Abstract
Objectives
To conduct a systematic review and meta-analysis to evaluate the risk and intensity of tooth sensitivity (TS) after topical application of desensitizers containing potassium nitrate before dental bleaching.
Methods
We searched PubMed, Scopus, Web of Science, LILACS, BBO, Cochrane Library, and SIGLE. We also surveyed gray literature without restrictions. We meta-analyzed the data using the random-effects model to compare potassium nitrate and placebo in terms of risk and intensity of TS and color change (∆SGU or ∆E). The quality of the evidence was rated using the GRADE approach. The risk of bias (RoB) of the included studies was analyzed using the Cochrane RoB tool.
Results
After the database screening, 24 articles remained. A significant 12% lower risk for the groups where desensitizing agents were applied (p = 0.02), with a risk ratio of 0.88 (95% CI 0.78 to 0.98). About the intensity of TS, a significant average mean difference of − 0.77 units of VAS units (95%CI − 1.34 to − 0.19; p = 0.01) in favor of the desensitizer group. In the NRS scale, a significant average mean difference of − 0.36 (95% CI − 0.61 to − 0.12; p value = 0.004) in favor of the desensitizer group. No significant difference was observed in color change (p > 0.28) in ∆SGU and ∆E.
Conclusions
Although a significant reduction in the risk and intensity of TS was observed in groups treated with a potassium nitrate at some point during the bleaching, the clinical significance of this reduction is subtle and clinically questionable. Color change is not affected by the use of agents.
Clinical relevance
The reduction in the risk and intensity of TS with the topical application of potassium nitrate–based desensitizing agents in dental bleaching is subtle and maybe clinically questionable.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patients’ facial appearance is affected by the smile and, when within esthetic standards, can improve self-esteem and social relationships [1, 2]. Although the attractiveness of a smile is related to the shape and position of the teeth, tooth color has a strong effect on the social perceptions, as brighter teeth are usually associated with good oral health status [3]. These factors are the reasons why tooth bleaching has been desired for many patients.
Currently, there are two dentist-supervised techniques available for dental bleaching: at-home bleaching [4, 5] and in-office bleaching [5, 6]. Although both techniques provide similar results [6,7,8,9], some patients prefer the in-office bleaching as they need faster bleaching results and are not willing to use bleaching trays for prolonged periods.
In a recent study, the authors reported that the risk of TS for in-office bleaching and at-home bleaching was quite similar [8]; however, the TS intensity was much higher for in-office bleaching (2.8 ± 2.9) than at-home (0.5 ± 0.9) when measured in a 0–4 pain scale [8].
This common and inconvenient adverse effect of TS has encouraged researches to investigate protocols to prevent or minimize its occurrence. Some of the approaches include the reduction of the concentration and usage time of the bleaching gel [10, 11], the application of topical desensitizing agents [12,13,14], the administration of systemic drugs [15,16,17,18], and the incorporation of desensitizing agents, into the formulation of the bleaching gels [19, 20].
Among all these approaches, the topical application of desensitizing agents showed promising results for the reduction of the risk and intensity of TS [21,22,23], but there are recent reports that do not reach the same conclusions [14, 22, 24].
Although a systematic review of the literature concluded that the application of desensitizing agents based on potassium nitrate and sodium fluoride reduces the bleaching-induced TS [25], there are significant methodological differences when compared to the present systematic review. Besides, other randomized controlled trials (RCTs) on this topic were published in the most recent years, and this systematic review requires updating.
Therefore, this systematic review aimed to answer the focused research question, based on the PICO acronym (Participant-Intervention-Comparator-Outcome): “Are the risk and intensity of TS lower when potassium nitrate–based desensitizers are applied before dental bleaching in adults, compared to a placebo?”
Methods
Protocol and registration
This study protocol was registered at the International Prospective Register of Systematic Reviews (PROSPERO - CRD 42018104598), and the present report follows the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement for report [26].
Information sources and search strategy
We used controlled vocabulary (MeSH terms) and free keywords for the concepts Participants and Intervention to define the search strategy for the following databases: Cochrane Library, MEDLINE via PubMed, Latin American and Caribbean Health Sciences Literature database (LILACS), and Brazilian Library in Dentistry (BBO). We also searched for some citation databases, such as Scopus and Web of Science. No restrictions on publication date or languages were made. Table 1 depicts the search strategies employed.
Some sources of gray literature were investigated: (1) abstracts of the annual conference of the International Association for Dental Research (1990–2020), (2) System for Information on Grey Literature in Europe (SIGLE), (3) dissertations and theses in ProQuest, (4) Periodicos Capes Theses database, and (5) clinical trial registries (current controlled trials, International Clinical Trials Registry Platform, ClinicalTrials.gov, ReBEC, and EU Clinical Trials Register).
Eligibility criteria
We included parallel and split-mouth randomized clinical trials (RCTs) that evaluated the application of potassium nitrate as a topical desensitizing agent on the risk and intensity of TS during in-office and at-home dental bleaching in adult patients. We excluded RCTs if studies (1) incorporated the potassium nitrate only into the bleaching gel; (2) evaluated dentifrices containing potassium nitrate; (3) evaluated desensitizing agents other than potassium nitrate; (4) did not have a placebo or no-desensitizing agent group for comparison; and (5) included both groups but did not compare bleaching gels with equivalent concentrations.
Initially, review authors removed duplicates and non-relevant articles by screening titles and abstracts. The full-text paper of the relevant articles was obtained, and subsequently, four reviewers (E.M., M.F., J.L.G., and M.R.) classified those that met the inclusion criteria. Each study received a study ID, combining the first author and the year of publication.
Details about study methods, designs and settings, participant’s characteristics, bleaching protocol, and desensitizing protocol were extracted from the eligible studies using customized extraction forms.
Risk of bias in individual studies
Four reviewers assessed, independently, the risk of bias of the eligible studies using the Cochrane Collaboration tool for assessing risk of bias in randomized trials [27]. This tool contains seven items: sequence generation, allocation concealment, blinding of the participants and the outcome assessors, incomplete outcome data, selective outcome reporting, and other sources of bias (not used in the present study). Any disagreements between the reviewers were resolved through discussion and, if necessary, by consulting a fifth reviewer (A.R.).
Each domain from the risk of bias tool was scored following recommendations as described in the Cochrane Handbook for Systematic Reviews of Interventions 5.1.0 (http://handbook.cochrane.org). The judgment for each entry involves the judgments of low risk of bias, high risk of bias, or unclear risk, indicating either lack of information or uncertainty over the potential for bias.
Summary measures and synthesis of the results
As the RCTs usually report TS intensity in different time assessments, we collected data from the worst scenario. When more than one experimental or placebo group was evaluated in the primary study, we merged the corresponding groups. In case medians and interquartile ranges were provided, the medians were used as the best estimates of means, and the interquartile ranges converted to standard deviation [SD] (the width of the interquartile range corresponds to approximately 1.35 SD for normally distributed data). Data provided in a numerical rating scale (NRS) and visual analog scale (VAS) were collected and evaluated separately.
In some cases, standard deviations were not reported in the primary articles. As standard deviations of pain scales usually range between half of the reported mean and the mean itself, we imputed a standard deviation equal to the mean in the missing cases. To evaluate the impact of such imputation, we conducted a sensitivity analysis to check if other imputations such as half of the mean could affect the overall conclusions.
Data were analyzed using Revman 5.3 (Review Manager Version 5.3, The Cochrane Collaboration, Copenhagen, Denmark). The risk of TS from the eligible studies was summarized by the risk ratio and the 95% confidence interval, while the intensity of TS was summarized by the mean difference and the 95% confidence interval.
Color change was also evaluated. Data from studies using the same color measurement tool was evaluated separately. The same approaches used for missing cases for the TS were applied for color change data.
For all meta-analysis, we used the random-effects models as this is the most appropriate model for studies performed in different populations. Subgroup analyses based on the type of bleaching (at-home and in-office) were performed. We evaluated the heterogeneity in all meta-analysis with at least four studies. For this purpose, we used the Cochran Q test (which test the null hypothesis that all studies share the same effect size), I2 statistics (which describe the proportion of the observed heterogeneity is due to real variation of the true effect sizes), and the 95% prediction interval (which is the dispersion of the observed effect sizes). Sensitivity analyses were conducted to investigate the reasons for high heterogeneity, whenever detected.
Assessment of the quality of the evidence using GRADE
We graded the quality of the evidence for primary outcomes across the studies (body of evidence) by using the Grading of Recommendations: Assessment, Development, and Evaluation (GRADE) (http://www.gradeworkinggroup.org/) to determine the overall strength of the evidence for each meta-analysis [28]. The GRADE pro Guideline Development Tool (available online at www.gradepro.org) was used to create a summary-of-findings table, as suggested in the Cochrane Handbook for Systematic Reviews of Interventions 5.0.2 (http://handbook.cochrane.org) for the primary outcomes in two study follow-ups.
The GRADE approach for RCTs addresses five reasons (risk of bias, imprecision, inconsistency, indirectness of evidence, and publication bias) to possibly downgrade the quality of the evidence (1 or 2 levels). Each of these topics was assessed as having “no limitations,” “serious limitations,” or “very serious limitations” to categorize the quality of the evidence into high, moderate, low, and very low.
Results
Study selection
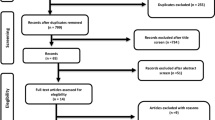
The search strategy was conducted initially on February 22, 2019, and updated twice (in December 14, 2019, and March 11, 2020). After database screening and duplicate removal, 4896 studies were identified (Fig. 1). After title screening, 206 studies remained, and after abstract screening, 34 studies remained. This number was reduced to 24 after careful examination of the full texts.
From the 34 studies, a total of ten studies were excluded since (1) they did not have a placebo group [13, 14, 29, 30]; (2) they did not use a desensitizer based on potassium nitrate [31, 32]; (3) they were not a RCT, but a clinical case report [33]; (4) they incorporated potassium nitrate only in dentifrices [34, 35]; and (5) they were ongoing studies without results [36].
Characteristics of the included studies
The characteristics of the 24 selected studies are listed in Table 2. The split-mouth design was used in six studies [37,38,39,40,41,42]. The parallel design was used in 18 studies [12, 21,22,23,24, 43,44,45,46,47,48,49,50,51,52,53,54,55]. VAS pain scale [21, 23, 37, 39, 40, 42, 46,47,48, 51,52,53, 55], and NRS pain scale [12, 21,22,23,24, 37, 38, 40, 41, 44, 45, 50] were the ones mostly employed. The study of Leonard [49] assessed patients’ risk of sensitivity through a questionnaire applied before and during bleaching.
For color evaluation, eight studies used only shade guides [12, 21, 23, 24, 42, 46, 50, 53], and six studies used shade guides and objective color measure instruments (spectrophotometer or colorimeter) [22, 37, 39, 40, 43, 55]. In two studies, only spectrophotometer was used [38, 52]. In other eight studies [41, 44, 45, 47,48,49, 51, 54], the authors did not evaluate color change.
The number of patients per group included in these studies ranged from 15 to 58. The mean age of all participants included in the clinical trials was approximately 25 ± 3 years, and the minimum age to participate in the study was 18. In six out of the twenty-four studies, most of the participants were female [22, 38, 41, 47, 49, 53]. Only one study reported that gender distribution was similar [12], and in other 12 studies, this information was not reported [21, 23, 37, 39, 42, 44, 46, 48, 50, 51, 54, 55].
Regarding the bleaching protocol, in 22 out of 24 studies, HP gels were employed [21,22,23,24, 37,38,39,40,41,42,43,44,45,46,47,48, 50,51,52,53,54,55] with HP concentrations varying from 20 to 35%. Two RCTs used 10% CP [49] and 16% carbamide peroxide (CP) [12]. The most used protocol with HP was two sessions with three applications of 15 min each [21,22,23,24, 37, 40, 41, 52, 53, 55]. However, other protocols were also employed. The use of CP ranged from 4 to 6 h daily for 2 to 5 weeks [12, 49]. Two studies used LED activation during HP bleaching [23, 55].
About the desensitizing protocol, 5% potassium nitrate was the most used, applied for 10 min before bleaching [12, 21,22,23,24, 37, 38, 41,42,43, 45,46,47, 50,51,52, 54], and 30 min before bleaching [49, 55]. Other concentrations and application times were also observed. For instance, a study applied 6% potassium nitrate for 30 min before bleaching [39], and another one applied 10% potassium nitrate 10 min before bleaching [40].
Assessment of the risk of bias
Regarding randomization, 16 out of the 24 studies evaluated reported the randomization method used [12, 21,22,23,24, 37, 39,40,41,42,43, 45, 50,51,52,53]. Only eight of the 24 studies reported the allocation concealment [22, 24, 37, 39, 40, 43, 52, 53].
Blinding was reported in 13 studies [12, 21,22,23,24, 37,38,39,40,41,42,43, 53]. At the study level, 17 studies [12, 21, 23, 24, 38, 41, 42, 44, 46,47,48,49,50,51,52, 54, 55] were judged as having an unclear risk of bias. One study was judged as having a high risk of bias [45] for selective outcome reporting. The authors reported that color change would be measured, but they did not present the results (Fig. 2).
Meta-analysis
Tooth sensitivity
The risk of TS was calculated from a total of 16 studies. This systematic review of the literature showed that desensitizing application produced a relative ratio reduction (RRR) of 12% in the risk of having TS (p = 0.02), with a risk ratio of 0.88 (95%CI 0.78 to 0.98) (Fig. 3). Heterogeneity was detected (p = 0.003), and more than half of the observed variability was due to variation in the true effect sizes (I2 = 59%).
To make this easier to understand, we can put it in other words. The RRR can be applied to different baseline risks of having TS. For example, approximately 80% of the participants who have their teeth bleached with in-office bleaching gels suffer from TS. If we apply this RRR of 12% over the baseline risk of 80% risk, we end up with an absolute reduction of 9.6%. This gives us the number needed to treat (NNT) of 10 (100/9.6), meaning that 10 patients will need to be treated for one patient to benefit from it. If we apply the same calculations to lower baseline risk of TS, such as for at-home bleaching, which has an approximately 50% risk, we end up with an absolute reduction of 6%, which is equivalent to a NNT of 17.
The intensity of TS was meta-analyzed using two different pain scales. In the VAS scale (Fig. 4), ten studies were included, giving a significant average mean difference of − 0.77 units of VAS units (95%CI − 1.34 to − 0.19; p = 0.01) in favor of the desensitizer group. Heterogeneity was detected (p = 0.01), and it was attributed mainly to variations in the true effect sizes (I2 = 57%).
To make the understanding of the reported reduction in the intensity of TS more familiar, we can rewrite it in other terms. The non-weighted average of the intensity of TS in the control groups of the eligible studies in this systematic review was approximately 4 units in a 0–10 VAS scale. The present meta-analysis showed that the use of a desensitizer can reduce this mean TS by an average of 0.77, which means that the desensitizer-treated patients would have a mean VAS pain of 3.23 units.
In the NRS scale (Fig. 5), a total of 14 studies were included, with a significant average mean difference of − 0.36 (95% CI − 0.61 to − 0.12; p = 0.004) in favor of the desensitizer group. Heterogeneity was detected (p value < 0.001), and it was mainly due to variations in the true effect sizes (I2 = 66%).
Color change
Data from color change were meta-analyzed in ∆SGU Vita Classical (six studies; MD = 0.14; 95% CI − 0.21 to 0.48), final SGU Vita Classical (six studies; MD = − 0.02; 95% CI − 0.29 to 0.24), ∆SGU Vita Bleachedguide (four studies; MD = 0.10; 95% CI − 0.35 to 0.54), and ∆E (seven studies; MD = − 0.22; 95% CI − 0.62 to 0.18). In all cases, no significant difference was observed in color change (p > 0.28). Heterogeneity was not detected in none of the meta-analysis (p > 0.25, Figs. 6, 7, 8, and 9).
Subgroup analysis
We performed subgroup analysis based on the type of bleaching protocol employed (at-home or in-office bleaching), as can be seen in the forest plots of the outcomes. The bleaching protocol did not explain the heterogeneity detected in the data for TS.
Sensitivity analysis
Imputations had to be made in some cases where standard deviations were not reported in the full texts, both in data from intensity of TS in VAS scale [48, 52, 54], intensity of TS in NRS scale [24], and color change in ∆E [55]. Sensitivity analysis using more extreme values of imputations were performed, but the overall conclusions were not affected.
Additionally, we suspected that the study of Dias [48] and Santos [54] shared the same experimental group with different comparators. In a sensitivity analysis, we excluded one or the other in the meta-analysis where they were included, but the conclusions remained the same.
Certainty of the evidence
A total of three outcomes for TS (risk of TS, intensity of TS in VAS scale, and intensity of TS in NRS scale) and four outcomes for color change (∆SGU Vita Classical, Final SGU Vita Classical, ∆SGU Bleachedguide, and ∆E) were evaluated. The certainty of evidence for all these outcomes is summarized in Table 3. Regarding TS, all outcomes were graded as low certainty of the evidence due to the fact that most articles included in the meta-analysis were at unclear risk of bias and due to unexplained heterogeneity. Data from the color change was graded as high (in the meta-analysis composed mostly with studies at low risk of bias) or moderate (when most of the studies included were at unclear risk of bias).
Discussion
Dental bleaching protocols employ hydrogen peroxide (HP) as the oxidizing agent. HP has a low molecular weight and thus penetrates the dental substrates fast and easily. However, this penetration is not only restricted to the dental hard tissues. HP can reach the pulp chamber within few minutes [56,57,58] and may cause inflammatory reaction [59, 60], oxidative stress, and cell damage.
This penetration is facilitated by factors such as the patient’s age, the concentration of the bleaching gel, the application time, the presence of restorations, and the end pH of the bleaching product [61,62,63,64]. Consequently, inflammatory mediators and stimulating nociceptors are released, causing pain transmission [65].
The presence of this undesirable side effect is the reason why topically applied desensitizing agents have been studied as preventive measures to avoid the undesirable bleaching-induced TS. Among these agents, potassium nitrate has been described as an alternative to minimize this side effect [25], although its exact mechanism of action to reduce TS in the dental bleaching is not well known [66].
As well as HP, potassium nitrate can penetrate the dental hard tissues and reach the pulp chamber [67, 68], and this penetration is considered time-dependent [68]. Theoretically, in the pulp tissue, potassium nitrate is believed to reduce dentinal sensory nerve activity by preventing nerve repolarization due to excess of K+ ions outside the nerve membrane. Without nerve repolarization, pain impulse does not progress through the length of the nerve fibers, and the patient may not feel the bleaching-induced TS [69,70,71].
Not included in this review, studies using toothpastes containing potassium nitrate in the composition, because the contact time and the concentration of the active ingredient is very different from the desensitizing potassium nitrate used for topical applications. In the majority of the studies, potassium-based desensitizers are usually applied for 10 min, while in the best scenario, dentifrices do not stay longer than 3 min in the buccal cavity. When included in desensitizers, potassium nitrate is incorporated in concentrations that range from 3 to 10% [40]; in dentifrices these concentrations are not superior to 5% [34]. Additionally, potassium nitrate–based dentifrices are leached out by the contact with saliva, which does not occur in professionally topical application of a desensitizer.
We also decided to exclude from this systematic review studies that included potassium nitrate in the bleaching agent. When incorporated into the gel, the potassium nitrate stays longer in contact with the dental structure, but it is delivered simultaneously to the hydrogen peroxide, differently to what occur to potassium nitrate desensitizers applied in a preventive manner [72]. The universe of eligible studies included in a systematic review should be narrow enough to collect studies with similar populations, treatment protocol, and comparator group to avoid a high heterogeneity of the data [73, 74].
Another systematic review was recently published addressing specifically the addition of desensitizers into the bleaching gels [72]; this study concluded that incorporating desensitizers in the bleaching gel did not reduce the risk of TS, and the quality of this evidence was considered moderate. On the other hand, the intensity of TS, color change, and risk of gingival irritation were similar between groups, but the quality of the evidence was graded as low or very low.
An earlier systematic review of the literature addressing this issue reported that potassium nitrate agents are effective in reducing the bleaching-induced TS [25]. In this systematic review, review authors included studies that evaluated the association of potassium nitrate with fluorides. It is claimed that fluorides can obliterate the dentinal tubules by precipitation of fluorine crystals, preventing the HP from reaching the pulp [75].
However, in most of the primary studies, patients with exposed dentin surfaces, where fluorides could have a beneficial effect, were excluded from the sample. Therefore, the benefit of fluorides in these cases is questionable, explaining why, in the present study, we included studies that evaluated potassium nitrate alone or in association with other agents.
It is also important to note that the systematic review published [25] presents flaws, and discussion about them is crucial to avoid these common mistakes in future systematic reviews. The data replicated from the same study population many times [22, 76, 77] overestimate the reported effect size in the study and increases the power of the systematic review unequivocally.
Additionally, the authors use a fixed-effect model for the meta-analysis, which is not the most appropriate to use [76, 78]. This fixed-effect model assumes that there is a single effect size, common to all studies. This assumption is unlikely to be true due to variations in the populations, bleaching protocols, and composition of the desensitizing agents. By using the fixed-effect model, the confidence intervals for the summary effect are smaller than under the random-effect model, which is the most appropriate statistical model [79]. Thus, the chances of finding statistically significant results are increased under the fixed-effect model. Finally, from the time this study was published until today, several new studies on the topic have been published, the reason why this study was conducted. In this case, we assume that each study is estimating different, yet related, intervention effects, and we want to know the average of all these different and related effect sizes along with the heterogeneity of these estimates.
In the present systematic review, we observed that potassium nitrate has a positive benefit in preventing the development of bleaching-induced TS. On average, patients treated with potassium nitrate–based agents have a 12% lower risk of presenting TS. In terms of pain intensity, the average reduction was less than 1 unit in VAS, and around 0.35 units in the 0-10 NRS scale. However, these figures should be interpreted with caution. Although these positive findings were statistically significant, they represent small effect sizes, with questionable clinical importance. An average of one unit in a VAS 0–10 or 0.35 unit in a 0–4 NRS scale does not represent a clinically relevant reduction in the intensity of the bleaching induced TS.
Although we could not identify, from a statistical perspective, the reasons for the heterogeneity that was observed in the meta-analysis, from a clinical view, the observed heterogeneity must be due to the different bleaching protocols and the different compositions of the potassium nitrate–based desensitizers. Although all studies applied potassium nitrate, the composition of these desensitizers varied. Changes in the concentration of the potassium nitrate and inclusion of other products, such as fluorides, as in most cases [12, 21,22,23,24, 38, 39, 41,42,43,44,45,46,47,48, 50, 52,53,54], glutaraldehyde [37], sodium monofluorophosphate [51], and nano-calcium phosphate crystals [24, 49], were observed. Additionally, variations of the bleaching protocols (varied HP concentrations, application times, frequency of application as well as number of clinical sessions) may also affect the overall bleaching-induced TS [6], and therefore, may impact the results.
We are confident to state that color change is not affected by the application of potassium nitrate–based agents. This conclusion was reached in the four meta-analyses of color change, and it is robust enough not to be affected by the type of color change instrument used. The HP and the potassium nitrate can penetrate the pulp tissue, but they do not compete for the same sites in the hard tissues, as each one has different mechanisms of action [14].
Most of the studies included in the present investigation were considered at unclear risk of bias in the domains sequence generation and allocation concealment, which agrees to what was previously reported in other bleaching studies [8, 80]. Adequate random sequence generation and allocation concealment are crucial to prevent selection bias, so that we can be confident that studies have comparable known and unknown characteristics at baseline. Thus, the differences obtained after the implementation of the treatments can only be attributed to the treatment itself. The fact that most studies were classified as unclear highlights the need for significant improvements in the conduction and report of future RCTs.
Future RCTs should focus on the investigation of other types of desensitizing agents and their combination to prevent or reduce the undesirable side effect of bleaching-induced TS. Apart from potassium nitrate, which has neural action, other types of desensitizing agents such as fluorides, bioactive agents, amorphous calcium phosphate, nano-hydroxyapatite, bioglass, or even their association could be the focus of further randomized controlled trials.
Conclusions
Although a significant reduction in the risk and intensity of TS was observed in groups treated with a potassium nitrate before dental bleaching, the clinical significance of this reduction is subtle, and it may be clinically questionable. Color change is not affected by the preliminary use of a potassium nitrate–based agent.
References
Koidou VP, Chatzopoulos GS, Rosenstiel SF (2018) Quantification of facial and smile esthetics. J Prosthet Dent 119:270–277. https://doi.org/10.1016/j.prosdent.2017.04.002
Llena C, Villanueva A, Mejias E, Forner L (2020) Bleaching efficacy of at home 16% carbamide peroxide. A long-term clinical follow-up study. J Esthet Restor Dent 32:12–18. https://doi.org/10.1111/jerd.12560
Kershaw S, Newton JT, Williams DM (2008) The influence of tooth colour on the perceptions of personal characteristics among female dental patients: comparisons of unmodified, decayed and 'whitened' teeth. Braz Dent J 204:256–257. https://doi.org/10.1038/bdj.2008.134
Alonso de la Pena V, Lopez Raton M (2014) Randomized clinical trial on the efficacy and safety of four professional at-home tooth whitening gels. Oper Dent 39:136–143. https://doi.org/10.2341/12-402-C
Rezende M, Loguercio AD, Reis A, Kossatz S (2013) Clinical effects of exposure to coffee during at-home vital bleaching. Oper Dent 38:E229–E236. https://doi.org/10.2341/12-188-C
de Geus JL, Wambier LM, Kossatz S, Loguercio AD, Reis A (2016) At-home vs In-office bleaching: a systematic review and meta-analysis. Oper Dent 41:341–356. https://doi.org/10.2341/15-287-LIT
Bernardon JK, Ferrari P, Baratieri LN, Rauber GB (2015) Comparison of treatment time versus patient satisfaction in at-home and in-office tooth bleaching therapy. J Prosthet Dent 114:826–830. https://doi.org/10.1016/j.prosdent.2015.05.014
Rezende M, Loguercio AD, Kossatz S, Reis A (2016) Predictive factors on the efficacy and risk/intensity of tooth sensitivity of dental bleaching:aA multi regression and logistic analysis. J Dent 45:1–6. https://doi.org/10.1016/j.jdent.2015.11.003
Tay LY, Kose C, Herrera DR, Reis A, Loguercio AD (2012) Long-term efficacy of in-office and at-home bleaching: a 2-year double-blind randomized clinical trial. Am J Dent 25:199–204
Mondelli RF, Azevedo JF, Francisconi AC, Almeida CM, Ishikiriama SK (2012) Comparative clinical study of the effectiveness of different dental bleaching methods - two year follow-up. J Appl Oral Sci 20:435–443. https://doi.org/10.1590/s1678-77572012000400008
Hewlett ER (2007) Etiology and management of whitening-induced tooth hypersensitivity. J Calif Dent Assoc 35:499–506
Kose C, Reis A, Baratieri LN, Loguercio AD (2011) Clinical effects of at-home bleaching along with desensitizing agent application. Am J Dent 24:379–382
Mehta D, Venkata S, Naganath M, LingaReddy U, Ishihata H, Finger WJ (2013) Clinical trial of tooth desensitization prior to in-office bleaching. Eur J Oral Sci 121:477–481. https://doi.org/10.1111/eos.12067
Martini EC, Parreiras SO, Szesz AL, Coppla FM, Loguercio AD, Reis A (2019) Bleaching-induced tooth sensitivity with application of a desensitizing gel before and after in-office bleaching: a triple-blind randomized clinical trial. Clin Oral Investig 24:385–394. https://doi.org/10.1007/s00784-019-02942-9
Coppla FM, Rezende M, de Paula E, Farago PV, Loguercio AD, Kossatz S, Reis A (2018) Combination of acetaminophen/codeine analgesics does not avoid bleaching-induced tooth sensitivity: a randomized, triple-blind two-center clinical trial. Oper Dent 3:E53–E63. https://doi.org/10.2341/17-092-C
Rezende M, Bonafe E, Vochikovski L, Farago PV, Loguercio AD, Reis A, Kossatz S (2016) Pre and postoperative dexamethasone does not reduce bleaching-induced tooth sensitivity: a randomized, triple-masked clinical trial. J Am Dent Assoc 147:41–49. https://doi.org/10.1016/j.adaj.2015.07.003
Charakorn P, Cabanilla LL, Wagner WC, Foong WC, Shaheen J, Pregitzer R, Schneider D (2009) The effect of preoperative ibuprofen on tooth sensitivity caused by in-office bleaching. Oper Dent 34:131–135. https://doi.org/10.2341/08-33
Paula E, Kossatz S, Fernandes D, Loguercio AD, Reis A (2013) The effect of perioperative ibuprofen use on tooth sensitivity caused by in-office bleaching. Oper Dent 38:601–608. https://doi.org/10.2341/12-107-C
Matis BA, Cochran MA, Eckert GJ, Matis JI (2007) In vivo study of two carbamide peroxide gels with different desensitizing agents. Oper Dent 32:549–555. https://doi.org/10.2341/07-10
Gallo JR, Burgess JO, Ripps AH, Bell MJ, Mercante DE, Davidson JM (2009) Evaluation of 30% carbamide peroxide at-home bleaching gels with and without potassium nitrate - a pilot study. Quintessence Int 40:e1–e6
Tay LY, Kose C, Loguercio AD, Reis A (2009) Assessing the effect of a desensitizing agent used before in-office tooth bleaching. J Am Dent Assoc 140:1245–1251. https://doi.org/10.14219/jada.archive.2009.0047
Bonafe E, Loguercio AD, Reis A, Kossatz S (2014) Effectiveness of a desensitizing agent before in-office tooth bleaching in restored teeth. Clin Oral Investig 18:839–845. https://doi.org/10.1007/s00784-013-1055-7
Reis A, Dalanhol AP, Cunha TS, Kossatz S, Loguercio AD (2011) Assessment of tooth sensitivity using a desensitizer before light-activated bleaching. Oper Dent 36:12–17. https://doi.org/10.2341/10-148-CR
Loguercio AD, Tay LY, Herrera DR, Bauer J, Reis A (2015) Effectiveness of nano-calcium phosphate paste on sensitivity during and after bleaching: a randomized clinical trial. Braz Oral Res 29:1–7. https://doi.org/10.1590/1807-3107BOR-2015
Wang Y, Gao J, Jiang T, Liang S, Zhou Y, Matis BA (2015) Evaluation of the efficacy of potassium nitrate and sodium fluoride as desensitizing agents during tooth bleaching treatment-a systematic review and meta-analysis. J Dent 43:913–923. https://doi.org/10.1016/j.jdent.2015.03.015
Moher D, Liberati A, Tetzlaff J, Altman DG (2010) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 8:336–341. https://doi.org/10.1016/j.ijsu.2010.02.007
Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA (2011) The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 343:d5928. https://doi.org/10.1136/bmj.d5928
Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, Norris S, Falck-Ytter Y, Glasziou P, Jaeschke R (2011) GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 64:383–394. https://doi.org/10.1016/j.jclinepi.2010.04.026
Kossatz S (2017) Efetividade e sensibilidade do clareamento caseiro com peróxido de hidrogênio 4% vs clareamento dental associado com peróxido de hidrogênio 35% e 4% com dessensibilizante - estudo clínico randomizado, ReBEC. http://www.ensaiosclinicos.gov.br/ Accessed 11 March 2020.
Nahsan F (2016) Potassium oxalate and potassium nitrate and post-bleaching sensitivity, https://clinicaltrials.gov/show/nct02941653 Accessed 11 March 2020.
Armenio RV, Fitarelli F, Armenio MF, Demarco FF, Reis A, Loguercio AD (2008) The effect of fluoride gel use on bleaching sensitivity: a double-blind randomized controlled clinical trial. J Am Dent Assoc 139:592–597. https://doi.org/10.14219/jada.archive.2008.0220
Barcellos DC, Batista GR, da Silva MA, Pleffken PR, Valera MC (2015) Clinical performance of topical sodium fluoride when supplementing carbamide peroxide at-home bleaching gel. Gen Dent 63:47–50
Rahal V, Azevedo F, Gallinari M, Silva N, Gonçalves R, Cintra L, Marson F, Briso A (2014) Avaliação sensorial quantitativa da sensibilidade dentária com o uso de um dessensibilizante. Rev Dent Press Estetic 11:108–117
Pintado-Palomino K, Peitl Filho O, Zanotto ED, Tirapelli C (2015) A clinical, randomized, controlled study on the use of desensitizing agents during tooth bleaching. J Dent 43:1099–1105. https://doi.org/10.1016/j.jdent.2015.07.002
Nahsan F (2018) Potassium nitrate influence on post in office bleaching sensitivity: a triple blinded randomized clinical trial, https://clinicaltrials.gov/ct2/show/NCT03523598 Accessed 11 March 2020.
Lima T (2019) Avaliação de agentes dessensibilizantes aplicados em consultório utilizados durante o clareamento com peróxido de hidrogênio a 35%: estudo clínico randomizado, ReBEC. http://www.ensaiosclinicos.gov.br/ Accessed 11 March 2020.
Parreiras SO, Szesz AL, Coppla FM, Martini EC, Farago PV, Loguercio AD, Reis A (2018) Effect of an experimental desensitizing agent on reduction of bleaching-induced tooth sensitivity: a triple-blind randomized clinical trial. J Am Dent Assoc 149:281–290. https://doi.org/10.1016/j.adaj.2017.10.025
Barbosa IF (2015) The effects of desensitizing agents previously applied during in-office bleaching, http://repositorio.unicamp.br/jspui/handle/REPOSIP/289627 Accessed 11 March 2020.
Abbud VP (2018) Efetividade do gel de nitrato de potássio 6% na sensibilidade do clareamento dentário de consultório - estudo clínico randomizado, https://tede.ufam.edu.br/handle/tede/6672 Accessed 11 March 2020.
Rezende M, Silva K, Miguel T, Farago PV, Loguercio AD, Reis A (2019) Prior application of 10% potassium nitrate to reduce post bleaching sensivity: a randomized, triple-blind clinical trial (in press). J Evid Based Dent Pract 20:101406. https://doi.org/10.1016/j.jebdp.2020.101406
de Paula B, Alencar C, Ortiz M, Couto R, Araujo J, Silva C (2019a) Effect of photobiomodulation with low-level laser therapy combined with potassium nitrate on controlling post-bleaching tooth sensitivity: clinical, randomized, controlled, double-blind, and split-mouth study. Clin Oral Invest 23:2723–2732. https://doi.org/10.1007/s00784-018-2715-4
Godoy CEM (2016) Efeito de dois agentes dessensibilizantes sobre o grau de clareamento e sensibilidade da dentina, aplicados previamente ao clareamento de consultório – Ensaio clínico controlado, randomizado, duplo-cego. http://tede.unioeste.br/handle/tede/3880 Accessed 11 March 2020.
de Paula E, Maran BM, Schmitt VL, Anquate-Netto C, Reis A, Naufel F (2019b) Prolonged application of a desensitizing gel before and after in-office bleaching to avoid bleaching-induced tooth sensitivity: a randomized, triple-blind controlled trial (submitted). J Dent.
Araújo F (2012) Clinical assessment of tooth sensitivity in patients undergoing in-office bleaching. IADR General Sessions, Final presentation ID: 2710.
Calheiros APC (2015) Efeito da terapia com laser de baixa potência na prevenção da sensibilidade pós clareamento dental de consultório. https://www.ibirapuera.br/bkp/mestrado/odontologia/pdf-teses/CALHEIROS2015.pdf Accessed 11 March 2020.
Cerqueira RR (2013) Efeito do uso de agente dessensibilizante na efetividade do clareamento e na sensibilidade dental. Rev Assoc Paul Cir Dent 67:64–67
Crescente CL (2016) Análise da sensibilidade após uso prévio de dessensibilizantes em clareamento dental. Rev Bras Odontol 73:34–38
Dias MSF (2017) Avaliação do efeito dos agentes dessensibilizantes após clareamento dentário em consultório, http://dspace.bc.uepb.edu.br/jspui/bitstream/123456789/18593/1/PDF-%20Malena%20Su%C3%AAnia%20Fernandes%20Dias.pdf Accessed 11 March 2020.
Leonard RH, Smith LR, Garland GE, Caplan DJ (2004) Desensitizing agent efficacy during whitening in an at-risk population. J Esthet Restor Dent 16:49–55. https://doi.org/10.1111/j.1708-8240.2004.tb00452
Martins G, Izidoro A, Meister L, Pereira S, Gomes O, Loguercio AD, Reis A (2011) Effect of a desensitizing agent used before in-office bleaching in the bleaching efficiency. Assoc Paul Cirur Dent 206.
Nanjundasetty JK, Ashrafulla M (2016) Efficacy of desensitizing agents on postoperative sensitivity following an in-office vital tooth bleaching: a randomized controlled clinical trial. J Conserv Dent 19:207–211. https://doi.org/10.4103/0972-0707.181927
Pierote JJA (2019) Effect of experimental desensitizer on the reduction of pain sensitivity associated with tooth bleaching: longitudinal controlled double-blind clinical trial. http://repositorio.unicamp.br/jspui/bitstream/REPOSIP/334434/1/Pierote_JosueJuniorAraujo_D.pdf Accessed 11 March 2020.
Tawfik S, Khairy M, ElBaz M, El Korashy M (2019) Evaluation of post-bleaching hypersensitivity using desensitizing agent before and /or after in-office bleaching: a randomized clinical trial. F1000Res 8. https://doi.org/10.12688/f1000research.20841.
Santos K (2017), Avaliação do efeito da laserterapia de baixa potência na sensibilidade dental induzida por clareamento dentário em consultório. http://dspace.bc.uepb.edu.br/jspui/handle/123456789/18591 Accessed 11 March 2020.
Pale M, Mayoral JR, Llopis J, Valles M, Basilio J, Roig M (2014) Evaluation of the effectiveness of an in-office bleaching system and the effect of potassium nitrate as a desensitizing agent. Odontology 102:203–210. https://doi.org/10.1007/s10266-013-0132-3
Marson FC, Goncalves RS, Silva CO, Cintra LT, Pascotto RC, Santos PH, Briso AL (2015) Penetration of hydrogen peroxide and degradation rate of different bleaching products. Oper Dent 40:72–79. https://doi.org/10.2341/13-270-L
Bowles WH, Ugwuneri Z (1987) Pulp chamber penetration by hydrogen peroxide following vital bleaching procedures. J Endod 13:375–377. https://doi.org/10.1016/S0099-2399(87)80196-6
Hanks CT, Fat JC, Wataha JC, Corcoran JF (1993) Cytotoxicity and dentin permeability of carbamide peroxide and hydrogen peroxide vital bleaching materials, in vitro. J Dent Res 72:931–938. https://doi.org/10.1177/00220345930720051501
Soares DG, Basso FG, Pontes EC, Garcia FL, Hebling J, Costa CAS (2014) Effective tooth-bleaching protocols capable of reducing H(2)O(2) diffusion through enamel and dentine. J Dent 42:351–358. https://doi.org/10.1016/j.jdent.2013.09.001
Costa CA, Riehl H, Kina JF, Sacono NT, Hebling J (2010) Human pulp responses to in-office tooth bleaching. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 109:e59–e64. https://doi.org/10.1016/j.tripleo.2009.12.002
Parreiras SO, Mena-Serrano A, Moreira CG, Otuki M, Loguercio AD, Reis A (2014) Penetration and cytotoxicity of a bleaching gel activated by LED/laser in restored teeth. Am J Dent 27:301–306
Mena-Serrano AP, Parreiras SO, do Nascimento EM, Borges CP, Berger SB, Loguercio AD, Reis A (2015) Effects of the concentration and composition of in-office bleaching gels on hydrogen peroxide penetration into the pulp chamber. Oper Dent 40:E76–E82. https://doi.org/10.2341/13-352-L
Acuna ED, Parreiras SO, Favoreto MW, Cruz GP, Gomes A, Borges CP, Loguercio AD, Reis A (2019) In-office bleaching with a commercial 40% hydrogen peroxide gel modified to have different pHs: Color change, surface morphology, and penetration of hydrogen peroxide into the pulp chamber. J Esthet Restor Dent 20:1–6. https://doi.org/10.1111/jerd.12453
Balladares L, Alegria-Acevedo LA, Montenegro-Arana A, Arana-Gordillo LA, Pulido C, Salazar-Gracez MT, Reis A, Loguercio AD (2019) Effects of pH and application technique of in-office bleaching gels on hydrogen peroxide penetration into the pulp chamber. Oper Dent 44:659–667. https://doi.org/10.2341/18-148-L
Cook SP, McCleskey EW (2002) Cell damage excites nociceptors through release of cytosolic ATP. Pain 95:41–47. https://doi.org/10.1016/s0304-3959(01)00372-4
Markowitz K (2010) Pretty painful: why does tooth bleaching hurt? Med Hypotheses 74:835–840. https://doi.org/10.1016/j.mehy.2009.11.044
Kwon SR, Dawson DV, Schenck DM, Fiegel J, Wertz PW (2015) Spectrophotometric evaluation of potassium nitrate penetration into the pulp cavity. Oper Dent 40:614–621. https://doi.org/10.2341/14-214-L
Kwon SR, Dawson DV, Wertz PW (2016) Time course of potassium nitrate penetration into the pulp cavity and the effect of penetration levels on tooth whitening efficacy. J Esthet Restor Dent 28:S14–S22. https://doi.org/10.1111/jerd.12192
Hodosh M (1974) A superior desensitizer - potassium nitrate. J Am Dent Assoc 88:831–832. https://doi.org/10.14219/jada.archive.1974.0174
Bartold PM (2006) Dentinal hypersensitivity: a review. Aust Dent J 51:212–218. https://doi.org/10.1111/j.1834-7819.2006.tb00431.x
Haywood VB, Caughman WF, Frazier KB, Myers ML (2001) Tray delivery of potassium nitrate-fluoride to reduce bleaching sensitivity. Quintessence Int 32:105–109. https://doi.org/10.4103/0972-0707.181927
Rezende M, Coppla FM, Chemin K, Chibinski AC, Loguercio AD, Reis A (2019) Tooth sensitivity after dental bleaching with a desensitizer-containing and a desensitizer-free bleaching gel: a systematic review and meta-analysis. Oper Dent 44:58–74. https://doi.org/10.2341/17-253-L
Charrois TL (2015) Systematic reviews: what do you need to know to get started? Can J Hosp Pharm 68(2):144–148. https://doi.org/10.4212/cjhp.v68i2.1440
Torres C, Zanatta RF, Silva TJ, Borges AB (2019) Effect of calcium and fluoride addition to hydrogen peroxide bleaching gel on tooth diffusion, color, and microhardness. Oper Dent 44:424–432. https://doi.org/10.2341/18-113-L
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 62:e1–e34. https://doi.org/10.1016/j.jclinepi.2009.06.006
Bashir Y, Conlon KC (2018) Step by step guide to do a systematic review and meta-analysis for medical professionals. Ir J Med Sci 187:447–452. https://doi.org/10.1007/s11845-017-1663-3
Browning WD, Chan DC, Myers ML, Brackett WW, Brackett MG, Pashley DH (2008) Comparison of traditional and low sensitivity whiteners. Oper Dent 33:379–385. https://doi.org/10.2341/07-134
Borenstein M (2019) Common mistakes in meta-analysis: and how to avoid them. Biostat Incorporated (2019).
Borenstein M, Hedges LV, Higgins JPT, Rothstein HR (2011) Introduction to meta-analysis Wiley.
Loguercio AD, Maran BM, Hanzen TA, Paula AM, Perdigao J, Reis A (2017) Randomized clinical trials of dental bleaching - compliance with the CONSORT Statement: a systematic review. Braz Oral Res 31:e60. https://doi.org/10.1590/1807-3107BOR-2017
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study, formal consent is not required.
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Martini, E.C., Favoreto, M.W., Rezende, M. et al. Topical application of a desensitizing agent containing potassium nitrate before dental bleaching: a systematic review and meta-analysis. Clin Oral Invest 25, 4311–4327 (2021). https://doi.org/10.1007/s00784-021-03994-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-021-03994-6