Abstract
Objectives
To answer whether the topical drug application can reduce in-office tooth bleaching sensitivity without impairing the color change.
Materials and methods
This review was registered on PROSPERO (CRD42024524171). Two reviewers screened PubMed, Web of Science, Scopus, Embase, and clinicaltrials.gov in March 2024 independently for randomized clinical trials investigating the efficacy of topical drug application to manage in-office tooth bleaching sensitivity. The risk of bias was assessed using Cochrane’s Risk of Bias tool (RoB2). Certainty of the evidence was assessed using the Grading of Recommendations: Assessment, Development, and Evaluation tool (GRADE). The meta-analyses evaluated the bleaching sensitivity and color change with RevMan 5.4 software.
Results
334 articles were retrieved. The final sample was composed of four articles. Tested drugs were Otosporin, Eugenol, Ibuprofen with arginine, and Dipyrone. The meta-analysis evidenced no difference in bleaching sensitivity up to 1 h (MD, -0.39; 95% CI, -0.89, 0.11), 24 h (MD, -0.26, 95% CI, -0.71, 0.18), or 48 h (MD, 0.00, 95% CI, -0.16, 0.16). Meta-analysis for color change evidenced no difference for color change (MD, 0.03; 95% IC, -0.56, 0.61). The risk of bias was low. The certainty of the evidence was rated moderate for bleaching sensitivity and high for color change.
Conclusions
Although topical drug application did not impair color change, it was ineffective in reducing in-office tooth bleaching sensitivity.
Clinical relevance
topical drug application on dental enamel is not an effective approach in reducing bleaching sensitivity, but several modifications can be made in future studies to possibly achieve a better outcome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tooth bleaching is effective in changing the color of the teeth, being a very satisfactory, conservative, safe treatment, and improving the patient’s self-confidence [1]. It can be performed by using different techniques: at-home bleaching, in-office bleaching, or the combined method, which uses both techniques together [2]. Although a statistically significant difference was not obtained, previous reviews show a tendency for higher sensitivity in the in-office bleaching technique, since a much higher bleaching agent concentration is used [3, 4]. Thus, more painless in-office bleaching protocols are necessary.
Since the inflammatory infiltrate due to in-office tooth bleaching with hydrogen peroxide is the most common cause associated with bleaching sensitivity [5], previous investigations evaluated the efficacy of oral anti-inflammatory drugs to decrease pulp inflammation and bleaching sensitivity. However, recent systematic reviews all concluded that the use of several oral anti-inflammatory drugs promoted no reduction in the risk and intensity of bleaching sensitivity [6,7,8]. One of the primary causes behind these findings originates from the restricted ability of drugs to penetrate the pulp chamber when administered orally [8]. Therefore, the ineffectiveness of oral drugs in reducing sensitivity caused by in-office bleaching suggests the need to explore alternative routes of administration.
Existing literature corroborates that administering drugs topically offers numerous advantages over the oral route, such as controlled application, avoidance of the first-pass metabolism in the liver, and reduction of gastrointestinal side effects [9, 10]. Drug topical administration is the first choice of treatment for noninvasive oral lesions of candidiasis [11]. The drug topical administration route, already explored in other fields of research for pain management, can achieve the targeted site directly while having a limited systemic absorption percentage [9, 12]. Therefore, a topical drug application over the dental surface could promote pulp absorption if the drug permeates through enamel and dentine, acting directly on the inflamed tissue.
Recent animal studies tested the efficacy of topical drug application to the enamel surface and showed positive results in reducing the inflammatory infiltrate, which suggests that this course of treatment could be effective in humans [13,14,15,16]. Likewise, randomized clinical trials have been carried out aiming to answer if the topical drug application would reduce in-office tooth bleaching sensitivity without impairing color change [17,18,19]. However, the diversity of the primary study results demands a comprehensive synthesis of the evidence to analyze, summarize, and establish the level of evidence on the efficacy of topical drug application in reducing in-office bleaching-mediated tooth sensitivity.
Therefore, this systematic review and meta-analysis aimed to answer whether topical drug application to the enamel surface can reduce in-office bleaching-mediated tooth sensitivity. In addition, this paper will also answer if this novel treatment will impair the color change. The following PICOS guided this article: (P) patients undergoing in-office tooth bleaching. (I) topical drug application. (C) control groups or placebo application. (O) bleaching sensitivity and color change. (S) randomized clinical trials.
Methods
This systematic review and meta-analysis were conducted according to the Cochrane Handbook for Systematic Reviews of Interventions [20] and reported using the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [21]. The protocol was registered on the PROSPERO platform under the identifier CRD42024524171.
Eligibility criteria
This review followed the PICOS question below.
P(opulation): patients undergoing in-office tooth bleaching.
I(ntervention): topical drug application.
C(omparison): control groups or placebo application.
O(utcomes): bleaching sensitivity and color change.
S(tudy design): randomized clinical trials.
Inclusion criteria: randomized clinical trials investigating the efficacy of topical drug application to the enamel surface to treat or prevent in-office tooth bleaching sensitivity and the effect on color change. No restriction was made regarding language or year of publication to comprehend an ample search in the literature.
Exclusion criteria: studies with systemic drug administration and studies with no control group.
Information sources
The electronic databases PubMed, Web of Science, Scopus, Embase, and clinicaltrials.gov were last searched in March 2024, comprehending articles published in any year. All searches were exported to the Rayyan (https://www.rayyan.ai) platform with the Ris or BibTex format.
Search strategy
The following search strategy was applied in all databases. Adaptations were made following the databases’ directions.
(sensitivity OR pain OR “pain management” OR ache OR discomfort)
AND.
((bleaching OR whitening) AND (in-office OR “in office” OR professional))
AND.
(“administration, topical” OR “topical drug administration” OR “topical administration” OR “topical administrations” OR “topical drug administrations” OR “administration buccal” OR “buccal drug administration” OR “buccal administration” OR “buccal administrations” OR “topical drug” OR “topical medication” OR “topical medicine” OR “topical application” OR gel OR nanogel OR nano-gel OR “nano gel” OR emulsion OR solution OR cream OR paste OR nanoemulsion OR nano-emulsion OR “nano emulsion”)
AND.
(NSAIDs OR corticosteroids OR antibiotics OR anti-inflammatory OR “anti-inflammatory” OR antinflammatory OR analgesic OR hydrocortisone OR acetaminophen OR paracetamol OR Ibuprofen OR Otosporin™ OR Carvedilol OR Dipyrone OR Eugenol OR diclofenac OR dexamethasone OR betamethasone OR tramal OR tramadol OR nimesulide OR opioids OR opioid OR codeine OR medication OR medications OR drugs OR medicine OR remedy)
Selection process
Two previously calibrated reviewers (FJDO and MJFC) conducted the selection process independently. Calibration was made using approximately 10% of the initial sample (30 articles) (k = 0.88).
After exportation to the Rayyan platform, the platform initially detected duplicates, and a reviewer (FJDO) assessed if the detection was accurate, solving the duplicate exclusion phase. Studies were then selected in a two-step independent evaluation: first, articles were selected by reading the title and abstract. Studies approved in this initial selection were then submitted to a second selection by full-text reading. Only the studies approved in the second selection were included in the final sample. Any disagreement between reviewers was solved by discussion or intervention from a third reviewer (BCDB), if necessary.
Data collection process
Studies were screened by one reviewer for the following information: drug used, product composition, study design, sample size, groups tested, bleaching technique, method and time of drug application, outcome assessed and evaluation time, and main results. This information is organized in Table 1.
Meta-analysis
The meta-analyses were conducted with the software Review Manager version 5.4 to evaluate bleaching sensitivity, and color change. In the primary studies, bleaching sensitivity was measured using a Visual Analog Scale (VAS) filled out by the patients, while color change was measured in ΔE using digital devices. Therefore, data extracted from the primary studies consisted of continuous variables: mean pain per VAS for bleaching sensitivity, and ΔE results for color change. The mean difference was the effect measure chosen to perform the meta-analysis because there were no methodological differences in the assessment of variables. The random-effects model was chosen. Analyses were made with a 95% confidence interval (CI). The statistical significance for the variable tested was considered when the meta-analysis resulted in p < 0.05.
The heterogeneity analysis was also performed from the Review Manager 5.4 using the value of the I2 test.
Risk of bias assessment
Two independent reviewers (FJDO and MJFC) conducted the risk of bias assessment using the Cochrane Collaboration tool (Rob 2) [22]. The assessment criteria comprised five domains: sequence generation, allocation concealment, blinding of outcome assessors, incomplete outcome data, and selective outcome reporting. The analysis was made for the two evaluated outcomes, bleaching sensitivity and color change. Throughout data selection and quality assessment, any disagreements between the reviewers were resolved through discussion, and if necessary, a third reviewer (BCDB) was consulted.
Certainty of evidence assessment
Two independent reviewers (FJDO and MJFC) evaluated the certainty of evidence for each outcome across studies using the Grading of Recommendations: Assessment, Development, and Evaluation (GRADE) [23]. The GRADE approach initially assigns a high level of evidence for randomized clinical trials, but this can be downgraded by one or two levels due to five criteria (methodological limitations, imprecision, inconsistency, indirectness of evidence, and publication bias). In addition, evidence can also be elevated by three criteria (high magnitude of effect, residual confounders, and dose-response gradient). The analysis was made for the two evaluated outcomes, bleaching sensitivity and color change. Throughout the process of certainty of evidence assessment, any disagreements between the reviewers were resolved through discussion, and if necessary, a third reviewer (BCDB) was consulted.
Results
General characteristics
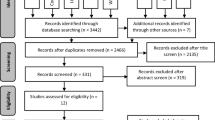
The database search initially resulted in 334 articles. After two-step selection, four articles were selected to compose the final sample. The selection process is detailed in the flowchart in Fig. 1.
The published data of the selected articles varied between 2018 and 2024. All articles were published in English. Regarding the country of the studies, all studies were from Brazil.
All studies were performed in a split-mouth design. In total, 258 patients were evaluated, resulting in 516 hemi-arches.
Drugs evaluated
The studies evaluated Otosporin, Ibuprofen in combination with arginine, dipyrone, and eugenol. The first was used as a solution, whereas all remaining three were used as a gel. In the study by Vilela et al. (2021) [18] they used a nanogel formulation.
All studies applied the drug before the bleaching session. The application was made for 10 min. HortKoff et al. (2024) [24] applied the gel for 20 s before leaving undisturbed for 10 min. Rezende et al. (2018) [19] actively applied the drug for 20 s after leaving undisturbed for 10 min.
Bleaching technique
All studies performed bleaching treatment in two sessions with a 1-week interval. The product was the same in all studies: 35% hydrogen peroxide (Whiteness HP Automixx, FGM, Joinville, SC, Brazil).
Bleaching sensitivity
All studies reported the absolute risk and intensity of bleaching sensitivity using the visual analog scale and the numeric rating scale at 1 h, 24 h, and 48 h after each bleaching session.
Two studies [17, 18] reported no statistically significant difference in the absolute risk or intensity of bleaching sensitivity between experimental and control groups. One study [19] reported a difference only at 1 h after favoring the experimental group. The study by Hortkoff et al. (2024) [24] reported a statistically significant reduction for both absolute risk and intensity of bleaching sensitivity, favoring the experimental group.
Color change
The color change was evaluated through both subjective and objective measurements. The first was performed with the value-oriented shade guide Vita Classical and the Vita Bleachedguide, and the latter through the spectrophotometer Vita Easyshade.
All studies reported no statistically significant difference between experimental and control groups regarding tooth color change.
Meta-analysis
Bleaching sensitivity
All studies were included in the meta-analysis for sensitivity evaluation up to 1 h, 24 h, and 48 h after bleaching. The results from all meta-analyses, shown in Figs. 2 and 3, and 4, respectively, evidence no statistically significant difference in bleaching sensitivity between experimental and control groups up to 1 h (MD, -0.39; 95% CI, -0.89, 0.11), 24 h (MD, -0.26, 95% CI, -0.71, 0.18), or 48 h (MD, 0.00, 95% CI, -0.16, 0.16).
Color change
All studies were included in the meta-analysis for color change evaluation. The results shown in Fig. 5 evidence no statistically significant difference in color change between experimental and control groups (MD, 0.03; 95% IC, -0.56, 0.61).
Risk of bias assessment
All studies evaluated were judged to have a low risk of bias for all domains analyzed and an overall bias for bleaching sensitivity and color change. The analysis considered information in the primary publication and the trial protocols. All studies demonstrated good methodological descriptions of the studies; hence, the overall bias was low. Judgment for all criteria is available in Figs. 6 and 7 for bleaching sensitivity and color change, respectively.
Certainty of the evidence
The overall certainty of the evidence was graded moderate for bleaching sensitivity and high for color change. Although initially graded high because of the design of the studies being randomized clinical trials, the evidence was reduced in one level for imprecision in bleaching sensitivity. No methodological limitations were pointed out since no bias was judged to be significantly present among primary studies. No indirectness was identified because all studies directly compared the intended intervention and the control. No inconsistency was judged because the heterogeneity, evaluated through I2 test, was low. No publication bias was suspected since a broad search was made, and negative and positive outcome trials were identified and included in the review. A funnel plot was not made to identify publication bias further because the meta-analysis included less than 10 articles. Regarding imprecision, evidence was downgraded one level for bleaching sensitivity evaluation because, even though confidence intervals were fairly narrow, the clinical recommendation would be significantly different considering the upper and lower end of the confidence interval since the result of the meta-analyses crossed the line of no effect. This judgment was made following GRADE guidelines for rating imprecision [25]. The color change evaluation was not downgraded for imprecision because the absence of difference between the two groups presents a positive effect for the tested intervention since the topical drug application should not interfere with color change. There was no upgraded level because of the high magnitude of effect, residual confounders, and dose-response gradient since none of these criteria was observed in the articles. The certainty of evidence assessment is detailed in Tables 2 and 3 for bleaching sensitivity and color change, respectively.
Discussion
To the author’s knowledge, this is the first systematic review evaluating the efficacy of topical drug application in reducing or preventing in-office tooth bleaching sensitivity, and whether this approach would impair color change. According to our results, the proposed course of treatment had no statistically significant efficacy in reducing bleaching sensitivity up to 1 h, 24 h, and 48 h. The application of drugs also caused no impairment in the color change. Given the benefits of topical application, the adverse outcomes reported demand a thorough analysis of potential causes of ineffectiveness.
Enamel structure is known to be a highly mineralized tissue that partially enables the passage of ions and molecules [26]. The diameter of the tunnels evidenced in enamel structure ranges from 1 to 3 μm, a very narrow space that makes difficult passage through this hard tissue [27]. Enamel organization must be considered when a new drug is proposed to pass through this challenging pathway and reach the pulp chamber.
Factors related to the drug composition can also affect its permeability into the dental tissues to reach the pulp. Low molecular weight and low affinity for the tissue are desired to increase the drug permeability [19, 28]. Hydrogen peroxides present a low molecular weight and minimal interaction with the organic matrix in enamel, which results in a homogenous distribution [19, 28]. Following data from PubChem, the molecular weight of the tested drugs is: Ibuprofen (206 g/mol), Dipyrone (311 g/mol), Eugenol (164 g/mol), and Hydrocortisone (362.5 g/mol), the anti-inflammatory present in Otosporin. All tested drugs present a higher molecular weight than hydrogen peroxide (34 g/mol), and no information is available regarding the specific interactions and affinity with dental tissue that can reduce penetration. All these factors combined can impair penetration and reduce the amount of drug reaching the pulp chamber, which may not be enough to act on inflammation properly.
Two studies in the present review used commercially available drugs that were not manipulated to sustain such tasks better. Although it is not possible to affirm that this was the cause, it is valid to suggest that future investigations involve testing of nanostructured drugs, because nanosized drugs have advantages such as controlled release, reduced toxicity, longer action time, and overall better permeability [29], an ideal characteristic for the challenge imposed by enamel penetration. In addition, the drug characteristics need to be considered to use the pharmacokinetics in favor of better outcomes.
It is well known that hydrogen peroxide enhances tooth permeability, especially when used in high concentrations, such as the ones used in-office bleaching [30]. All four studies applied the drugs for 10 min before in-office bleaching. Still, considering hydrogen peroxide’s effect on dental enamel, suggesting that an application after the procedure could have better positive outcomes in future trials is valid.
Regarding possible causes for bleaching sensitivity, it is important to emphasize that the exact mechanism behind this type of pain is yet to be uncovered. Theories for this phenomenon cover, firstly, a possible association with reversible pulpitis, since bleaching agents penetrate the pulp chamber, causing irritation [31]. Second, a negative effect from the osmotic gradient caused by the water content on the bleaching product, drying the tooth structure [31]. Third, a possibility that bleaching sensitivity may be a form of dentin hypersensitivity, which is more acceptably explained by the hydrodynamic theory, in which several stimuli can activate intradental nerve endings, causing pain [31]. The uncertainty that covers the exact cause of bleaching sensitivity makes treatment difficult.
Previous animal studies reported that topical drug application to the enamel surface reduced inflammation [13,14,15,16]. The inflammatory process is one of the causes attributed to the in-office bleaching-mediated sensitivity. Of course, the findings of animal studies cannot be carelessly extrapolated, but if these drugs reduce inflammation, it is valuable to consider other causes for bleaching sensitivity. In this context, the transient receptor potential ankyrin 1 (TRPA1) is believed to play a critical role in bleaching sensitivity because the procedure increased the protein levels of hyperalgesia factors (TRPA1 and PANX1) [32]. Only one study had statistically significant results favoring experimental treatment with an Ibuprofen gel [24]. The study by De Logu et al. (2019) [33] pointed out that an ibuprofen metabolite, ibuprofen-acyl glucuronide, selectively antagonizes TRPA1, which can help understand why this drug was the most efficient [29].
As general limitations identified by this systematic review, it is important to highlight that, although an ample literature search was made, all identified studies were conducted in Brazil. Other countries should conduct similar clinical studies so researchers can understand if ethnic heterogeneity can influence different outcomes. Current literature already supports that pain is an individual experience that is possibly influenced by numerous demographic factors, such as sex, age, and ethnicity [34]. For example, a cohort study showed that several suprathreshold orofacial pain measures had significant racial differences, likely related to variances in central nociceptive processing among different ethnicities [34]. Therefore, the perception of bleaching sensitivity can be influenced by such ethnic differences.
Another limitation regarding methodological heterogeneity concerns the variety of the drugs tested, which can lead to variation in the results presented by each primary study. In addition, as the topical drug application approach to manage in-office bleaching sensitivity is new, a few studies are still available in the literature. The limited number of studies calls for careful consideration when extrapolating the findings of the present review to the proposed population.
In light of the present results, future studies should consider developing nanostructured drugs with nanotechnology with a high affinity for the TRPA1. A recent study [35] evaluating the in-silico affinity of several drugs with TRPA1 suggested that codeine and dexamethasone showed high binding affinity to TRPA1. Thus, further investigations might test nanostructured topical drugs based on codeine or dexamethasone regarding their permeability into the tooth and protective biological effect in cell cultures. In addition, such drugs should be applied before and after bleaching treatment when the enamel permeability, one of the biggest challenges with this course of treatment, is enhanced. This way, it is possible to improve further the potential of the drug to reach the pulp chamber and maybe obtain a more desirable effect on bleaching sensitivity. Therefore, considering the estimated effect favoring the experimental group, although a statistically significant difference was not reached, this promising field of research needs to be further explored with the feasible modifications highlighted previously.
Based on the evidence available in the literature, this work’s findings have proposed several directions for future studies. Dental care providers worldwide would benefit greatly from understanding the new proposed courses of treatment and whether they are, in fact, effective. Moreover, shedding light on a new research topic can provide visibility to better studies on a novel treatment strategy.
Conclusion
Based on the evidence in the literature, it can be concluded that topical drug application is ineffective in reducing or preventing in-office tooth bleaching sensitivity and does not impair the efficacy of color change. However, this novel treatment modality requires further investigation. Future research should consider working with nanostructured drug formulations with affinity for the TRPA1 receptor.
Data availability
No datasets were generated or analysed during the current study.
References
Joiner A, Luo W (2017) Tooth colour and whiteness: a review. J Dent 67:3–10. https://doi.org/10.1016/j.jdent.2017.09.006
Pereira R, Silveira J, Dias S et al (2022) Bleaching efficacy and quality of life of different bleaching techniques — randomized controlled trial. Clin Oral Investig 26:7167–7177. https://doi.org/10.1007/s00784-022-04678-5
Aidos M, Marto CM, Amaro I et al (2024) Comparison of in-office and at-home bleaching techniques: an umbrella review of efficacy and post-operative sensitivity. https://doi.org/10.1016/j.heliyon.2024.e25833. Heliyon
de Geus J, Wambier L, Kossatz S et al (2016) At-home vs In-office bleaching: a systematic review and Meta-analysis. Oper Dent 41:341–356. https://doi.org/10.2341/15-287-LIT
Vaz MM, Lopes LG, Cardoso PC et al (2016) Inflammatory response of human dental pulp to at-home and in-office tooth bleaching. J Appl Oral Sci 24:509–517. https://doi.org/10.1590/1678-775720160137
Almassri HNS, Zhang Q, Yang X, Wu X (2019) The effect of oral anti-inflammatory drugs on reducing tooth sensitivity due to in-office dental bleaching. J Am Dent Assoc 150:145–157. https://doi.org/10.1016/j.adaj.2019.05.023
da Silva A, Muniz R, de Assis C et al (2022) Effect of preoperative use of nonsteroidal anti-inflammatory drugs on dental sensitivity induced by dental bleaching in the dental office: a systematic review and meta-analysis. Oper Dent 47:503–513. https://doi.org/10.2341/21-088-LIT
Carregosa Santana ML, Leal PC, Reis A, Faria-e-Silva AL (2019) Effect of anti-inflammatory and analgesic drugs for the prevention of bleaching-induced tooth sensitivity: a systematic review and meta-analysis. J Am Dent Assoc 150:818–829. https://doi.org/10.1016/j.adaj.2019.05.004
Klinge SA, Sawyer GA (2013) Effectiveness and safety of topical versus oral nonsteroidal anti-inflammatory drugs: a comprehensive review. Phys Sportsmed 41:64–74. https://doi.org/10.3810/psm.2013.05.2016
Sanz R, Calpena A, Mallandrich M, Clares B (2015) Enhancing topical analgesic administration: review and prospect for transdermal and transbuccal drug delivery systems. Curr Pharm Des 21:2867–2882. https://doi.org/10.2174/1381612821666150428145627
Rai A, Misra SR, Panda S et al (2022) Nystatin effectiveness in oral candidiasis treatment: a systematic review & meta-analysis of clinical trials. Life 12:1677. https://doi.org/10.3390/life12111677
Leppert W, Malec–Milewska M, Zajaczkowska R, Wordliczek J (2018) Transdermal and topical drug administration in the treatment of pain. Molecules 23:681. https://doi.org/10.3390/molecules23030681
Benetti F, Briso ALF, Ferreira LL et al (2018) In vivo study of the action of a topical anti-inflammatory drug in rat teeth submitted to dental bleaching. Braz Dent J 29:555–561. https://doi.org/10.1590/0103-6440201802177
Louzada LM, Briso ALF, Benetti F et al (2019) Anti-inflammatory potential of a carvedilol gel in the pulpal tissue of rats after dental bleaching: a histopathological evaluation. J Investig Clin Dent. https://doi.org/10.1111/jicd.12401
de Oliveira Gallinari M, Ângelo Cintra LT, Benetti F et al (2019) Pulp response of rats submitted to bleaching and the use of different anti-inflammatory drugs. PLoS ONE 14:1–15. https://doi.org/10.1371/journal.pone.0210338
Moura SKSCF, dos Santos MLV, do Nascimento LA et al (2022) Design of a thermosensitive ibuprofen-loaded nanogel as smart material applied as anti-inflammatory in tooth bleaching: an in vivo study. J Drug Deliv Sci Technol. https://doi.org/10.1016/j.jddst.2022.103123
Favoreto MW, Vochikovski L, Terra RMO et al (2022) Topical application of Otosporin® before in-office bleaching: a split mouth, triple-blind, multicenter randomized clinical trial. Clin Oral Investig 26:2555–2564. https://doi.org/10.1007/s00784-021-04224-9
Vilela AP, Rezende M, Terra RMO et al (2021) Effect of topical application of nanoencapsulated eugenol on dental sensitivity reduction after in-office dental bleaching: a randomized, triple-blind clinical trial. J Esthet Restor Dent 33:660–667. https://doi.org/10.1111/jerd.12728
Rezende M, Chemin K, Vaez SC et al (2018) Effect of topical application of dipyrone on dental sensitivity reduction after in-office dental bleaching: a randomized, triple-blind multicenter clinical trial. J Am Dent Assoc 149:363–371. https://doi.org/10.1016/j.adaj.2017.11.003
Higgins J, Thomas J, Chandler J et al (2020) Cochrane handbook for systematic reviews of interventions. Chichester, United Kingdom
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. https://doi.org/10.1371/journal.pmed.1000097
Higgins JPT, Altman DG, Gotzsche PC et al (2011) The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343:5928–5928. https://doi.org/10.1136/bmj.d5928
Guyatt G, Oxman AD, Akl EA et al (2011) GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 64:383–394. https://doi.org/10.1016/j.jclinepi.2010.04.026
Hortkoff D, da Silva KL, Farago PV et al (2024) Effect of topical application of ibuprofen/arginine on the in-office bleaching-induced tooth sensitivity: a randomized, triple-blind controlled trial. J Dent 142:104875. https://doi.org/10.1016/j.jdent.2024.104875
Guyatt GH, Oxman AD, Kunz R et al (2011) GRADE guidelines 6. Rating the quality of evidence—imprecision. J Clin Epidemiol 64:1283–1293. https://doi.org/10.1016/j.jclinepi.2011.01.012
Ragit R, Fulzele P, Rathi NV et al (2022) Iontophoresis as an effective drug delivery system in dentistry: a review. https://doi.org/10.7759/cureus.30658. Cureus
Kunin AA, Evdokimova AY, Moiseeva NS (2015) Age-related differences of tooth enamel morphochemistry in health and dental caries. EPMA J 6:3. https://doi.org/10.1186/s13167-014-0025-8
Yu Y-Q, Yang X, Wu X-F, Fan Y-B (2021) Enhancing permeation of drug molecules across the skin via delivery in nanocarriers: novel strategies for effective transdermal applications. Front Bioeng Biotechnol. https://doi.org/10.3389/fbioe.2021.646554
Daniel AN, Sartoretto SM, Schmidt G et al (2009) Anti-inflammatory and antinociceptive activities a of eugenol essential oil in experimental animal models. Rev Bras Farmacogn 19:212–217. https://doi.org/10.1590/S0102-695X2009000200006
Rauen CA, Chidoski Filho JC, Bittencourt BF et al (2015) Effect of bleaching agents containing fluoride or calcium on enamel microhardness, roughness and permeability. Braz J Oral Sci 14:262–266. https://doi.org/10.1590/1677-3225v14n4a02
Kielbassa AM, Maier M, Gieren A-K, Eliav E Tooth sensitivity during and after vital tooth bleaching: a systematic review on an unsolved problem. Quintessence Int 46:881–897. https://doi.org/10.3290/j.qi.a34700
Chen C, Huang X, Zhu W et al (2021) TRPA1 triggers hyperalgesia and inflammation after tooth bleaching. Sci Rep 11:17418. https://doi.org/10.1038/s41598-021-97040-w
De Logu F, Li Puma S, Landini L et al (2019) The acyl-glucuronide metabolite of ibuprofen has analgesic and anti-inflammatory effects via the TRPA1 channel. Pharmacol Res 142:127–139. https://doi.org/10.1016/j.phrs.2019.02.019
Ostrom C, Bair E, Maixner W et al (2017) Demographic predictors of pain sensitivity: results from the OPPERA Study. J Pain 18:295–307. https://doi.org/10.1016/j.jpain.2016.10.018
Costa MJF, Sette-de-Souza PH, Borges BCD (2023) In silico affinity between analgesic/anti-inflammatory drugs and the transient receptor potential A1 to predict potential pharmacological managing approaches for bleaching sensitivity. Acad Bras Cienc. https://doi.org/10.1590/0001-3765202320230555
Acknowledgements
We thank Maria Luisa Pacheco and Thauan Vitor for their initial contribution to the research project.
Funding
This work was supported by the Coordination for the Improvement of Higher Education Personnel (CAPES) (grants 88887.706007/2022-00 and 88887.907567/2023-00). The funding source did not specifically participate in the design, collection, analysis, interpretation of data, writing, or decision to submit for publication.
Author information
Authors and Affiliations
Contributions
The initial idea is attributed to B.C.D.B.F.J.D.O. and M.S.B performed the literature search.F.J.D.O. and M.J.F.C. performed data analysis.F.J.D.O. drafted the manuscript.B.C.D.B. critically revised the work.
Corresponding author
Ethics declarations
Ethical approval
Not Applicable.
Informed consent
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Oliveira, F.J.D., Costa, M.J.F., de Bessa, M.S. et al. Efficacy of topical drug application to manage in-office bleaching sensitivity: a systematic review and meta-analysis of randomized clinical trials. Clin Oral Invest 28, 452 (2024). https://doi.org/10.1007/s00784-024-05851-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00784-024-05851-8