Abstract
Objectives
There is increasing evidence that bisphosphonates affect orthodontic tooth movement. The object of the study was to investigate the changes produced by tensile strain on human periodontal ligament fibroblasts (HPdLFs) treated with clodronate or zoledronate.
Materials and methods
HPdLF were cultured with 5 and 50 μM clodronate or zoledronate for 48 h and applied to tensile strain (TS) (5 and 10 %) for 12 h in vitro. Viability was verified by MTT assay and apoptosis rate via caspase 3/7 assay. Gene expression of receptor activator of nuclear factor kappa-B ligand (RANKL) and osteoprotegerin (OPG) was investigated using real-time PCR. OPG was also analyzed by ELISA and RANKL by immunocytochemical staining.
Results
Zoledronate (50 μM) reduced the viability of HPdLF (76 vs 100 %) and combined with 5 % TS to 53 %. TS of 10 % and clodronate reduced viability to 79 % with increased caspase 3/7 activity. Clodronate (5 μM) led to a slight increase of OPG gene expression, zoledronate (5 μM) to a slight decrease. Combined with 5 % TS, both increased OPG gene expression (2–3-fold) and OPG synthesis. Zoledronate increased gene expression of RANKL (4-fold). Combined with 5 % of TS, this increase was abolished. TS of 10 % in combination amplified increase of RANKL ending up with a 9-fold gene expression by clodronate and high RANKL protein synthesis.
Conclusions
This study shows for the first time that mechanical loading alters the effects of bisphosphonates on viability, apoptosis rate, and OPG/RANKL system of HPdLF dependent on the applied strength. Low forces and bisphosphonates increase factors for bone apposition, whereas high forces combined with bisphosphonates stimulate osteoclastogenesis.
Clinical relevance
Mechanical loading of periodontal ligament with high strengths should be avoided during bisphosphonate therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many reviews published over the years have focused on the effects of several drugs during orthodontic tooth movement [1, 2]. Due to the demographic change and the altered attitude toward orthodontic treatment in adults, the number of adult patients receiving orthodontic treatment has enormously increased in the last years [3]. Bisphosphonates are the most commonly prescribed medications for patients with osteoporosis [4]. So far, there are only a few case reports or animal studies analyzing the orthodontic treatment under bisphosphonate therapy [5–7]. Thus, this is a very new topic in the field of orthodontics.
Bisphosphonates are internalized in osteoclasts, inducing their apoptosis and eventually inhibiting bone resorption [8, 9]. Bisphosphonates are divided into two groups: non-nitrogen-containing bisphosphonates, such as clodronate, and nitrogen-containing bisphosphonates, such as zoledronate. The Mechanism of action is different between both groups [10]. Nitrogen-containing bisphosphonates are highly potent but also exhibit more negative side effects regarding viability and apoptosis of different cell lines such as osteoblasts or fibroblasts. This might play a role in the development of osteonecrosis of the jaw, one of the most dreaded complications under bisphosphonate therapy [11–14].
During orthodontic treatment, forces are applied to the tooth transmitted by the periodontal ligament, which is composed of cellular and extracellular components and localized between the tooth cementum and the alveolar bone. The predominant cell types are fibroblasts, epithelial cells, and undifferentiated mesenchymal cells. The margins of the periodontal ligament are composed of cementoblasts, osteoblasts, and osteoclasts [15].
Mechanical forces are needed to enable orthodontic tooth movement governed by changes of the periodontal tissue and alveolar bone. Bone remodeling is a complex process and essential for tooth movement [16]. The forces induce mechanical strain in the extracellular matrix, leading to changes in the cells, for example, in the cell membrane, the nuclear protein matrix, and the genome [17, 18]. This eventually results in changes to the gene expression, which can cause alterations in cell viability, proliferation, and differentiation, all necessary for tooth movement [19, 20].
The effect of bisphosphonates on orthodontically induced tooth movement has been observed in a number of animal experiments [21–27, 5]. Results show reduced tooth movement, high bone mineralization, and fewer osteoclasts in histological staining. In line with this, some case reports describe difficulties at space closure, widened periodontal ligaments, high bone mineralization, and longer treatment times for orthodontic patients under bisphosphonates [6, 7]. We observed similar characteristics in radiographs of our patients during orthodontic treatment under bisphosphonates (Fig. 1).
The first study analyzing the side effects of orthodontic tooth movement and bisphosphonates is from 1994 [21], but exact interface of the molecular mechanism between bisphosphonates and mechanical loading is not yet known. This is of current interest due to the mechanical loading of periodontal ligament and alveolar bone not only by orthodontic tooth movement but also due to mastication. Our hypothesis was that there is an interaction between bisphosphonates and mechanical loading with a strong influence on bone remodeling of the alveolar bone via the osteoprotegerin (OPG)/receptor activator of nuclear factor kappa-B ligand (RANKL) system. OPG and RANKL are antagonists regarding the mechanism of bone turnover. RANKL increases osteoclast differentiation and activates them, leading to increased bone resorption, whereas OPG extinguishes the RANKL effect since it works as a decoy receptor for RANKL. As one of the best known system for bone turnover [11, 28, 29], these proteins might play an important key role on which mechanical loading as well as bisphosphonates might have a strong impact.
Therefore, the aim of the present study was to investigate effects of the combination of bisphosphonates and mechanical loading on the metabolism of human periodontal fibroblasts regarding their viability, apoptosis rate, and the OPG/RANKL system.
Material and methods
Cell culture
Cell cultures were prepared and maintained according to standard cell culture procedures. Commercially available human periodontal ligament fibroblasts (HPdLFs) (Lonza, Basel, Switzerland) were maintained in Dulbecco’s Modified Eagle Medium (DMEM; Invitrogen, Carlsbad, USA) containing 10 % FCS, 1 % l-glutamine, and 1 % penicillin/streptomycin/neomycin. We observed cell morphology under light microscope for typical fibroblast morphology. Additionally, we analyzed alkaline phosphatase, which might be characteristic for ligament fibroblasts but not for gingival fibroblasts [30]. The cells were cultured in an incubator with 5 % CO2 and 95 % air at 37 °C. Cells were passaged at regular intervals depending on their growth characteristics using 0.05 % Trypsin-EDTA solution (PAA, Pasching, Austria). Cells were used for experiments at passages 4 to 6.
Mechanical strain devices and incubation with bisphosphonates
As an experimental model of tensile strain, HPdLFs were seeded at a density of 1.0 × 105 cells/well on flexible-bottomed six-well plates (Bioflex® Plates, Flexcell® International Corporation, Hillsborough, USA) and cultured in supplemented DMEM. After 2 days for cell attachment, spread and growth to subconfluency medium was replaced by medium with 1 % fetal calf serum (starvation medium) and with two different bisphosphonates (clodronate or zoledronate) at two concentrations of 5 and 50 μM for 36 h [31]. Then, a Flexercell Strain Unit (Modell FX 3000, Dunn Labortechnik Gmbh, Asbach, Germany), which is capable of controlling the magnitude, type, and frequency of cell deformation, was used to generate tensile strain (TS) in HPdLF. Cells were subjected to static TS of 5 % (3 cN/mm2) and 10 % (5.2 cN/mm2) in an incubator for 12 h to simulate moderate and high strengths of constant strain. Experiments were repeated three times to confirm reproducibility, and three wells were used for each group.
MTT assay
Cell viability of HPdLF was evaluated in the six-well plates with the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) colorimetric assay (Sigma, München, Germany). Viable cells ferment tetrazolium bromide to formazan, which can be measured after cell lysis photometrically at 550 nm. The experiments were performed in triplicate.
Caspase assay
The apoptosis rate of HPdLF was assessed via caspase activity in cells with the caspase-Glo 3/7 system (Promega, Madison, WI, USA) following the manufacturer’s protocol using a microplate reader (Synergy HT, BioTek, Winooski, USA). The experiments were performed in triplicate.
Messenger RNA extraction and reverse transcriptase polymerase chain reaction (RT-PCR)
Cells were detached with 0.05 % Trypsin-EDTA solution directly after stretching and individually harvested. Messenger RNA (mRNA) was isolated using the peqGOLD Total RNA KIT (peqLab Biotechnologie Gmbh, Erlangen, Germany). This included a DNAse digestion step. Both the quantity (260 nm) and quality (ratio 260/280 nm) of the RNA were determined by using a NanoDrop-Spectrophotometer ND-100 (peqLab Biotechnologie Gmbh, Erlangen, Germany). Reverse transcription (RT) of RNA (100 ng) was performed by standard protocols with Gene Amp PCR System 2400 (Perkin Elmer, MA, USA) and iscript cDNA Synthesis Kit (Biorad Laboratories, Hercules, USA) in a total volume of 20 μl.
OPG and RANKL primers (Eurofins MWG Operon, Ebersberg, Germany) were designed using the NCBI-nucleotide library and Primer3-design to detect the mRNA levels (Table 1). All primers had been matched to the mRNA sequences of the target genes (NCBI Blast software). As housekeeping genes, actin and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were evaluated. We were able to show the most stable expression for actin and GAPDH using specialized freeware called GeNorm.
Quantitative real-time PCR was performed with the IQ5-I-Cycler and IQ5 Optical System software version 2.0 (Bio-Rad Laboratories, Hercules, USA) according to the manufacturer’s instructions, which included an initial denaturation at 95 °C, annealing temperature of 56 °C, and an elongation temperature of 71 °C over 40 cycles. q-PCR amplification was conducted with a reaction mixture containing SYBR Green Supermix (BioRad Laboratories, Hercules, USA), an appropriate amount of paired primers, and 2-μl template cDNA. The background was to determine the threshold at the SYBR green fluorescence curve at the exponential part. This method was applied to calculate the cycle number and C T value for quantitation.
Furthermore, the C T values of the actin and GAPDH housekeeping genes and the individual primer efficacy were taken into account. Single-product formation was confirmed by melting point analysis. Data were obtained from three individual experiments and normalized to the C T of actin and GAPDH. cDNA from individual cell experiments was analyzed in triplicate PCR. The relative expression levels of each mRNA were evaluated by using a modification of the ΔΔCT method [32].
Enzyme-linked immunosorbent assay (ELISA)
OPG were measured in supernatants by Quantikine Human Immunoassay for OPG (R&D Systems, Inc., Minneapolis, USA) according to the manufacturer’s instructions using a microplate reader (Metertech, Inc., Taipei, Taiwan). The assays were performed in triplicate, and the limits of detection for the immunoassay were 8 pg/ml.
Immunocytochemical staining
To illustrate the localization of RANKL, HPdLFs were fixed with methanol/acetone (1:1) at −20 °C for 10 min. Cells were blocked with 0.25 % casein/0.1 % bovine serum albumin for 30 min at room temperature. The antibody against RANKL (1:50; Santa Cruz Biotech, Heidelberg, Germany) was incubated overnight at 4 °C. Staining was visualized by using a serum against rabbit IgG conjugated with Alexa 594 (1:50; Lifetechnologies, Darmstadt, Germany). Counterstaining (nuclear staining) was performed with DAPI (4′,6-diamidine-2-phenylindole). Photomicrographs of immunofluorescent staining were made using a Keyence fluorescence microscope (BZ-9000, Keyence, Osaka, Japan).
Statistical analysis
SPSS 19.0 (IBM-SPSS, Ehningen, Germany) was used for statistical analyses. To detect the difference between the groups, one-way ANOVA was used with the post hoc Tukey test. A p value <0.05 was considered statistically significant. Results of immunocytochemical staining were described descriptively.
Results
Viability and apoptosis
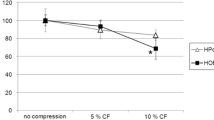
Viability assay demonstrated that 10 % TS led to a slight decrease (83.7 %) in HPdLF cell viability compared to control cells without TS (100 %). HPdLF did not show any changes of viability at 5 % TS. HPdLF incubated with 50 μM clodronate showed a strength-dependent decrease in viability, which turned out to be significant at 10 % (78.9 %, p = 0.003). Zoledronate of 50 μM led to decreased cell viability (75.9 %, p < 0.001). When combined with TS, this effect was enhanced, with the lowest cell viability at 5 % TS with zoledronate (52.7 %, p < 0.001) (Fig. 2).
MTT assay. Cell viability test for HPdLFs exposed to 50 μM clodronate or zoledronate for 48 and to 12 h of TS at strengths of 5 and 10 % compared to control cells (no strain) set to 100 % (x-axis = different strengths of TS, y-axis = cell viability compared to control group in percent). The assays were performed in triplicate. Significant changes compared to unstretched control cells (no strain) are marked with an asterisk (*p < 0.05). The whiskers at each value represent the standard deviation
In correlation with the viability assay, HPdLF did not show any increased caspase activity at 5 or 10 % TS compared to unstretched control cells. HPdLF incubated with 50 μM clodronate exposed significantly increased caspase activity only when combined with 10 % TS, whereas 50 μM zoledronate led to increased caspase activity of HPdLF at 5 and 10 % TS (Fig. 3).
Caspase 3/7 assay. Apoptosis rate of HPdLFs exposed to 50 μM clodronate (clodro.) or zoledronate (zoledro.) for 48 and to 12 h of TS at strengths of 5 and 10 % compared to control cells (no strain) (x-axis = different strengths of TS and bisphosphonate groups, y-axis = caspase 3/7 activity in relative light units (RLU)). The assays were performed in triplicate, *p < 0.05. The black bar in the middle of each box represents the median. The box includes all values between the 25th and 75th percentiles. Whiskers indicate values still within the 1.5 interquartile range, circles represent outliers. An asterisk represents a significant difference between the values under the clamb, n.s. underlines not statistically significant changes
At reduced concentrations of clodronate and zoledronate of 5 μM, there were no significant changes in the cell viability or apoptosis rate of HPdLF, with or without TS (data not shown).
Osteoprotegerin gene expression and protein level
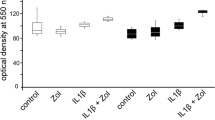
Due to the fact that apoptotic cells can particularly distort protein assays, we only performed the experiments for gene expression and protein level with 5 μM clodronate and zoledronate. Clodronate led to a slight increase of OPG gene expression, and zoledronate led to a slight decrease. TS of 5 % led to a 3-fold increase of OPG gene expression. In combination with the bisphosphonates, this increase was enhanced, with the highest (4-fold) gene expression in combination with clodronate. TS of 10 % did not enhance the OPG gene expression compared to 5 %; however, when combined with clodronate and zoledronate, gene expression levels decreased to levels similar to the control group without TS (Fig. 4).
Quantitative RT-PCR results for osteoprotegerin (OPG) of HPdLFs exposed to 5 μM clodronate (clodro.) or zoledronate (zoledro.) for 48 and to 12 h of TS at strengths of 5 and 10 % as fold of control cells with no strain. Data were obtained from three individual experiments (means ± SD). Significant changes compared to unstretched control cells (no strain) are marked with an asterisk (*p < 0.05). The whiskers at each bar represent the standard deviation
Protein synthesis of OPG confirmed the results at the gene level. Neither clodronate nor zoledronate had a significant influence on OPG production of HPdLF. TS of 5 % increased OPG production significantly (13.7 vs 4.4 ng, p < 0.001), with an additional effect when combined with clodronate (16.0 ng) and zoledronate (15.4 ng). The increase of OPG production at 10 % TS (9.9 ng) was not as strong as at 5 % TS, and combining with bisphosphonates in fact showed a slightly reduced effect (Fig. 5).
Osteoprotegerin (OPG) secretion in supernatant liquor of HPdLFs analyzed after incubation with 5 μM clodronate (clodro.) or zoledronate (zoledro.) for 48 and 12 h of TS at strengths of 5 and 10 % compared to control cells. The assays were performed in triplicate, and data are given in nanogram per milliliter. The black bar in the middle of each box represents the median. The box includes all values between the 25th and 75th percentiles. Whiskers indicate values still within the 1.5 interquartile range. An asterisk represents a significant difference between the values under the clamb
RANKL gene expression and immunofluorescence
Zoledronate increased the gene expression of RANKL about 5-fold compared to control group, whereas clodronate led to a slight decrease of RANKL gene expression. When combined with 5 % of TS, the zoledronate-induced RANKL gene expression increase was abolished. TS of 10 % slightly increased gene expression of RANKL. When combined with bisphosphonates, this increase was enhanced to 10-fold gene expression by clodronate and 6-fold gene expression by zoledronate. Ratio of RANKL/OPG gene expression showed the highest values for zoledronate with a nearly 10-fold increase compared to control cells. Clodronate reduced the RANKL/OPG ratio by half. Increased OPG gene expression and unchanged RANKL gene expression led to lowest values for the ratio of RANKL/OPG at 5 % TS. Combined with 10 % TS, zoledronate as well as clodronate led to a 6-fold increase of the RANKL/OPG ratio (Fig. 6).
a Quantitative RT-PCR-results of receptor activator of nuclear factor kappa-B ligand (RANKL) and b RANKL/OPG ratio of HPdLFs exposed to 5 μM clodronate (clodro.) or zoledronate (zoledro.) for 48 and to 12 h of TS at strengths of 5 and 10 % as fold of control cells with no strain. Data were obtained from three individual experiments (means ± SD). Significant changes compared to unstretched control cells (no strain) are marked with an asterisk (*p < 0.05)
Immunofluorescence confirmed the results of RANKL gene expression and showed a small amount of RANKL in the cytoplasm of HPdLF incubated with 5 μM zoledronate. When combined with 5 % TS, no RANKL protein was detectable. Increasing levels were detectable in HPdLF at 10 % TS in combination with 5 μM clodronate or zoledronate, whereas the other groups did not show any RANKL protein synthesis (Fig. 7).
The expression of receptor activator of the nuclear factor-kappa ligand (RANKL) was demonstrated on the protein level using immunofluorescence (red). Nuclei were counterstained using DAPI (blue). See text for further details. HPdLFs exposed to 5 μM clodronate or zoledronate for 48 and to 12 h TS at strengths of 5 and 10 % compared to control cells without TS (no strain) and without bisphosphonates (control)
Discussion
This study investigated the influence of TS at two different strengths and two bisphosphonates in two different concentrations on HPdLF regarding their viability, apoptosis, and the OPG/RANKL system. HPdLF cell viability was slightly decreased by high-strength TS, but overall tensile alone did not significantly affect either the viability or apoptosis rate of HPdLF. This is in accordance with previous studies analyzing the effects of biomechanical loading on different cell types of the periodontal ligament [33–35].
Various studies observed negative side effects regarding viability and apoptosis of different cell lines by nitrogen-containing bisphosphonates at high concentrations, which might have an impact on the development of osteonecrosis of the jaw [12–14]. Our study confirms these results for HPdLF and zoledronate at the higher concentration. We additionally show that these negative side effects are enhanced when TS is combined with zoledronate at high concentrations and also occur at high concentrations of clodronate combined with TS of high strength. Due to the association with the development of osteonecrosis of the jaw, we recommend that mechanical loading, in particular high strength, should be avoided under high concentrations of bisphosphonates.
The OPG/RANKL system as an indicator of bone remodeling plays an important role in orthodontic tooth movement [34, 28]. In the present study, TS led to increased gene expression and protein synthesis of OPG, resulting in reduced activation of osteoclasts, indicating the formation of new bone on the tension site of tooth movement. These results correspond with the results of Tsuji et al., who observed an increase OPG synthesis and an unaltered RANKL expression of periodontal ligament cells under cyclic mechanical strain [29].
Recent studies have shown that bisphosphonates not only effect osteoclasts but also the OPG/RANKL system of osteoblasts. Koch et al. found increased RANKL gene expression of osteoblasts after incubation with zoledronate but no significant increase after incubation with clodronate. OPG gene expression in osteoblasts was not stimulated neither by zoledronate nor clodronate at low concentrations similar to our study [36]. They analyzed osteoblasts, but results of their study are similar to the results from the present study with periodontal fibroblasts. This supports the fact that periodontal fibroblasts contain osteogenic potential and play an important role in the remodeling of the alveolar bone [37, 38]. By now, it is unclear how much of the administered bisphosphonates reach the periodontal fibroblasts. But, it is known that drugs and hormones can affect periodontal fibroblasts and they do communicate with osteoblasts; this plays an important role in particular when mechanical loading is performed such as that during orthodontic tooth movement [39, 40].
TS of 5 % and bisphosphonates had an additive effect on the OPG gene expression and protein synthesis of HPdLF. TS of 5 % also abolished elevated gene expression and protein synthesis of RANKL by zoledronate. TS of 10 %, however, increased zoledronate-induced RANKL gene expression and led to an elevated expression of RANKL in combination with clodronate and zoledronate. Taken together, these attributes demonstrate that TS of moderate strength can support bone formation, whereas TS of high strength combined with bisphosphonate increases the risk for bone loss via the OPG/RANKL system.
It is known that upregulation of cyclooxygenase-2 and increased prostaglandin E2 levels can cause an increased RANKL expression [41]. Liu et al. analyzed the effect of clodronate to periodontal ligament cells under compressive mechanical stress. They observed inhibitory effects of clodronate on stress-induced prostaglandin E2, cyclooxygenase-2, and RANKL. In our study, clodronate demonstrated a positive effect on OPG gene expression but also increased RANKL expression when combined with TS of high strength. In both studies, clodronate alone had no significant effects on RANKL. Anti-inflammatory effects of clodronate in the study of Liu et al. were highest at 125 μM. We only used 5 μM of both bisphosphonates for PCR and ELISA assays. Thus, lower concentrations and different mechanical stress protocols could be responsible for the contradictory results regarding RANKL gene expression [31].
It is recognized, however, that a lack of cell-cell and cell-matrix interactions, which are typical for integrated cell populations in living tissues, constrains the ability of these in vitro results to be extrapolated to the more complex cell behavior in vivo. Another critical point is the difficulty of applying the same strength of TS to all cells cultured on the flexible membrane. Gilbert et al. found out that the applied TS might be differently transferred to the cells depending on their region of the membrane. Strain magnitude on the border is slightly higher than that on the center of the well [42].
However, Stadelmann et al. analyzed the effect of zoledronate and mechanical loading on bone formation of mouse tibia in vivo. They found a positive effect with an increase of cortical thickness and bone area of the tibia for low forces but a negative interaction when high forces appear in combination with zoledronate [43]. They conclude that there is an upper limit on bone adaptation rate induced by zoledronate when mechanical stimulations reach high intensity.
Braith et al. could show that bone loss of the lumbar spine in patients after lung transplantation can be prevented by bisphosphonates compared to the control group. They analyzed a third group receiving a combination of bisphosphonates and mechanical loading performed by resistence training. This group developed the highest values of bone mineral density. They made the conclusion that combination of bisphosphonates and physiological mechanical loading induced a gain of bone mass of the lumbar spine [44].
Both in vitro and in vivo studies give evidence of an interface between mechanical loading and bisphosphonates. Our in vitro study reveals that the RANKL/OPG system might play a key role for the observed in vivo results due to its different manipulations by the combination of bisphosphonates and mechanical loading dependent on the strength of strain.
Conclusions
For the first time, we have found out that TS increases the negative effects of bisphosphonates at high concentrations in terms of the viability and apoptosis of HPdLF. Mechanical loading should therefore be avoided immediately after bisphosphonate intake. This study also reveals for the first time that bisphosphonates at low concentrations and TS interact on the OPG/RANKL system of HPdLF dependent on the applied strength. Low forces and bisphosphonates might synergistically increase bone apposition, whereas high forces combined with bisphosphonate stimulate factors for osteoclastogenesis. This plays an important role for patients receiving bisphosphonates during orthodontic therapy, and mechanical loading of periodontal ligament with high strengths should be avoided during bisphosphonate therapy.
References
Tyrovola JB, Spyropoulos MN (2001) Effects of drugs and systemic factors on orthodontic treatment. Quintessence Int 32(5):365–371
Krishnan V, Davidovitch Z (2006) The effect of drugs on orthodontic tooth movement. Orthod Craniofacial Res 9(4):163–171
Chaison ET, Liu X, Tuncay OC (2011) The quality of treatment in the adult orthodontic patient as judged by orthodontists and measured by the Objective Grading System. Am J Orthod Dentofac Orthop 139(4 Suppl):S69–S75
Deeks ED, Perry CM (2008) Zoledronic acid: a review of its use in the treatment of osteoporosis. Drugs Aging 25(11):963–986
Sirisoontorn I, Hotokezaka H, Hashimoto M, Gonzales C, Luppanapornlarp S, Darendeliler MA, Yoshida N (2012) Orthodontic tooth movement and root resorption in ovariectomized rats treated by systemic administration of zoledronic acid. Am J Orthod Dentofac Orthop 141(5):563–573
Krieger E, d'Hoedt B, Scheller H, Jacobs C, Walter C, Wehrbein H (2013) Orthodontic treatment of patients medicated with bisphosphonates-a clinical case report. J Orofac Orthop 74(1):28–39
Krieger E, Jacobs C, Walter C, Wehrbein H (2013) Current state of orthodontic patients under bisphosphonate therapy. Head Face Med 9:10
Flanagan AM, Chambers TJ (1991) Inhibition of bone resorption by bisphosphonates: interactions between bisphosphonates, osteoclasts, and bone. Calcif Tissue Int 49(6):407–415
Rogers MJ, Gordon S, Benford HL, Coxon FP, Luckman SP, Monkkonen J, Frith JC (2000) Cellular and molecular mechanisms of action of bisphosphonates. Cancer 88(12 Suppl):2961–2978
Green JR (2004) Bisphosphonates: preclinical review. Oncologist 9(Suppl 4):3–13
Koch FP, Wunsch A, Merkel C, Ziebart T, Pabst A, Yekta SS, Blessmann M, Smeets R (2011) The influence of bisphosphonates on human osteoblast migration and integrin aVb3/tenascin C gene expression in vitro. Head Face Med 7(1):4
Pabst AM, Ziebart T, Koch FP, Taylor KY, Al-Nawas B, Walter C (2012) The influence of bisphosphonates on viability, migration, and apoptosis of human oral keratinocytes—in vitro study. Clin Oral Investig 16(1):87–93
Walter C, Klein MO, Pabst A, Al-Nawas B, Duschner H, Ziebart T (2010) Influence of bisphosphonates on endothelial cells, fibroblasts, and osteogenic cells. Clin Oral Investig 14(1):35–41
Walter C, Pabst A, Ziebart T, Klein M, Al-Nawas B (2011) Bisphosphonates affect migration ability and cell viability of HUVEC, fibroblasts and osteoblasts in vitro. Oral Dis 17(2):194–199
Hassell TM (1993) Tissues and cells of the periodontium. Periodontol 2000(3):9–38
Proff P, Romer P (2009) The molecular mechanism behind bone remodelling: a review. Clin Oral Investig 13(4):355–362
Krishnan V, Davidovitch Z (2009) On a path to unfolding the biological mechanisms of orthodontic tooth movement. J Dent Res 88(7):597–608
Krishnan V, Davidovitch Z (2006) Cellular, molecular, and tissue-level reactions to orthodontic force. Am J Orthod Dentofac Orthop 129(4):469 e461–469 e432
Mabuchi R, Matsuzaka K, Shimono M (2002) Cell proliferation and cell death in periodontal ligaments during orthodontic tooth movement. J Periodontal Res 37(2):118–124
Meikle MC (2006) The tissue, cellular, and molecular regulation of orthodontic tooth movement: 100 years after Carl Sandstedt. Eur J Orthod 28(3):221–240
Adachi H, Igarashi K, Mitani H, Shinoda H (1994) Effects of topical administration of a bisphosphonate (risedronate) on orthodontic tooth movements in rats. J Dent Res 73(8):1478–1486
Alatli I, Hellsing E, Hammarstrom L (1996) Orthodontically induced root resorption in rat molars after 1-hydroxyethylidene-1,1-bisphosphonate injection. Acta Odontol Scand 54(2):102–108
Igarashi K, Adachi H, Mitani H, Shinoda H (1996) Inhibitory effect of the topical administration of a bisphosphonate (risedronate) on root resorption incident to orthodontic tooth movement in rats. J Dent Res 75(9):1644–1649
Kaipatur NR, Wu Y, Adeeb S, Stevenson TR, Major PW, Doschak MR (2013) Impact of bisphosphonate drug burden in alveolar bone during orthodontic tooth movement in a rat model: a pilot study. Am J Orthod Dentofac Orthop 144(4):557–567
Karras JC, Miller JR, Hodges JS, Beyer JP, Larson BE (2009) Effect of alendronate on orthodontic tooth movement in rats. Am J Orthod Dentofac Orthop 136(6):843–847
Liu L, Igarashi K, Haruyama N, Saeki S, Shinoda H, Mitani H (2004) Effects of local administration of clodronate on orthodontic tooth movement and root resorption in rats. Eur J Orthod 26(5):469–473
Ortega AJ, Campbell PM, Hinton R, Naidu A, Buschang PH (2012) Local application of zoledronate for maximum anchorage during space closure. Am J Orthod Dentofac Orthop 142(6):780–791
Lossdorfer S, Gotz W, Jager A (2011) PTH(1-34)-induced changes in RANKL and OPG expression by human PDL cells modify osteoclast biology in a co-culture model with RAW 264.7 cells. Clin Oral Investig 15(6):941–952
Tsuji K, Uno K, Zhang GX, Tamura M (2004) Periodontal ligament cells under intermittent tensile stress regulate mRNA expression of osteoprotegerin and tissue inhibitor of matrix metalloprotease-1 and -2. J Bone Miner Metab 22(2):94–103
Giannopoulou C, Cimasoni G (1996) Functional characteristics of gingival and periodontal ligament fibroblasts. J Dent Res 75(3):895–902
Liu L, Igarashi K, Kanzaki H, Chiba M, Shinoda H, Mitani H (2006) Clodronate inhibits PGE(2) production in compressed periodontal ligament cells. J Dent Res 85(8):757–760
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25(4):402–408
Grunheid T, Zentner A (2005) Extracellular matrix synthesis, proliferation and death in mechanically stimulated human gingival fibroblasts in vitro. Clin Oral Investig 9(2):124–130
Jacobs C, Grimm S, Ziebart T, Walter C, Wehrbein H (2013) Osteogenic differentiation of periodontal fibroblasts is dependent on the strength of mechanical strain. Arch Oral Biol 58(7):896–904
Yamamoto E, Kogawa D, Tokura S, Hayashi K (2005) Effects of the frequency and duration of cyclic stress on the mechanical properties of cultured collagen fascicles from the rabbit patellar tendon. J Biomech Eng 127(7):1168–1175
Koch FP, Merkel C, Ziebart T, Smeets R, Walter C, Al-Nawas B (2012) Influence of bisphosphonates on the osteoblast RANKL and OPG gene expression in vitro. Clin Oral Investig 16(1):79–86
Li L, Han M, Li S, Wang L, Xu Y (2013) Cyclic tensile stress during physiological occlusal force enhances osteogenic differentiation of human periodontal ligament cells via ERK1/2-Elk1 MAPK pathway. DNA Cell Biol 32:488–497
Tang N, Zhao Z, Zhang L, Yu Q, Li J, Xu Z, Li X (2012) Up-regulated osteogenic transcription factors during early response of human periodontal ligament stem cells to cyclic tensile strain. Arch Med Sci 8(3):422–430
Diercke K, Kohl A, Lux CJ, Erber R (2011) Strain-dependent up-regulation of ephrin-B2 protein in periodontal ligament fibroblasts contributes to osteogenesis during tooth movement. J Biol Chem 286(43):37651–37664
Lossdorfer S, Yildiz F, Gotz W, Kheralla Y, Jager A (2010) Anabolic effect of intermittent PTH(1-34) on the local microenvironment during the late phase of periodontal repair in a rat model of tooth root resorption. Clin Oral Investig 14(1):89–98
Romer P, Kostler J, Koretsi V, Proff P (2013) Endotoxins potentiate COX-2 and RANKL expression in compressed PDL cells. Clin Oral Investig 17(9):2041–2048
Gilbert JA, Weinhold PS, Banes AJ, Link GW, Jones GL (1994) Strain profiles for circular cell culture plates containing flexible surfaces employed to mechanically deform cells in vitro. J Biomech 27(9):1169–1177
Stadelmann VA, Bonnet N, Pioletti DP (2011) Combined effects of zoledronate and mechanical stimulation on bone adaptation in an axially loaded mouse tibia. Clin Biomech (Bristol, Avon) 26(1):101–105
Braith RW, Conner JA, Fulton MN, Lisor CF, Casey DP, Howe KS, Baz MA (2007) Comparison of alendronate vs alendronate plus mechanical loading as prophylaxis for osteoporosis in lung transplant recipients: a pilot study. J Heart Lung Transplant 26(2):132–137
Acknowledgments
We thank Dr. Jutta Goldschmidt, Jutta Bühler, Ute Zerfass, and Lotte Groothusen for their assistance in the laboratory and Kathy Taylor for orthographic correction of the article.
Conflict of interest
The authors declare that no competing financial interest exists.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jacobs, C., Walter, C., Ziebart, T. et al. Mechanical loading influences the effects of bisphosphonates on human periodontal ligament fibroblasts. Clin Oral Invest 19, 699–708 (2015). https://doi.org/10.1007/s00784-014-1284-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-014-1284-4