associated with shorter operative time, lesser postoperative pain and was not different to SMF in terms of peri-operative complications, length of stay and hernia recurrence.
Other relevant variables
Authors intended to analyse data of other relevant and important variables like cost-effectiveness, mesh fixation time and surgical site infection following the use of TMF and SMF but unfortunately due to either insufficiently investigated or reported data, these calculations were virtually impossible to perform.
Discussion
Based on this review, TMF in LIVH repair is associated with shorter operative time and lesser postoperative pain which is consistent with previously published randomised, controlled trials [19, 20], non-randomised trials [21, 22] and other cohort studies [28–30]. The incidence of postoperative complications has been reported higher in SMF in two randomised [19, 20] studies but lower in two non-randomised studies [21, 22]. However, statistically this difference was not significant according to our review. TMF is comparable with SMF in terms of length of hospital stay and hernia recurrence. Therefore, TMF may be used in LIVH repair.
Authors are fully aware of the fact that combined outcome of this review is based on merely 207 patients undergoing LIVH repair in two randomised and two non-randomised trials. Both types of trials are of moderate strength and therefore, conclusions of this review may be considered weaker and biased. Although statistically there was no “significant” heterogeneity among the trials but methodologically there was significant diversity among studies. The Cochrane collaboration recommends overcoming the problem of weak power of the Chi-square test for heterogeneity by using the significance level 0.10. The problem is inherent here, because of the small number of studies. By using this level in the statistical analysis of this review article, the principle conclusion did not change. Measuring scales for postoperative pain were not homogenous among included studies leading to potentially a less reliable conclusion. In addition, timing of postoperative pain data recording was variable among studies. The shorter duration of follow-up in included studies seems insufficient to detect any given rate of hernia recurrence. The size of hernia in recruited patients was also relatively small which may have contributed into variable readings of postoperative pain and other morbidities. The reported randomised, controlled trials were not homogenous in terms of inclusion criteria, exclusion criteria and mesh fixation protocols. For example, fixations in the TMF group were without four corner sutures in Beldi et al. [20], but four polypropylene sutures were used in the study reported by Bansal et al. [19]. This review did not explore the role of absorbable versus non-absorbable tacks or tacks versus tacks plus sutures fixation due to either insufficiently reported or investigated variables. However, TMF in conjunction with suture fixation in case of larger defects is also a viable option but further investigation is required before the routine use. It is impossible to tell surgeons to apply TMF in all ventral hernias repaired laparoscopically based on the analysed data. If a patient has a 250-cm2 defect, it is unlikely tacks alone would be successful. Additionally, it is not just the size of the defect that affects recurrence rates; it is also the shape and location of the defect and the size/type of prosthetic mesh relative to the defect that is influential. Other contributing factor would be whether or not the defect was near the pubis and whether or not the mesh was anchored to Cooper’s ligaments. Another influential factor is whether the mesh was placed over the fat of the umbilical ligament and/or falciform ligaments, or whether these were dissected off the abdominal wall prior to the mesh placement. This review is not capable to answer the concerns raised by these questions. Therefore, further studies in the form of a major multicentre randomised, controlled trials recruiting higher number of patients are mandatory. High quality trials are required to validate the findings of this review as well as to explore the role of absorbable versus non-absorbable tacks in addition to other influencing factors.
References
Lomanto S, Iyer G, Shabbir A, Cheah WK (2006) Laparoscopic versus open ventral hernia mesh repair: a prospective study. Surg Endosc 20:1030–1035
Mudge M, Hughes LE (1985) Incisional hernia: a 10-year prospective study of incidence and attitudes. Br J Surg 72:70–71
Lewis RT, Wiegand FM (1989) Natural history of vertical abdominal parietal closure: prolene versus dexon. Can J Surg 32:196–200
Hoer J, Lawong G, Klinge U (2002) Factors influencing the development of incisional hernia: a retrospective study of 2983 laparotomy patients over a period of 10 years. Chrirug 73:474–480
Franchi M, Ghezzi F, Buttarelli M, Tateo S, Balestreri D, Bolis P (2001) Incisional hernia in gynaecologic oncology patients: a 10-year study. Obstet Gynecol 97(5 Pt 1):696–700
Sørensen LT, Hemmingsen UB, Kirkeby LT, Kallehave F, Jørgensen LN (2005) Smoking is a risk factor for incisional hernia. Arch Surg 140:119–123
National Centre for Health Statistics (1996) Combined surgery data (NHDS and NSAS) data highlights. Available at: http://www.cdc.gov/nchs/about/major/hdasd
Sauerland S, Walgenbach M, Habermalz B, Seiler CM, Miserez M (2011) Laparoscopic versus open surgical techniques for ventral or incisional hernia repair. Cochrane Database Syst Rev 16(3):CD007781
Forbes SS, Eskicioglu C, McLeod RS, Okrainec A (2009) Meta-analysis of randomized controlled trials comparing open and laparoscopic ventral and incisional hernia repair with mesh. Br J Surg 96:851–858
Goodney PP, Birkmeyer JD (2002) Short term outcomes of laparoscopic and open ventral hernia repair: a meta-analysis. Arch Surg 137:1161–11655
Kapischke M, Schulz T, Schipper T, Tensfeld J, Caliebe A (2008) Open versus laparoscopic incisional hernia repair: something different from a meta-analysis. Surg Endosc 22:2251–2260
Müller-Riemenschneider F, Roll S, Friedrich M, Zieren J, Reinhold T, von der Schulenburg JM, Greiner W, Willich SN (2007) Medical effectiveness and safety of conventional compared to laparoscopic incisional hernia repair: a systematic review. Surg Endosc 21:2127–2136
Pham CT, Perera CL, Watkin DS, Maddern GJ (2009) Laparoscopic ventral hernia repair: a systematic review. Surg Endosc 23:4–15
Sajid MS, Bokhari SA, Mallick AS, Cheek E, Baig MK (2009) Laparoscopic versus open repair of incisional/ventral hernia: a meta-analysis. Am J Surg 197:64–72
Wassenaar E, Schoenmaeckers E, Raymakers J, van der Palen J, Rakic S (2010) Mesh-fixation method and pain and quality of life after laparoscopic ventral or incisional hernia repair: a randomized trial of three fixation techniques. Surg Endosc 24:1296–1302
Costanza MJ, Heniford BT, Arca MJ, Mayes JT, Gagner M (1998) Laparoscopic repair of recurrent ventral hernias. Am Surg 64:1121–1125
Samuel K, Miller SK, Carey SD, Rodriguez FJ, Smoot RT Jr (2003) Complications and their management. In: LeBlanc KA (ed) Laparoscopic hernia surgery: an operative guide. Arnold, London, pp 161–169
Eriksen JR, Poornoroozy P, Jørgensen LN, Jacobsen B, Friis-Andersen HU, Rosenberg J (2009) Pain, quality of life and recovery after laparoscopic ventral hernia repair. Hernia 13:13–21
Bansal VK, Misra MC, Kumar S, Keerthi Rao Y, Singhal P, Goswami A, Guleria S, Arora MK, Chabra A (2011) A prospective randomized study comparing suture mesh fixation versus tacker mesh fixation for laparoscopic repair of incisional and ventral hernias. Surg Endosc 25:1431–1438
Beldi G, Wagner M, Bruegger LE, Kurmann A, Candinas D (2011) Mesh shrinkage and pain in laparoscopic ventral hernia repair: a randomized clinical trial comparing suture versus tack mesh fixation. Surg Endosc 25:749–755
Greenstein AJ, Nguyen SQ, Buch KE, Chin EH, Weber KJ, Divino CM (2008) Recurrence after laparoscopic ventral hernia repair: a prospective pilot study of suture versus tack fixation. Am Surg 74:227–231
Nguyen SQ, Divino CM, Buch KE, Schnur J, Weber KJ, Katz LB, Reiner MA, Aldoroty RA, Herron DM (2008) Postoperative pain after laparoscopic ventral hernia repair: a prospective comparison of sutures versus tacks. JSLS 12:113–116
Lepere M, Benchetrit S, Bertrand JC, Chalbet JY, Combier JP, Detruit B, Herbault G, Jarsaillon P, Lagoutte J, Levard H, Rignier P (2008) Laparoscopic resorbable mesh fixation. Assessment of an innovative disposable instrument delivering resorbable fixation devices: I-Clip(TM). Final results of a prospective multicentre clinical trial. Hernia 12:177–1783
Clarke T, Katkhouda N, Mason RJ, Cheng BC, Algra J, Olasky J, Sohn HJ, Moazzez A, Balouch M (2011) Fibrin glue for intraperitoneal laparoscopic mesh fixation: a comparative study in a swine model. Surg Endosc 25:737–748
van’t Riet M, de Vos van Steenwijk PJ, Kleinrensink GJ, Steyerberg EW, Bonjer HJ (2002) Tensile strength of mesh fixation methods in laparoscopic incisional hernia repair. Surg Endosc 16:1713–1716
Karakousis CP, Volpe C, Tanski J, Colby ED, Winston J, Driscoll DL (1995) Use of a mesh for musculoaponeurotic defects of the abdominal wall in cancer surgery and the risk of bowel fistulas. J Am Coll Surg 181:11–16
Kaufman Z, Engelberg M, Zager M (1981) Fecal fistula: a late complication of Marlex mesh repair. Dis Colon Rectum 24:543–544
Wassenaar EB, Raymakers JT, Rakic S (2008) Impact of the mesh fixation technique on operation time in laparoscopic repair of ventral hernias. Hernia 12:23–25
Kurian A, Gallagher S, Cheeyandira A, Josloff R (2010) Predictors of in-hospital length of stay after laparoscopic ventral hernia repair: results of multivariate logistic regression analysis. Surg Endosc 24:2789–2792
Barzana D, Johnson K, Clancy TV, Hope WW (2010) Hernia recurrence through a composite mesh secondary to transfascial suture holes. Hernia Sep 12 (Epub ahead of print)
Higgins JPT, Green S (eds) (2008) Cochrane handbook for systematic reviews of interventions Version 5.0.0 (updated February 2008). The Cochrane Collaboration. http://www.cochrane-handbook.org. Accessed 15 April 2011
Review Manager (RevMan) [Computer program]. Version 5.0. The Nordic Cochrane Centre, The Cochrane Collaboration: Copenhagen, 2008
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188
DeMets DL (1987) Methods for combining randomized clinical trials: strengths and limitations. Stat Med 6:341–350
Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21:1539–1558
Egger M, Smith GD, Altman DG (2006) Systematic reviews in healthcare. BMJ Publishing, London
Deeks JJ, Altman DG, Bradburn MJ (2001) Statistical methods for examining heterogeneity and combining results from several studies in meta-analysis. Systemic reviews in health care: meta-analysis in context, 2nd edn. BMJ Publication group, London
Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ (1996) Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 17:1–12
Chalmers TC, Smith H Jr, Blackburn B, Silverman B, Schroeder B, Reitman D (1981) A method for assessing the quality of a randomized control trial. Control Clin Trials 2:31–49
SIGN guidelines. Accessed 12 April 2011. Available from: http://www.sign.ac.uk/guidelines/fulltext/50/checklist3
Rangel SJ, Kelsey J, Colby CE, Anderson J, Moss RL (2003) Development of a quality assessment scale for retrospective clinical studies in pediatric surgery. J Pediatr Surg 38:390–396
http://ims.cochrane.org/revman/other-resources/gradepro/download. Accessed 3 May 2011
Conflict of interest
None to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sajid, M.S., Parampalli, U. & McFall, M.R. A meta-analysis comparing tacker mesh fixation with suture mesh fixation in laparoscopic incisional and ventral hernia repair. Hernia 17, 159–166 (2013). https://doi.org/10.1007/s10029-012-1017-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10029-012-1017-z