Abstract
The aims of the study were to evaluate by spectrophotometer the in vivo colour changes resulting from the application of an in-office tooth bleaching system containing 28 % H2O2 by light-emitting diode (LED) activation and to determine whether the application of 5 % potassium nitrate 30 min before bleaching decreased tooth sensitivity. Thirty-two individuals were assigned randomly to two groups (n = 16). Group A received 5 % potassium nitrate as a desensitizing agent 30 min before bleaching with 28 % hydrogen peroxide activated by LED. Group B received glycerin as a placebo and the same bleaching protocol was applied. The colour of the right central incisor of each patient was measured visually and by spectrophotometer before bleaching, immediately thereafter, 15 days and 3 months later. Differences in L* a* b* values were tested with a repeated measures analysis of variance (ANOVA). Differences in ΔΕ values were tested with ANOVA statistical analysis at a 0.05 level of significance. Significant (p < 0.05) differences were detected in L*, as well as in b* values, between initial (I) and post bleaching (PB) and between initial (I) and 3 months post-op. In contrast, there was no significant difference between PB and 3 months post-op. The a* values showed no statistically significant differences among the different time points. Tooth sensitivity decreased significantly when potassium nitrate was applied. In-office bleaching system gave quantitatively stable results over a 3-month period. Tooth sensitivity was reduced significantly, when a desensitizing agent was applied 30 min before treatment, but the efficacy of bleaching decreased.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent decades, dentistry has undergone many changes, especially in the area of aesthetics. Influenced by the media, which places a great emphasis on health and beauty, patients commonly desire white teeth [1], because whiter teeth are perceived to be associated with health and beauty [2, 3]. As compared with the use of resin-bonded composites, porcelain veneers, and crowns [4, 5], bleaching is the best conservative treatment for lightening teeth to improve the appearance of the smile [1].
Popular demand for tooth whitening has existed for more than 125 years. There are three major approaches to the bleaching of vital teeth: in-office bleaching, clinician-supervised “night guard” bleaching (administrated by the clinician for home use by the patient), and commercial bleaching products (applied by the patient) [6]. In-office bleaching procedures predominated until 1989, when the first at-home “night guard” bleaching system was introduced by Haywood and Heymann [1, 7, 8].
“Power bleaching” is an in-office whitening technique that was developed to bleach teeth during a single dental visit using a combination of a whitening agent such as hydrogen peroxide and an auxiliary activator such as light. All of the smile-line teeth are whitened simultaneously. This system includes rapid lightening, use of peroxide gels of lower concentration for shorter periods, a protection of the gingiva with a barrier material is used, and the degree of bleaching is controlled by the dentist [7, 9].
The latest bleaching agents that are intended for professional application are based on 35–50 % hydrogen peroxide with photosensitive components that initiate and catalyse the reaction upon exposure to a light source. Different light sources are used and include blue-coloured halogen curing lamps, light-emitting diodes (LEDs), infrared CO2 lasers, blue-coloured plasma arc lamps, blue argon lasers, and 980-nm gallium-aluminium-arsenium (GaAlAs) lasers [10]. LEDs are a cost-effective alternative to lasers, because they require less energy to generate light. The efficiency of LEDs is also higher than that of halogen light-curing units, and they produce less heat [10].
Evaluation of the efficacy and safety of tooth whitening systems has received considerable attention. Concern has been expressed about limitations to efficacy, such as the colour rebound time and the intensity of the stain to be removed, and about issues of safety, including an increase in tooth sensitivity, soft-tissue irritation, and systemic effects of the bleaching agent [7]. Increased tooth sensitivity is usually reversible, and resolves itself over time or with the help of a desensitizing agent (DSA) [5]. Two DSAs that are used widely to treat tooth sensitivity during “night guard” vital bleaching are neutral fluoride and 3–5 % potassium nitrate. Fluoride is used to occlude the tubules. Potassium nitrate works primarily by compromising the ability of nerves to transmit pain [5, 11, 12]. The use of more stable and less caustic hydrogen peroxide bleaching materials or 10 % carbamide peroxide that contains fluoride and potassium nitrate might help to meet the demands of patients to achieve whiter teeth more quickly, with more predictable results and reduced risk of tooth sensitivity [4]. However, little previous research has investigated the effectiveness of bleaching and the reduction of sensitivity that is associated with the use of DSAs.
Methods to measure tooth discolouration and the degree of bleaching range from subjective methods of comparison to objective instrumental techniques [13]. The visual determination of colour by comparing a patient’s tooth with a colour standard (i.e. a commercially available shade guide) is the method that is applied most frequently in clinical dentistry. However, the determination of tooth colour by visual means is considered to be highly subjective [14]. A number of different methods enable the objective evaluation of discolouration and the colour change that occurs during tooth whitening procedures; these include spectrophotometry, colorimetric, and computer analysis of digital images. The use of quantitative light-induced fluorescence (QLF) in vitro has also been described in the literature. Spectrophotometers are highly precise, and are relatively simple and easy to use. They measure the reflectance or transmittance of an object a single wavelength at a time and are based on the CIE L*a*b* colour space (CIELAB) system, which was defined by the International Commission on Illumination in 1967. L* represents the value (lightness or darkness), a* is the measurement along the red–green axis, and b* is the measurement along the yellow–blue axis. A positive a* value indicates red, whereas a negative a* value indicates green; a positive b* value indicates yellow and a negative b* value indicates blue [13].
The aims of the in vivo study were to test and evaluate quantitatively whether an in-office tooth bleaching system that used 28 % H2O2 activated by LED, gave results that were stable at 3-month follow-up examination, and whether the use of 5 % potassium nitrate 30 min before bleaching decreased tooth sensitivity during the first 24 h post-treatment, when compared with a placebo.
Materials and methods
A total of 32 individuals participated in the study. The participants were recruited from among patients who received care at the dental clinic of the International University of Catalonia. The Ethics Committee of the International University of Catalonia, Barcelona, reviewed and approved the research protocol and the informed consent form. All subjects underwent a dental screen and dental prophylaxis before the bleaching procedure. Informed consent was obtained before the study began. We assigned all the participants randomly to two groups:
-
Group A (n = 16): received 5 % potassium nitrate (Flashwhite, Corpora®, Barcelona, Spain) as a DSA and underwent in-office bleaching using 28 % hydrogen peroxide (Flashwhite, Corpora®, Barcelona, Spain).
-
Group B (n = 16): received glycerin as a placebo and underwent in-office bleaching with 28 % hydrogen peroxide (Flashwhite, Corpora®, Barcelona, Spain).
Inclusion and exclusion criteria
Table 1 lists the inclusion and exclusion criteria used in the study. Table 2 describes the characteristics of the materials used in the study.
Study design
After the prophylaxis procedure, we took alginate impressions of the maxillary and mandibular arches for each subject. The impressions were poured in dental stone and trimmed, and the resultant cast was prepared for a custom stent. The trays were manufactured from a soft tray material in a heat/vacuum tray-forming machine and were trimmed to fit each model perfectly before they were given to the participants.
Subsequently, before the bleaching procedure, we applied a uniform layer of 5 % potassium nitrate or glycerin (depending on the group assignment) in both trays and placed them for a total time of 30 min into the subject’s mouth.
Once the DSA had been rinsed away with abundant water, we started the bleaching procedure. Vaseline was applied to the patient’s lips and around the corners of their mouth. Cheek retractors were positioned to retract the skin and lips from the treatment area. The area was isolated with gauzes placed on either side of the buccal vestibules in both arches. To ensure protection of the maxillary and mandibular gingiva, and exposed dentin and cementum, a light-curable resin dam was applied over the entire gingival area and photopolymerized in accordance with the manufacturer’s instructions. The patient and the clinician wore protective eyewear throughout the procedure. The whitening gel (28 % hydrogen peroxide; Flashwhite, Corpora®, Barcelona, Spain) was applied to the tooth surfaces of both arches, including the second premolars. A 460-nm wavelength powerLED illuminator unit Flashwhite, Corpora®, Barcelona, Spain) was positioned to illuminate the maxillary and mandibular teeth simultaneously for a 15-minute period. Upon completion of the period the gel was removed from the teeth surfaces with suction. The entire procedure was performed three times, for a total of 45 min. Once the procedure was finished, the bleaching material was removed with high-speed suction and the mouth rinsed with water. The resin dam was also removed. Finally, the patients were asked to brush their teeth at least twice a day with a non-whitening toothpaste.
Evaluation of shade
A baseline shade was established before bleaching, as described below. Immediately after bleaching, a second measurement was obtained. The definitive shade was assessed 2 weeks and 3 months after the cessation of bleaching to evaluate the rebound in colour. The shade of the teeth was always evaluated at the right central incisor, using two methods:
-
(1)
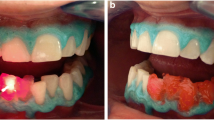
A photograph was taken of the teeth and a Vitapan Classical Shade Guide (Vita Zahnfabrik, Bad Sackingen, Germany) positioned next to the teeth (Fig. 1)
-
(2)
The colour of the middle third of the teeth was determined with an MHT Optic Research AG SpectroShade spectrophotometer (Zurich, Switzerland) (Fig. 2).
All recordings were taken in the same light environment.
With the second method, the colour of the teeth (before, immediately after, 15 days and 3 months after bleaching) was determined on the basis of the CIELAB system. The total difference in colour, or distance between two colours (ΔE), was calculated using the formula below: [8].
We calculated ΔΕ1 (difference in colour between post-bleaching and initial values), ΔΕ2 (difference in colour between 15 days after post-bleaching and initial values), and ΔΕ3 (difference in colour between 3 months after post-bleaching and initial values).
These measurements were performed using a reflectance spectrophotometer (SpectroShade, Handy 147 Dental Type, MHT, Arbizzano, Italy) using the CIE L*a*b* system. The SpectroShade consisted of a D65 light source (6,500 K). This light was split so that the specimen could be illuminated simultaneously from a 45° angle using an intraoral camera. Before each measurement session, the spectrophotometer was calibrated according to manufacturer recommendations using the supplied calibration standards [15].
Evaluation of tooth sensitivity
The Husskison Lancet Visual Analog Scale (VAS) was used to evaluate the level of tooth sensitivity. Each patient was asked to report his or her degree of sensitivity, experienced before and at 24 h post bleaching (PB). The patients graded their maximum level of sensitivity during each period on a scale from 0 to 100 mm (0 = no sensitivity, 100 = unbearable sensitivity) [16].
Statistical analysis
As the data was normally distributed (as determined by the Kolmogorov–Smirnov test), the values were submitted to parametric statistical tests. Differences in L*a*b* values between initial/post-bleaching and post-bleaching/3 months were tested with a repeated measures ANOVA. Differences in ∆E values between initial and post-bleaching (∆E1), between 15 days post-op and initial (∆E2), and between 3 months post-op and inicial (∆E3) were tested with a factorial ANOVA. All tests were carried out at a 95 % level of significance.
Results
Evaluation by spectrophotometry
Figure 3 shows the values of ΔΕ1, ΔΕ2, and ΔΕ3. The bleaching treatment produced an increase in L* values and a decrease in a* and b* values in both groups, but the changes were of varying amplitude. The results were similar either in group A (5 % potassium nitrate) or group B (placebo). The L* value reached a maximum immediately after bleaching and the a* value a minimum 15 days PB; then the two values remained constant during the 3-month post-operative period (Figs. 4 and 5). In contrast, the b* values decreased after the bleaching regimen and had rebounded slightly 3 months postoperatively (Fig. 6). Overall, the bleaching treatment produced a change in tooth chroma immediately after the bleaching regimen, as well as at 3 months postoperatively.
Global colour changes (ΔΕ) between group A (5 % potassium nitrate) and group B (placebo) in ΔΕ1 (differences in colour between post-bleaching and initial values), ΔΕ2 (differences in colour between 15 days post-bleaching and initial values), and ΔΕ3 (differences in colour between 3 months post-bleaching and initial values). Asterisk (*) indicates significant differences (p < 0.05)
With respect to the L* and b* values, significant differences were observed between the initial values and those obtained immediately PB, as well as between the initial values and those obtained 15 days PB or 3 months PB. In contrast, there was no significant difference between the values obtained PB and those obtained 15 days PB or 3 months PB. The a* values showed no statistically significant differences among the different time points. With respect to ΔΕ, no differences were observed between ΔΕ1, ΔΕ2, and ΔΕ3. However, there were statistically significant differences between groups, as the placebo group experimented a higher ΔΕ than the group with 5 % potassium nitrate (p < 0.05).
Dental sensitivity
When the two groups were compared, the group given 5 % potassium nitrate showed a statistically significant lower mean VAS score during the first 24 h PB than the placebo group (p = 0.0037) (Fig. 7).
In both groups, the mean VAS score for the first 24 h PB was significantly higher than that obtained before bleaching and for the 15 days PB (Fig. 7).
Discussion
Bleaching agents lighten discoloured teeth structures, because they can diffuse through the organic matrix of the enamel and dentin [9]. The exact mechanism of action of hydrogen peroxide during bleaching is not understood completely, but it is decomposed into free radicals. The free radicals break down large pigmented molecules that reflect a specific wavelength of light through oxidation and reduction and thus break down the stain in enamel into smaller less pigmented molecules [17].
The power bleaching method has been refined to accelerate the bleaching process, and as a result has grown in popularity [18]. The major advantage of this method, in addition to the shorter duration of treatment, increased patient comfort, and immediate results [10], is that the light source heats the hydrogen peroxide. This increases the rate of decomposition of oxygen to form oxygen free radicals and enhances the release of the staining molecules. Most bleaching agents that have been developed for use with light sources are combined with an activator or colourant (for example carotene, manganese sulphate or transition metals) to improve light absorption or to reduce heating of the teeth [17, 19].
Some studies have concluded that teeth were lightened to nearly the same degree when bleaching gel alone was used as when light was also applied [20, 21]. However, other studies reported that the application of light improved the whitening efficacy of bleaching materials significantly [7–9], even though the effects depended on the mode of activation and were not perceptible or measurable in all cases under examination. From these studies, only that of Tavares et al. [7] was performed in vivo, and this demonstrated that light can increase the effects of peroxide tooth whitening.
In traditional clinical practice, the shade of teeth has been evaluated visually for many years. However, general variables such as external light conditions, experience, age, and human eye fatigue, as well as physiological variables such as colour blindness, lead to inconsistencies. In addition, standardized verbal communication of colour characteristics that are assessed visually is limited. Despite these limitations, the human eye is very efficient at detecting even small differences in colour between two objects [14].
Spectrophotometers, which are extremely precise instruments and are relatively simple and easy to use, measure the light wavelengths that are reflected from an object at many points along the visual spectrum, and thus provide spectral colour data. Tooth colour can be expressed easily according to commonly used shade guides and to CIELAB colour parameters. Paul et al. [14] demonstrated that the accuracy of image capture was not sensitive to discrepancies in angulations of up to ± 12° or in placement in the horizontal and vertical planes of up to ± 5 mm. The spectrophotometer generates a highly accurate spectral curve that indicates the exact tooth colour before and after bleaching. The measurements can also be cross-referenced to existing shade tabs. The SpectroShade spectrophotometer (MHT) has been used in several dental research studies, including studies that involved the detection of colour differences and evaluation of bleaching effects. The use of the split-screen option and the ability to synchronize images obtained before and after bleaching enable a very accurate assessment of the change in tooth colour, which can be quantified and the ΔΕ differences assessed [22]. Given that the ΔΕ value describes the global colour change and includes the three dimensions of the CIELAB system, it was chosen to evaluate the colour stability of the bleaching technique used in the present study.
The results showed that the ΔΕ values before PB (3.9 for group A and 4.78 for group B), before 15 days PB (3.75 for group A and 4.87 for group B), and before 3 months PB (3.30 for group A and 4.55 for group B) were well above the limit of human perception, which is reported to be 3.3 [23]. The colour stability after 3 months showed no statistically significant differences (p > 0.05) in relation to colour immediately PB, although it showed a tendency to rebound in both groups. The same tendency was observed by Marson et al. [24], in a study in which they evaluated the alteration of colour, colour stability, dental sensitivity, and gingival irritation clinically in patients who underwent dental bleaching using various methods of bleaching and light-activation sources. The authors concluded that there were no differences in colour stability among the groups until the sixth month of evaluation. Rosenstiel et al. [25] monitored, in vitro, the modification of colour and the stability of the modification after one session of in-office bleaching with 35 % hydrogen peroxide activated with light for 30 min. The results of the study showed that the colour rebounded 7 days after treatment, and thus this finding differed from those of the present study. In the present study, the obtained pre-treatment values L* (lightness) and b* (yellow/blue) were shown to be affected consistently by the bleaching procedures, but no significant differences were found in relation to the a* (red/green) values among the different time points. Our results are similar to those obtained by Joiner et al. [18] and Luk et al. [26], who also observed changes in L* and b* values and no significant differences with respect to a*.
Deliperi et al. [4] have agreed that 2–3 weeks is an ideal amount of time after which to re-evaluate shade. By that time, the remanent oxygen, the product of the reaction of the bleaching gel, has been released completely and should not interfere with the optical properties of the tooth structure. However, Mokhlis et al. [27] recommended re-evaluation 4 weeks after the cessation of bleaching. To evaluate the stability of the colour, we re-evaluated tooth shade after 15 days and after 3 months PB.
In addition, tooth sensitivity was evaluated post-treatment by applying a VAS, as cited by Huskisson in 1976 [28]. This is a simple way to evaluate and quantify the sensitivity of a tooth. Given that it uses a continuous scale, all the intermediate values can be evaluated. Revill et al. demonstrated that reproducible values can be obtained and that a true opinion of the patient can be registered. However, among other authors, Charakorn et al. [16] stated that the use of this scale is controversial, because it is considered to be a highly subjective measurement technique. Nevertheless, the scale has been used to measure pain in numerous studies, including the one described herein [16, 29].
In the present study, we observed that both the experimental and control groups experienced their maximum sensitivity during the first 24 h PB and then the pain decreased to values similar to those obtained before the procedure, as described in the literature. In general, tooth sensitivity persists for up to 4 days after bleaching, but durations of up to 39 days have been reported [30]. In the study by Mondelli et al. [31], after 24 h, the degree of sensitivity had lowered considerably and returned to normal levels after 1 week for all groups evaluated. However, in the present study, the group that was administered 5 % potassium nitrate 30 min before the bleaching procedure experienced less sensitivity than the placebo group (mean values of 22.22 for group A and 26.97 for group B). These findings agree with those of Goldberg, who reported that sensitivity after bleaching can be prevented or decreased by treating the teeth 30 min before whitening with DSAs that contain 3 % potassium nitrate and 0.11 % fluoride on the basis of weight [32]. Leonard et al. [11] also demonstrated that pre-treatment with DSAs benefits patients who are at risk of developing tooth sensitivity. The potassium nitrate and fluoride formulation that has been introduced into some carbamide peroxide gels might play an important role in preventing an increase in tooth sensitivity.
Although A. Reis [33] and Tay LY [34] affirm that the use of a desensitizing agent as 5 % potassium nitrate before in-office bleaching did not affect the bleaching efficacy, in this study we observed differences between groups. While in both groups the teeth were lighten significantly, the placebo group experimented higher values of lightening that the group with 5 % potassium nitrate (p < 0.05).
It is not understood fully why some patients experience tooth sensitivity during bleaching procedures and others do not. Leonard et al. [11] have reported that the pain is almost certainly multifactorial and is not related solely to the whitening solution. In the present study, tooth sensitivity probably occurred as a result of the length of time for which the bleaching gel was applied (45 min) and the use of light and heat sources, which led to higher temperatures in the pulp. Some studies [24, 30, 35] have concluded that a higher incidence of tooth sensitivity (from 67 to 78 %) occurs after bleaching with H2O2 in combination with heat; however, Tavares et al. [7] concluded that tooth sensitivity after bleaching was linked primarily to the use of peroxide and not to that of light.
Markowitz et al. [36] elucidated the differences between dentine sensitivity (DS) and post-bleaching sensitivity (BS). Although pain can be evoked in bleached teeth by thermal or other stimuli (as occurs in DS), most patients complain of tingling or shooting pain (“zingers”) in the absence of provoking stimuli. Markowitz et al. hypothesized that BS arises as a consequence of the penetration of the tooth structure by peroxide, which causes direct activation of neuronal receptors, rather than through a hydrodynamic mechanism. This has important implications for pain management. Therapies for DS can reduce pain either by reducing stimulus-evoked shifts in dentinal fluid or by reducing the neuronal response that is triggered by these stimuli. Agents such as potassium salts that depress nerve excitability should be more effective than tubule-occluding agents in reducing BS. Incorporating agents that contain potassium into bleaching protocols seems to reduce the severity of BS without compromising the aesthetic results. Potassium-containing dentifrices or gels can also be applied in the bleaching tray, either as part of the bleaching composition or separately. Application of potassium salts in the tray gives the potassium ions more time to diffuse through the structure of the tooth. However, the optimum concentration and mode of application has yet to be determined [36].
Conclusion
In summary, the hypothesis addressed in the present study was confirmed, namely, that an in-office bleaching treatment system using a gel that contained 28 % hydrogen peroxide, with activation by light, gave quantitatively stable results for a period of 3 months. When a DSA that contained 5 % potassium nitrate was applied before the 30-minute bleaching treatment, the sensitivity of the teeth after bleaching was reduced significantly, but the results of the bleaching efficacy were also decreased.
References
Rodrigues JA, Marchi GM, Ambrosano GM, Heymann HO, Pimenta LA. Microhardness evaluation of in situ vital bleaching on human dental enamel using a novel study design. Dent Mater. 2005;21(11):1059–67.
Matis BA, Cochran MA, Wang G, Eckert GJ. A clinical evaluation of two in-office bleaching regimens with and without tray bleaching. Oper Dent. 2009;34(2):142–9.
Lee BS, Huang SH, Chiang YC, Chien YS, Mou CY, Lin CP. Development of in vitro tooth staining model and usage of catalysts to elevate the effectiveness of tooth bleaching. Dent Mater. 2008;24(1):57–66.
Deliperi S, Bardwell DN, Papathanasiou A. Clinical evaluation of a combined in-office and take-home bleaching system. J Am Dent Assoc. 2004;135(5):628–34.
Matis BA, Cochran MA, Eckert GJ, Matis JI. In vivo study of two carbamide peroxide gels with different desensitizing agents. Oper Dent. 2007;32(6):549–55.
Minoux M, Serfaty R. Vital tooth bleaching: biologic adverse effects-a review. Quintessence Int. 2008;39(8):645–59.
Tavares M, Stultz J, Newman M, Smith V, Kent R, Carpino E, et al. Light augments tooth whitening with peroxide. J Am Dent Assoc. 2003;134(2):167–75.
Ontiveros JC, Paravina RD. Colour change of vital teeth exposed to bleaching performed with and without supplementary light. J Dent. 2009;37(11):840–7.
Yazici AR, Khanbodaghi A, Kugel G. Effects of an in-office bleaching system (ZOOM) on pulp chamber temperature in vitro. J Contemp Dent Pract. 2007;8(4):19–26.
Carrasco LD, Guerisoli DM, Rocha MJ, Pecora JD, Froner IC. Efficacy of intracoronal bleaching techniques with different light activation sources. Int Endod J. 2007;40(3):204–8.
Leonard RH Jr, Smith LR, Garland GE, Caplan DJ. Desensitizing agent efficacy during whitening in an at-risk population. J Esthet Restor Dent. 2004;16(1):49–55 Discussion 6.
Porto IC, Andrade AK, Montes MA. Diagnosis and treatment of dentinal hypersensitivity. J Oral Sci. 2009;51(3):323–32.
Adeyemi AA, Jarad FD. de Josselin de Jong E, Pender N, Higham SM. The evaluation of a novel method comparing quantitative light-induced fluorescence (QLF) with spectrophotometry to assess staining and bleaching of teeth. Clin Oral Investig. 2010;14(1):19–25.
Paul S, Peter A, Pietrobon N, Hammerle CH. Visual and spectrophotometric shade analysis of human teeth. J Dent Res. 2002;81(8):578–82.
Ardu S, Braut V, Gutemberg D, Krejci I, Dietschi D. A long term laboratory test on staining susceptibility of esthetic composite resin materials. Quintessence Int. 2010;41:695–702.
Charakorn P, Cabanilla LL, Wagner WC, Foong WC, Shaheen J, Pregitzer R, et al. The effect of preoperative ibuprofen on tooth sensitivity caused by in-office bleaching. Oper Dent. 2009;34(2):131–5.
Taher NM. The effect of bleaching agents on the surface hardness of tooth coloured restorative materials. J Contemp Dent Pract. 2005;6(2):18–26.
Joiner A. The bleaching of teeth: a review of the literature. J Dent. 2006;34:412–9.
Cassoni A, Rodrigues JA. Argon laser: a light source alternative for photopolymerization and in-office tooth bleaching. Gen Dent. 2007;55(5):416–9.
Marson P, Bagatella P, Bortolati M, Tison T, De Silvestro G, Fabris F, et al. Plasma exchange for the management of the catastrophic antiphospholipid syndrome: importance of the type of fluid replacement. J Intern Med. 2008;264(2):201–3.
Papathanasiou AKS, Perry RD, Kugel G. Clinical evaluation of a 35% hydrogen peroxide in-office whitening system. Compend Contin Educ Dent. 2002;23:334–5.
Chu SJ. Use of a reflectance spectrophotometer in evaluating shade change resulting from tooth-whitening products. J Esthet Restor Dent. 2003;15(Suppl 1):S42–8.
Dietschi D, Benbachir N, Krejci I. In vitro colourimetric evaluation of the efficacy of home bleaching and over-the-counter bleaching products. Quintessence Int. 2010;41(6):505–16.
Marson FC, Sensi LG, Vieira LC, Araujo E. Clinical evaluation of in-office dental bleaching treatments with and without the use of light-activation sources. Oper Dent. 2008;33(1):15–22.
Rosenstiel SF, Gegauff AG, McCafferty RJ, Johnston WM. In vitro tooth colour change with repeated bleaching. Quintessence Int. 1991;22(1):7–12.
Luk K, Tam L, Hubert M. Effect of light energy on peroxide tooth bleaching. J Am Dent Assoc. 2004;135(2):194–201 quiz 28-9.
Mokhlis GR, Matis BA, Cochran MA, Eckert GJ. A clinical evaluation of carbamide peroxide and hydrogen peroxide whitening agents during daytime use. J Am Dent Assoc. 2000;131(9):1269–77.
Huskisson EC, Jones J, Scott PJ. Application of visual-analogue scales to the measurement of functional capacity. Rheumatol Rehabil. 1976;15(3):185–7.
Scott PJ, Huskisson EC. Measurement of functional capacity with visual analogue scales. Rheumatol Rehabil. 1977;16(4):257–9.
Tredwin CJ, Naik S, Lewis NJ, Scully C. Hydrogen peroxide tooth-whitening (bleaching) products: review of adverse effects and safety issues. Br Dent J. 2006;200(7):371–6.
Mondelli RF, Azevedo JF, Francisconi AC, Almeida CM, Ishikiriama SK. Comparative clinical study of the effectiveness of different dental bleaching methods—2 year follow-up. J Appl Oral Sci. 2012;20(4):435–43.
Goldberg M, Grootveld M, Lynch E. Undesirable and adverse effects of tooth-whitening products: a review. Clin Oral Investig. 2010;14(1):1–10.
Reis A, Dalanhol AP, Cunha TS, Kossatz S, Loguercio AD. Assessment of tooth sensitivity using a desensitizer before light-activated bleaching. Oper Dent. 2011;36(1):12–7.
Tay TY, Kose C, Loguercio AD, Reis A. Assessing the effect of a desensitizing agent used before in-office tooth bleaching. J Am Dent Assoc. 2009;140(10):1245–51.
He LB, Shao MY, Tan K, Xu X, Li JY. The effects of light on bleaching and tooth sensitivity during in-office vital bleaching: a systematic review and meta-analysis. J Dent. 2012;40(8):644–53 Epub 2012 Apr 21.
Markowitz K. Pretty painful: why does tooth bleaching hurt? Med Hypotheses. 2010;74(5):835–40.
Acknowledgments
The authors would like to thank to Corpora® for providing the study material.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Palé, M., Mayoral, J.R., Llopis, J. et al. Evaluation of the effectiveness of an in-office bleaching system and the effect of potassium nitrate as a desensitizing agent. Odontology 102, 203–210 (2014). https://doi.org/10.1007/s10266-013-0132-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10266-013-0132-3